Variation Laws and Influencing Factors of pH in Fishery Waters in Quzhou City
2021-07-08QinxuanSHIXuepingYEDongrenZHOUShengGAOQifangWUFengLINTingYE
Qinxuan SHI Xueping YE Dongren ZHOU Sheng GAO Qifang WU Feng LIN Ting YE
Abstract [Objectives]This study was conducted to strengthen the understanding of the variation laws of pH in fishery waters. [Methods]Based on the investigation data of pH, dissolved oxygen (Do), water temperature (T) and chlorophyll a (Chl. a) of the fishery waters in Quzhou City from 2017 to 2020, the seasonal variation characteristics and influencing factors of pH in the fishery waters were analyzed and discussed. [Results]The pH of the fishery waters in Quzhou City was in the range of 6.40-11.3, and was characterized by the highest seasonal variation in spring and the lowest in winter. The correlation analysis showed that water temperature and pH were significantly positively correlated in spring and summer (P<0.05), and the photosynthesis of phytoplankton was the main reason that affected the pH of water bodies in spring and summer. There was a negative correlation between water temperature and pH in winter, and the pH in the natural waters had a significant negative correlation with water temperature (P<0.05). The balance of carbonate system in the water bodies was the main reason that affected the pH of water in winter. The level of water eutrophication (Chl. a) affected the pH of fishery waters to a certain extent. When Chl. a<10 μg/L, there was a significant positive correlation between the pH of fishery waters and Chl. a (P<0.05). Various environmental factors had different effects on the pH of different types of fishery waters. The stepwise regression analysis showed that the pH of natural waters was mainly affected by Chl. a, and the pH of aquaculture waters was mainly affected by water temperature and dissolved oxygen. [Conclusions]This study provides certain theoretical support for actual production management.
Key words Fishery waters; pH; Influencing factors; Correlation
pH is an important indicator to characterize the acidity and alkalinity of water bodies, as well as an important factor affecting the ecological environment quality of water areas[1-2]. The lowering of pH in aquatic ecosystems not only affects the inorganic environment, but also affects the biodiversity in the waters and disrupts the balance of the ecosystem. The study of Zhuang[3]showed that different groups of organisms had different sensitivity to pH, and organisms at different developmental stages had different sensitivity to pH. In addition, the impact of low pH on the aquatic ecosystem is also manifested in the change of community structure and the destruction of the biological food chain. When water is acidified, the diversity and biomass of plankton will decrease, and the entire aquatic food chain will be destroyed[4].
Quzhou City is located in the upper reaches of the Qiantang River where many important fishery waters in Zhejiang Province are distributed. This region has many hilly mountains and belongs to the subtropical monsoon climate zone. The runoff is controlled by the monsoon, and the seasonal variation is large. Meanwhile, with the rapid economic development, the population density has increased rapidly, and the eutrophication of fishery waters has also increased significantly. The preliminary investigation found that the fishery waters and reservoirs in Quzhou area are generally mesotrophic, while the reservoirs with aquaculture function are characterized by eutrophication, and pH and dissolved oxygen are accompanied by simultaneous changes in water eutrophication[5]. Existing studies[6]have shown that in eutrophic water bodies, pH has a certain correlation with dissolved oxygen and chlorophyll a. By monitoring pH, phenomena such as "water bloom" can be predicted and early warned. Therefore, it is of great significance to study the pH variation of fishery waters in Quzhou. In the past, the research on the variation laws of pH of water bodies mostly focused on rivers, lakes, reservoirs and estuaries. There were few studies on fishery waters, and there was no related research on the variation laws of water pH and its influencing factors in Quzhou area. In this study, based on the investigation data of pH, dissolved oxygen (Do), water temperature (T) and chlorophyll a (Chl. a) of the fishery waters in Quzhou City from 2017 to 2020, we analyzed and explored the distribution, seasonal variation characteristics and influencing factors of pH in fishery waters (natural waters and aquaculture waters), aiming to strengthen the understanding of the variation laws of pH in fishery waters and provide certain theoretical support for actual production management.
Materials and Methods
According to the water system distribution characteristics of the fishery waters in Quzhou City, 5 natural water monitoring areas and 10 aquaculture areas were set up. The natural waters included germplasm resource protection waters―Qianjiangyuan protection area S1 (2 monitoring points),spawning cordage waters―Kaihua Changshan section S2 (4 monitoring points S2-1, S2-2), reproduction and releasing waters―Huangtankou Reservoir S3 (2 monitoring points), Xin'an Lake S4 (2 monitoring points) and Tongshanyuan Reservoir S5 (4 monitoring points). The aquaculture water areas included 5 reservoirs S6-S10 (in each of which 2 monitoring points were set up), and 5 aquaculture family farms S11-S15 (in each of which 2 aquaculture pond monitoring points were set up). The specific distribution of each monitoring point is shown in Fig. 1.
From 2017 to 2020, water quality samples were collected in spring (April), summer (July), autumn (September), and winter (November). The pH and dissolved oxygen in the water were measured on-site using the HACHHQ40D multifunctional water quality tester, and the water temperature was measured and recorded at the same time. A Ruttner water collector was used to collect surface water samples, and a 250 ml water sample was collected at each sampling point, added with a magnesium carbonate suspension and sealed in a brown glass bottle for chlorophyll a determination. After being labelled, the samples were stored in a low temperature incubator and transported back to the laboratory for determination by acetone extraction and spectrophotometry. In the whole analysis process, 3 samples were collected in parallel for each sample, and the relative standard deviation between the parallel samples was less than 5%. Meanwhile,the accuracy of chlorophyll a was carried out with a quality control sample.
Results and Analysis
Seasonal variation of pH in fishery waters
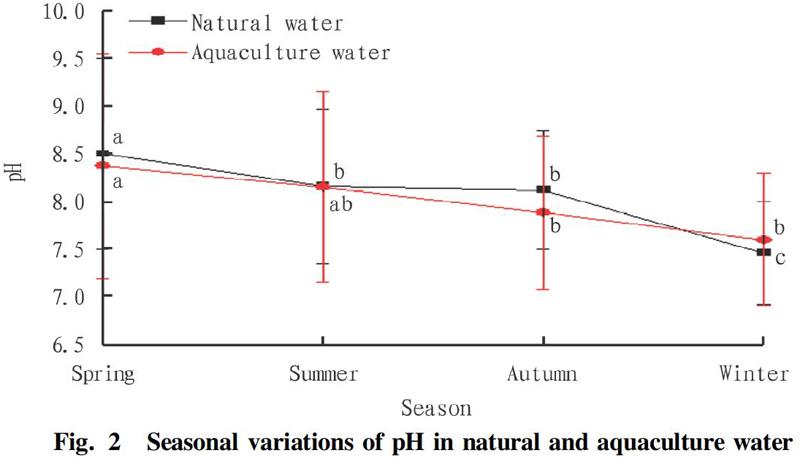
According to the investigation results, the pH range of natural fishery waters was 6.48-10.40, with an average value of 8.07±0.82. The pH values of natural fishery waters in different seasons were different (Fig. 2), showing a trend of gradually decreasing in spring, summer, autumn and winter, with the highest value in spring at 8.50±1.00 and the lowest value in winter at 7.46±0.54, which accords with the variation of water pH in most lakes in the middle and lower reaches of the Yangtze River in autumn and winter[7]. However, the variation laws of pH in spring and summer were different. The analysis of variance showed that the pH of the fishery waters in Quzhou in spring was significantly higher than that in other seasons (P<0.05), which is also different from the seasonal variation of the waters adjacent to the Yangtze River Estuary[8].
The pH range of the aquaculture waters was 6.40-11.30, with an average value of 7.99. From the perspective of different seasons (Fig. 2), the pH of the aquaculture waters showed a gradually decreasing trend in spring, summer, autumn and winter, with the highest pH in spring at 8.37±1.18 and the lowest in winter at 7.60±0.70, which was similar to the variation trend of pH in natural waters, which might be related to the fact that the city's aquaculture mainly uses natural waters such as rivers, reservoirs, and lakes as water sources. The analysis of variance showed that the pH of aquaculture waters was significantly higher in spring than in winter (P<0.05), and the differences between other seasons were not significant (P>0.05). It indicated that compared with natural waters, aquaculture waters were subject to artificial regulation, pH changed little with the seasons, and the water quality was relatively stable.
Interannual variation of pH in fishery waters
Comparing the variation laws in the pH of fishery waters during the four years (Fig. 3), the average pH values of natural waters from 2017 to 2020 were 7.76±0.96, 8.16±0.99, 8.13±0.59, and 8.20±0.62, respectively, and the analysis of variance showed that there were no significant differences in the pH of the water in the 4 years (P>0.05), which indicated that the natural fishery waters were relatively stable and were less affected by human factors such as production and life in the past 4 years. The average pH values of the aquaculture waters from 2017 to 2020 were 7.98±1.02, 8.30±1.25, 7.90±0.71, and 7.90±1.00, respectively, and the analysis of variance showed that the pH of the water bodies had no significant differences within 4 years (P>0.05), which was consistent with that of natural waters.
Correlation analysis of water pH and other environmental factors
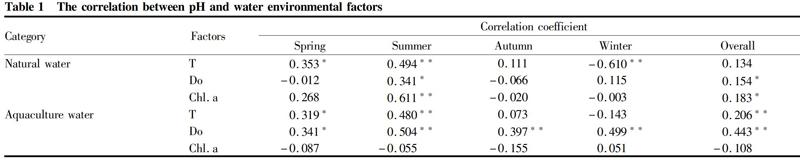
The correlation analysis between the pH of the fishery waters and other environmental factors (water temperature T, dissolved oxygen Do, Chl. a) is shown in Table 1. Since the pH of the fishery waters varied little from 2017 to 2020, and the interannual differences were not significant, so we analyzed the correlation of pH with other environmental factors in natural waters and aquaculture waters in different seasons.
From the analysis results, it can be seen that the pH of natural waters did not vary significantly with T, but the pH of the aquaculture waters was significantly positively correlated with T (P<0.05), indicating that the pH of aquaculture waters was significantly affected by the water temperature. From the perspective of different seasons, the pH of natural waters increased with the increase of T in the three seasons of spring, summer and autumn, and there was a significant positive correlation between pH and T in spring and summer (P<0.05), while a significant negative correlation was found between the pH and T of the water bodies in winter (P<0.05). Similar to natural waters, the pH of aquaculture waters increased with the increase of T in the three seasons of spring, summer and autumn, and there was a significant positive correlation between the pH and T in spring and summer (P<0.05), while in winter, it showed no significant correlation between pH and T(P>0.05). Since the water temperature of fishery waters is low in winter, the influence of water temperature on the biological effects of phytoplankton is weak, making the water temperature affect the first and second apparent dissociation constants of carbonic acid. As a result, the pH of fishery waters in winter increased with the decrease of water temperature. On the other hand, water temperature may affect the conversion of carbonate minerals, photosynthesis, respiration, and other physical, chemical and biological processes, which in turn affect pH[9-10]. Therefore, the pH of fishery waters in the three seasons of spring, summer and autumn increased with water temperature increasing, which is similar to the results of Shi et al.[8].
Further analysis of the correlation between pH and Do found that the pH and Do of fishery waters (natural waters + aquaculture waters) showed a significant positive correlation (P<0.05), which is consistent with the existing research results[11-14]. From the perspective of different seasons, the variation laws of pH with Do of different fishery waters varied with the seasons. The pH of natural waters was only positively correlated with Do in summer (P<0.05), while there was a significant positive correlation with Do in all four seasons in the aquaculture waters (P<0.05), which might be related to the fact that the pH of natural waters is affected by the process of CO2 exchange at the water-air interface to a certain degree. Do is affected by the exchange process at the water-gas interface. The higher the temperature, the lower the solubility of oxygen, carbon dioxide and other gases, which leads to the increase of water pH[9]. Due to the low nutrient content of some natural waters, the CO2 exchange process at water-gas interface may have some influences on the pH and Do of natural waters.
The correlation between Chl. a and pH characterizes the influences of phytoplankton and other biological actions on the pH of water bodies. The correlation analysis showed that there was a significant positive correlation between the pH of natural waters and Chl. a (P<0.05), which was mainly manifested in summer, indicating that the biological effects of phytoplankton and other organisms significantly affected the pH of natural waters in summer. On the contrary, the correlation between pH and Chl. a in aquaculture waters is not significant (P>0.05), which is consistent with the research results of Huang Suiliang et al.[15]on aquaculture waters. The respiration of aquatic organisms and residual bait oxidation and decomposition of organic matter in excrement may be the main reason for the above phenomenon. On the contrary, the correlation between pH and Chl. a in aquaculture waters was not significant (P>0.05), which is consistent with the research results of Huang et al.[15]on aquaculture waters. The respiration of aquatic organisms and the oxidation and decomposition of organic matter in residual baits and excrement in the breeding process may be the main reasons for the above phenomena.
Discussion
According to the Bulletin of China's Environmental Status, precipitation in the south of the Yangtze River is mostly acidic[16]. The fishery waters of Quzhou City are located in the acid rain area of the middle and lower reaches of the Yangtze River, but the pH of waters was investigated and analyzed to be weakly alkaline, and was free of acidification (pH<5.6), which is consistent with the investigation results of lakes in the middle and lower reaches of the Yangtze River[17-18], which may be because this area is located in the subtropical zone, the water bodies have a relatively high water temperature, and are rich in nutrients, and the photosynthesis of phytoplankton in the water bodies provides a certain buffering capacity for the water bodies' pH[19]. Secondly, the pH of the fishery waters in Quzhou City showed the variation characteristic of being the highest in spring and the lowest in winter, which is similar to the overall trend of the lakes in the middle and lower reaches of the Yangtze River in 2016, where the pH is the highest in summer and the lowest in winter, but there are some differences[7]. On the one hand, the pH of fishery waters was significantly positively correlated with water temperature in both spring and summer (P<0.05), indicating that photosynthesis of phytoplankton was the main factor pushing up the pH of fishery waters in spring and summer. On the other hand, the pH of fishery waters (natural waters + aquaculture waters) in summer and that in aquaculture waters in spring were significantly positively correlated with dissolved oxygen (P<0.05), while the pH of natural waters was negatively correlated with dissolved oxygen to a certain extent in spring, suggesting that the higher pH in spring might be related to the CO2 exchange process at the water-gas interface. The pH of fishery waters was the lowest in winter, but different types of waters had different conditions. The pH of natural waters was significantly negatively correlated with water temperature (P<0.05), but not correlated with dissolved oxygen (P>0.05), indicating that the decrease of water temperature caused the increase of water pH when the carbonate system of natural waters remained unchanged in winter[20]. The pH of the aquaculture waters was not correlated with the water temperature (P>0.05), but it had a significant positive correlation with the dissolved oxygen (P<0.05), which is similar to the research results of the Panjiakou Reservoir enclosure[15], Xiangshan Bay pond[21]and other culture waters, which may be related to biological processes such as phytoplankton growth. The large amounts of nutrients brought by aquaculture activities are conducive to the growth of phytoplankton, and changes in primary productivity caused by increased organic matter content and eutrophication may cause the variation laws in the pH of water bodies to be different from those in natural waters.
In order to further explore the impact of various environmental factors on the pH of different types of fishery waters, based on the correlation analysis (Table 1), stepwise regression analysis was carried out by selecting significantly correlated factors to obtain optimized regression equations (Table 2). The results showed that the pH of natural waters was mainly affected by chlorophyll a, and the pH of aquaculture waters was mainly affected by water temperature and dissolved oxygen. Compared with natural waters, in addition to being affected by photosynthesis of phytoplankton, in aquaculture waters, the respiration of cultured aquatic organisms and the high content of organic matter in the culture system would decompose and produce CO2, resulting in a complex relationship between water pH and chlorophyll a and weakening of the correlation.
In addition, eutrophic water bodies (Chl. a>10) accounted for 17.4% and 71.2% of natural waters and aquaculture waters, respectively, so the eutrophication level of water bodies might also be the cause of the differences in environmental impact factors in different waters. The analysis showed (Table 3) that when Chl. a<10 μg/L, the pH of natural waters and aquaculture waters both had a significant positive correlation with Chl. a (P<0.05), and when Chl. a>10 μg/L the correlation between the pH of natural waters and Chl. a was not significant (P>0.05), and the pH of aquaculture waters was significantly negatively correlated with Chl. a (P<0.05), which is different from the research results of Huang et al.[22]who found that pH and chlorophyll a had a significant positive correlation when the water body was in a eutrophic state. After some areas of natural waters receive the influx of industrial, agricultural and domestic sewage and some bait residues and aquatic animal excrement remain in aquaculture waters, the content of organic matter in the fishery waters is relatively high, resulting in a poor correlation between the pH of the water and chlorophyll a. The related influence mechanism needs to be further studied.
Conclusions
Based on the investigation data of pH, dissolved oxygen, water temperature and chlorophyll a in fishery waters in Quzhou from 2017 to 2020, we analyzed and explored the distribution, seasonal variation characteristics and influencing factors of pH in fishery waters (natural waters and aquaculture waters), obtaining following conclusions:
The pH of the fishery waters in Quzhou showed the seasonal variation characteristic of being the highest in spring and the lowest in winter, and the pH in spring, summer, autumn and winter showed a trend of gradually decreasing. The interannual variation in the pH of fishery waters in the 4 years was relatively small, and the water quality was relatively stable.
The variation of pH in fishery waters was affected by environmental factors such as water temperature and dissolved oxygen, and showed a certain seasonal variation. The correlation analysis showed that water temperature and pH were significantly positively correlated in spring and summer (P<0.05), and the photosynthesis of phytoplankton was the main reason that affected the pH of water bodies in spring and summer. There was a negative correlation between water temperature and pH in winter, and the pH in the natural waters had a significant negative correlation with water temperature (P<0.05). The balance of carbonate system in the water bodies was the main reason that affected the pH of water in winter.
The level of water eutrophication (Chl. a) affected the pH of fishery waters to a certain extent. When Chl. a<10 μg/L, there was a significant positive correlation between the pH of fishery waters and Chl. a (P<0.05); and when Chl. a>10 μg/L, the correlation between pH and Chl. a in natural waters was not significant (P>0.05), and there was a significant negative correlation between pH and Chl. a in aquaculture waters (P<0.05).
Various environmental factors had different effects on the pH of different types of fishery waters. The stepwise regression analysis showed that the pH of natural waters was mainly affected by Chl. a, and the pH of aquaculture waters was mainly affected by water temperature and dissolved oxygen.
References
[1]TANG QS, CHEN ZD, YU KF, et al. The effects of ocean acidification on marine organisms and ecosystem[J]. Chinese Science Bulletin, 2013, 58(14): 1307-1314. (in Chinese)
[2]LIN BY. Concise principles of environmental geochemistry[M]. Beijing: Metallurgical Industry Press, 1990. (in Chinese)
[3]ZHUANG DH. Effects of acid rain on some organisms in aquatic ecosystem[J]. Journal of Lake Sciences, 1993, 5(1): 85-91. (in Chinese)
[4]NILSSEN JP. Acidification of freshwater and limnetic organisms: Complex biotic interactions or statistics[J]. 1980.
[5]SHI QX, HAO GJ, YE T, et al. Research on eutrophication and its driving factors in reservoirs of the Quzhou Area[J]. Progress in Fishery Sciences, 2020. (in Chinese)
[6]WANG ZH, CUI FY, AN Q, et al. Study on influence of pH on the advance of eutrophication in reservoir [J]. Water & Wastewater Engineering, 2004, 30(5): 37-41. (in Chinese)
[7]LIU L. Effects of acid rain on the pH and zooplankton in lakes with different nutrient levels[D]. Hefei: Anhui Agricultural University, 2018. (in Chinese)
[8]SHI X, SONG JM, LI XG, et al. Seasonal change of ph in the waters off Changjiang River estuary and its impact factors[J]. Oceanologia et Limnologia Sinica, 2019, 50(5): 1033-1042. (in Chinese)
[9]SONG JM. Biogeochemical processes of biogenic elements in China Marginal Seas[M]. Berlin, Heidelberg: Springer, 2010.
[10]SONG JM, QU BX, LI XG, et al. Carbon source and sink in the Yellow Sea and East China Sea: atmospheric exchange, water dissolution and sediment burial[J]. Scientia Sinica (Terrae), 2018, 48(11): 1444-1455. (in Chinese)
[11]LUO DL. Study on the distribution of dissolved oxygen in Shenhu Bay and its relationship with phytoplankton and suspended matter[J]. Marine Science Bulletin, 2002, 21(1): 31-36. (in Chinese)
[12]ZHANG PL, SUN CJ. The influence of algae growing on pH and DO in surface water[J]. Environmental Monitoring in China, 2004, 20(4): 49-50. (in Chinese)
[13]ZHANG WT. An analysis of limiting factors of eutrophication in Dashahe Reservoir[J]. Guangdong Water Resources and Hydropower, 2009, 1(9): 26-28. (in Chinese)
[14]WANG XP, JIA XP, LIN Q, et al. The distribution of feature and relationship between the dissolved oxygen, salinity, ph and nutrition salts in the Waters of Hong Hai Bay[J]. Marine Science Bulletin, 1999, 18(5): 35-40. (in Chinese)
[15]HUANG SL, ZANG CJ, DU SL, et al. Study on the relationships among pH, dissolved oxygen and chlorophyll a I: Aquaculture water[J]. Chinese Journal of Environmental Engineering, 2011, 5(2): 1201-1208. (in Chinese)
[16]ZHAO YX, HOU Q. An analysis on spatial/temporal evolution of acid rain in China (1993-2006) and its causes[J]. Acta Meteorologica Sinica, 2008, 66(6): 1032-1042. (in Chinese)
[17]LIU RQ, ZHANG SY. Multivariable analyzing and comparing of water quality of shallow lakes in middle and lower reaches of Changjiang River[J]. Acta Hydrobiologica Sinica, 2000(5): 439-445. (in Chinese)
[18]LI SN, SHI XL, XIE WW, et al. Genetic diversity of picoeukaryotic phytoplankton in the lakes along the middle-lower reaches of the Yangtze River[J]. Environmental Science, 2013, 34(9): 3416-3422. (in Chinese)
[19]TAO DJ, WANG C. Investigation research of chemical properties of acidic precipitation and its influence to the pH value of Tai Lake water body[J]. Jiangsu Environmental Science and Technology, 2000, 13(2): 1-3. (in Chinese)
[20]GIESKES JM. Effect of temperature on the pH of seawater[J]. Limnology and Oceanography, 1969, 14(5): 679-685.
[21]XU YJ, FANG JG, WEI W. Application of Gracilaria lichenoides (Rhodophyta) for alleviating excess nutrients in aquaculture[J]. Appl. Phycol., 2008, 20(2): 199-203.
[22]HUANG SL, ZANG CJ, DU SL, et al. Study on the relationships among pH, dissolved oxygen and chlorophyll a I: Non-aquaculture water[J]. Chinese Journal of Environmental Engineering, 2011, 5(8): 1681-1688. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Review on Effects of Sunlight on the Internal Quality of Peach Fruit
- Research Progress on Genetic Breeding of Sweet Sorghum Related to Sugar Traits
- Screening of Red-flesh Small Watermelon Varieties for Substrate Cultivation in Spring Greenhouses
- Planting Techniques of Pennisetum giganteum in Huanghuai Area
- Bibliometric Analysis of Status Quo and Trend of the Research on Duck Based on the Web of Science Database
- Preparation and Insecticidal Activity of Sea Anemone Peptide AP-GI from Aiptasia pallida
