Indoor Propagation of Transgenic Glyphosate-tolerant Rapeseed (Brassica napus L.) and Transfer Breeding for Herbicide Tolerance
2021-07-08XiaoyingZHOUYaoyaoWUQiPENGSanxiongFUWeiZHANGHongliZHUJiefuZHANGSongCHEN
Xiaoying ZHOU Yaoyao WU Qi PENG Sanxiong FU Wei ZHANG Hongli ZHU Jiefu ZHANG Song CHEN

Abstract [Objectives]This study was conducted to investigate the possibility of screening for herbicide resistance in the early stage of rapes growth. [Methods]Eight treatments were set for a herbicide concentration experiment. [Results]Rape sprouts were highly sensitive to the herbicide glyphosate, and even a very low concentration of glyphosate solution (187 mg/L) was sufficient to kill most of the rape seedlings, while the genetically modified herbicide-tolerant rape could tolerate higher concentrations of the herbicide. Low concentrations of glyphosate could be used for screening at the sprout stage, and the results of herbicide-tolerance screening were consistent with PCR testing. [Conclusions]This study lays a technical and material foundation for accelerating the cultivation of new herbicide-resistant rape varieties suitable for rapeseed production areas in the lower reaches of the Yangtze River.
Key words Transgenic herbicide-tolerant rape; Indoor generation adding; Transfer breeding; Inheritance
Rape is one of the crops that are more severely damaged by weeds. The area damaged by weeds in the winter rape area in China reaches 45%-48%, and the area with moderate or severe damage reaches 22%-23%. The area of weeds in spring rape area is as high as 80%. Weeds have become an important factor restricting the improvement of rapeseed production in China[1]. Manual weeding in traditional cultivation mode requires a lot of labor input, which increases planting costs. The use of chemical herbicides has a certain effect on rape weed control. However, because monocot and dicot weeds often grow in the rape field in a mixed condition, the application time and dosage are strict, and it is often difficult to achieve the desired effect. Therefore, it is necessary to breed rape varieties resistant to broad-spectrum non-selective herbicides[2].
There are mainly two types of herbicide-resistant rape varieties currently cultivated. One is the herbicide-resistant mutant rape obtained by chemical or physical mutagenesis. There have been research reports at home and abroad: Swanson et al.[3]mutagenized rape microspores with the mutagen ethyl nitrosourea to obtain p-imidazolinone herbicide-resistant rape Pl. Hu et al. treated the Shuang 9 seeds of Brassica napus L. with EMS mutagens and obtained three tribenuron-methyl-resistant herbicide mutants K1, K4, K5[4]. Li et al.[5]selected herbicide-resistant mutants in rape somatic cell culture using Basta solution as a screening agent, the explants were taken from the seeds of Xiangyou 15 irradiated with 60Co-γ rays. Studies have found that such mutants often show a negative effect on yield[5-6].
The other is the herbicide-resistant rape varieties obtained by genetic engineering technology, among which the most widely used is transgenic glyphosate-resistant rape[7]. Glyphosate is a broad-spectrum systemic herbicide that blocks the pathway of shikimic acid metabolism in cells by inhibiting the activity of 5-enolpyruvyl shikimate-3-phosphate synthase (EPSPS), thereby hindering the synthesis of aromatic amino acids[8-9], leading to plant death. Compared with other herbicides, glyphosate has the advantages of good herbicidal effect and low environmental residues, and is widely used in weed control. Glyphosate can be closely adsorbed by soil particles and quickly degraded by soil microorganisms. Its half-life is less than 30 d, which is shorter than most herbicides[10]. Studies have shown that one month after spraying glyphosate, the contents of glyphosate and the metabolite AMPA (aminomethylphosphonic acid) of glyphosate in the soil are almost negligible[11]. At present, most of the resistance genes of glyphosate-resistant crops are from the CP4-mEPSPS gene of Agrobacterium[11].
Since 1996, genetically modified glyphosate-resistant crops with a single glyphosate resistance gene events (GR gene) or stacked gene events (herbicide tolerance and other trait, such as insect resistance) have accounted for about 80% of the global planting area of more than 175 million hm2 of genetically modified crops. The efficient application of genetically modified glyphosate-resistant crops has saved global farmers more than US$45 billion in costs from 1996 to 2012. Genetically modified soybeans and rapeseed crops have increased farmers' income by nearly 5%. Because planting genetically modified glyphosate-resistant crops has such obvious economic benefits, they have been extremely rapidly popularized in many countries outside the Americas and the Western Hemisphere[12]. The research on herbicide-resistant (tolerant) crops in China started early, from the 1990s, and bromoxynil-resistant rape was reported as early as 1997[13]. After years of accumulation, a batch of transgenic herbicide-resistant (tolerant) crops with the characteristic of stable expression have been obtained in recent years, such as glyphosate-resistant cotton[14-16], tobacco[16], glyphosate-resistant soybeans[17-19]and rice[20].
In 2018, a major special project for the cultivation and industrialization of new genetically modified rape varieties was officially launched. The research of genetically modified herbicide-resistant rape has entered the stage of breeding new varieties. Huazhong Agricultural University cloned the glyphosate resistance gene from Isoptericola variabilis[2]. The protein encoded by this gene does not have the 4 conservative sequences protected by the CP4-EPSPS patent, but it retains a high tolerance to glyphosate[14]. The rice transformant created by the I. variabilis-EPSPS gene can tolerate high doses of glyphosate at 8 400 g/hm2, showing good application prospects[14]. Liu introduced the modified glyphosate resistance gene mEPSPS into B. napus line Jia 572 through genetic transformation mediated by Agrobacterium tumefaciens to obtain transgenic rape plants. The first selfed generation of trans-mEPSPS rape could still grow normally under the spraying conditions of 100 times dilution of the 41% Roundup isopropylamine salt preparation (containing glyphosate 3 039 mg/L), while the control plants without the transgene all died after being sprayed with 200 times dilution of Roundup (containing 1 519 mg/L of glyphosate)[2]. At present, the transgenic glyphosate-tolerant rape germplasm is widely used as a herbicide-resistant parent material with independent intellectual property rights in the breeding of new glyphosate-tolerant rape varieties.
The cultivation of genetically modified rape that has not yet obtained an environmental safety certificate requires strict isolation conditions, and there are few experimental sites that can carry out genetically modified rape cultivation in China. Therefore, the research work related to the propagation and transfer breeding of transgenic rape is mostly limited to laboratory conditions. In this study, indoor multi-generation reproduction was successfully realized through artificial vernalization on rape seedlings under a low-temperature environment simulated with light incubators, and the glyphosate-tolerant gene was transferred to rape varieties in the ecological zone of the lower reaches of the Yangtze River through the conventional breeding techniques of hybridization and backcrossing combined with herbicide resistance screening, molecular detection and other mean. The preliminary experimental results were summarized as below.
Materials and Methods
Experimental materials
The transgenic glyphosate-resistant rape was transgenic HX-6, provided by Professor Zhou Yongming of Huazhong Agricultural University; and the parental rapeseed varieties (Ningyou 18, Ningyou 20, Ningyou 22, Ningyou 26, etc.) for transformation were bred and preserved by Rapeseed Research Laboratory, Institute of Industrial, Jiangsu Academy of Agricultural Sciences.
Glyphosate herbicide was a glyphosate ammonium salt produced by Jiangsu Haoshoucheng Weien Agrochemical Co., Ltd., with an effective ingredient content of 30%. The content of glyphosate ammonium salt was 33%.
The rape growth substrate with N, P, K≥2.5% was produced Jiangsu Xingnong Substrate Technology Co.Ltd.
The plant light incubators were E-41L2 of Percival Technology Company, USA.
Experimental methods
Planting of parents, acquisition of the F1 generation
The parent materials were all planted in pots at the end of September 2018, and they were naturally overwintered and vernalized outdoors. At the flowering period in March 2019, they were crossed with genetically modified herbicide-resistant rape HX-6, and F1 was harvested in mid-to-late May.
Indoor generation-adding and backcross transformation
At the end of May 2019, the seeds of genetically modified rape HX-6, parent P, and rape F1 were sown in trays filled with the cultivation substrate, and germinated in an artificial climate chamber with a photoperiod of 16/8 h at a day and night temperature of 22/15 ℃. When the seedlings grew to the stage of 4 and 5 leaves, they were transplanted to pots and trained at a low temperature for 3-4 d, during which the day and night temperature was 12/8℃ and the photoperiod was 16/8 h. Next, the temperature was further lowered to the day and night temperature of 8/5 ℃, at which the seedlings were grown for 25-30 d with the photoperiod of 16/8 h. Then, the day and night temperature was increased to 24/18 ℃, with the photoperiod remained at 16/8 h. Until the rape blossomed, selfing and backcrossing were carried out to obtain F2, BC1F1 and other generation materials.
Response of rape to the herbicide glyphosate at the seedling stage
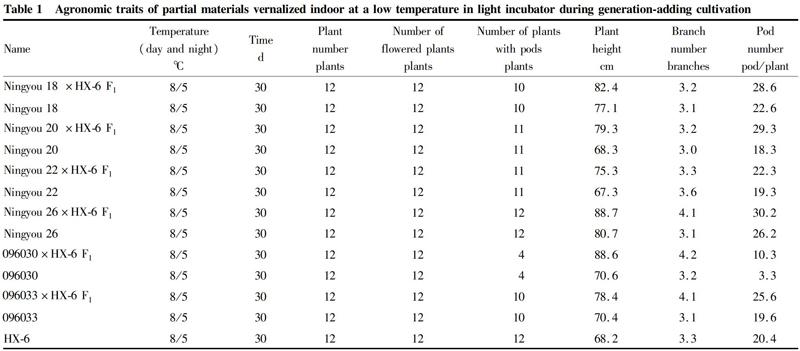
Eight treatments were set as ×100, ×200, ×400, ×800, ×1 000, ×1 200 and ×1 600 dilutions of glyphosate herbicide, and pure water, which could be converted into effective herbicide concentrations of 3 000, 1 500, 750, 375, 300, 250, 187.5 mg/L, and 0, respectively. The non-transgenic and transgenic rape HX-6 were treated at the seedling stage, in three repetitions. The seedlings were cultured in a plant growth chamber at a temperature of (22±2) ℃. After 2 weeks, the growth of rape and the rate of dead seedlings were investigated, and a response curve of the rate of dead seedlings and herbicide concentration was drawn.
Responses of transgenic rape progeny to herbicide resistance
The rape seedlings of each hybrid generation were sprayed with a half-lethal dose concentration, and the resistance of rape to the herbicide was investigated after 2 weeks.
PCR detection of genetically modified herbicide-resistant rape
According to the sequence of the herbicide glyphosate-resistant gene, specific primers for PCR were designed: EPSPS-F: ATTAGCGCTAGGGACGTGAG; EPSPS-R: ATTAGCGCTCCCACATCCTGTC, and the genetically modified herbicide-resistant rape was detected by PCR. The PCR reaction program was started with pre-denaturation at 94 ℃ for 5 min, followed by 35 cycles of denaturation at 94 ℃ for 1 min, annealing at 58 ℃ for 30 s and extension at 72 ℃ for 30 s, and completed with extension at 72 ℃ for 5 min. The PCR products were detected by 1% agarose gel electrophoresis. The product with a size of about 600 bp indicated a positive plant.
Results and Analysis
Indoor generation-adding rape propagation
In this study, artificial climatechambers were used to create a low-temperature environment for rape growth. When the rape was grown to the stage of 4 and 5 leaves, the rape seedlings were treated at a low temperature for vernalization. After 30 d of low-temperature treatment, all tested materials were vernalized, that is, bolted and flowered normally. Only several materials had poor fecundity. The test results are shown in Table 1.
Response of rape sprouts to glyphosate at different concentrations
In this study, 7 glyphosate solutions with different dilution multiples and with water were sprayed to rape at the seedling stage (with flat cotyledons, heart leaf just emerging). At 3-4 d after spraying, sprouts of the control rape basically stopped growing, and began to die one after another 1 week later. Especially the treatments with higher concentrations, the phenomenon of seedling death appeared earlier, but every concentration of treatment in this study showed a higher rate of seedling death, even if the seedlings sprayed with the glyphosate solution with a dilution factor of 1 600, the death rate of sprouts also reached more than 90%, indicating that the rape material was quite sensitive to glyphosate herbicide during the sprout stage (Fig. 1). The transgenic rape seedlings showed strong herbicide tolerance: even under the treatment of high concentrations of ×100 and ×200, some of the seedlings survived. Plotting the death rate with the concentration of the herbicide, a response curve was obtained (Fig. 2). The response curves of the non-genetically modified rape and the genetically modified rape to the herbicide were quite different. The former was relatively steep, and the death rate reached a high value almost at very low concentrations, while the latter changed relatively slowly. After preliminary calculations, the half-lethal dose of glyphosate to common rape during the sprout stage was about 100 mg/L, and the half-lethal dose for the genetically modified herbicide-tolerant rape was about 1 400 mg/L.
The blue curve is for the non-genetically modified rape, and the orange is for the genetically modified herbicide-tolerant rape.
Response of transformed progeny to glyphosate
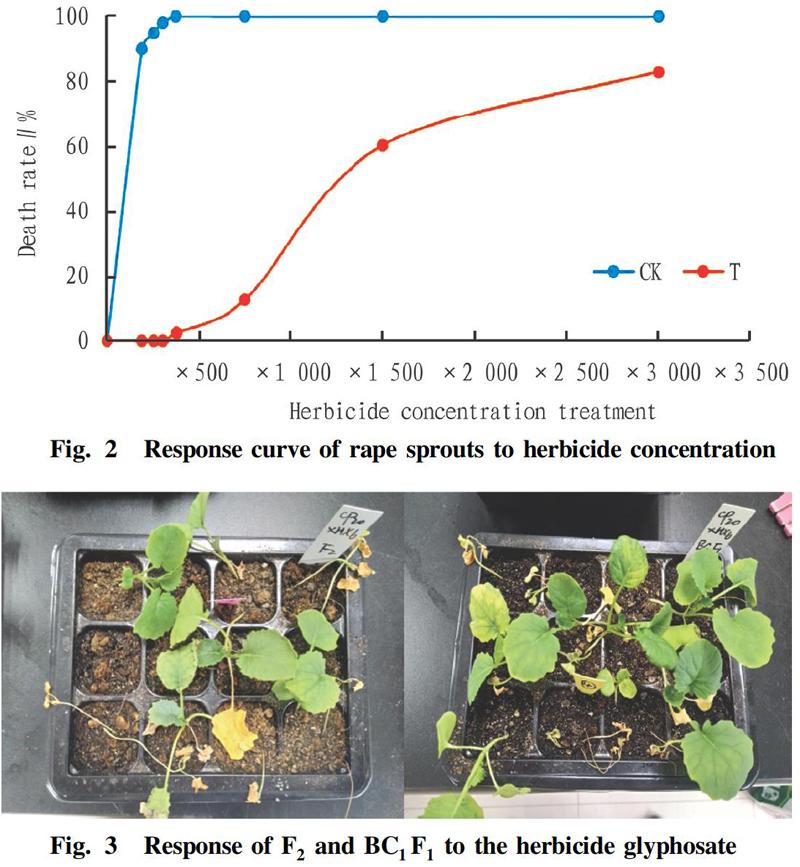
Approximately 200-fold dilution of the herbicide (1 500 mg/L) was sprayed on the genetically modified rape (F2 and BC1F1) at the sprout stage. The results showed that the rape seedlings gradually showed phytotoxicity symptoms, including yellow leaves, stagnant growth and death. This result also indicated that the rape sprouts were highly sensitive to glyphosate herbicide during the sprout stage. However, some seedlings resumed growth, and these seedlings showed strong herbicide tolerance.
PCR detection of genetically modified herbicide-resistant rape
Specific PCR primers were designed according to the gene sequence to establish a PCR identification method. The genomic DNA of part of the rapeseed seedlings treated with herbicides in "Response of rape sprouts to glyphosate at different concentrations" was extracted and identified by PCR. The results showed that the survived rape all could show specific bands after amplification, while the dead rape seedlings were mostly negative. Based on this result, we used PCR to molecularly identify genetically modified herbicide-resistant rape.
PCR analysis for the positive ratios in F2, F1, and BC1F1
The F1, F2, and BC1F1 plants obtained by crossing transgenic herbicide-resistant rape with conventional rape were detected by PCR. The results are shown in Table 2. The test results of the F1 generation plants showed that all plants were able to show the positive band in amplification, but only the negative control failed to show the positive band (Fig. 4). The test results of the F2 population showed that most plants showed the positive band during amplification, while a small number of plants failed to show the positive band (Fig. 5), and the ratio of positive plants to negative plants was close to 3∶1. After the chi-square test, the segregation ratio of positive plants to negative plants in the tested F2 population conformed to a ratio of 3∶1. The test results of the BC1F1 population showed (Fig. 6) that the segregation ratio of positive plants to negative plants conformed to 1∶1.
Conclusions and Discussion
In view of the fact that the genetically modified herbicide-tolerant rape has not yet obtained environmental release permits, experimental studies need to be conducted in a controlled room. In order to speed up the breeding process of genetically modified herbicide tolerance, we carried out indoor rape vernalization experiments. The growth cycle of B. napus is generally about 220-240 days. Winter and semi-winter B. napus usually need to go through a period of low temperature growth before they can bloom and bear fruit, which is called vernalization. The vernalization conditions of different rape varieties are slightly different. There were more research reports on artificial vernalization of rape in the past. Shi et al.[21]showed that after treating the test-tube plantlets of semi-winter rape in a light incubator at 6-8 ℃ for 32 d, all plants could be vernalized.
With reference to previous studies, combining the existing plant lighting incubator equipment, we designed the following indoor generation-adding program: seedlings were raised in pots, subjected to low-temperature artificial vernalization at a temperature of 8/5 ℃ with a photoperiod of 16/8 h for 30 d continuously when they reached the stages of 4 to 5 leaves, and then moved to a plant growth room with a temperature of about 22 ℃ for further growth. In our research, most of the tested materials were vernalized, that is, they bolted and flowered when grown at room temperature for about 20-30 d. Through auxiliary pollination, or hybrid pollination, most materials produced normal pods. Only some materials had poor fecundity, even produced no pods or as few as 1-3 seeds per pod. After the growth process designed in this study, the full growth period of the tested materials were shortened to about 125 d, which was nearly half of the time earlier than the rape grown in the natural environment.
In this study, the feasibility of screening for herbicide resistance in the early stage of rape growth was investigated. Through the herbicide concentration test, the research results showed that rape sprouts were highly sensitive to herbicide glyphosate, even the glyphosate solution with very low concentration (187 mg/L) was also sufficient to kill most of the rape seedlings, while the genetically modified herbicide-tolerant rape could tolerate higher concentrations of the herbicide. We tried to screen the F2 and BC1F1 rape populations harboring the glyphosate-tolerant gene with 1 000-1 500 mg/L glyphosate dilutions, and the survived rape seedlings all could be detected with the characteristic band of the resistance gene, which provides convenience for molecular marker-assisted screening.
The F2 and BC1F1 populations were analyzed and detected for the herbicide resistance gene by PCR. The results showed that the transgenic glyphosate-resistant rape harbored one copy of the transgene, and the herbicide resistance was a dominant inheritance controlled by a pair of genes. It indicated that the transgenic herbicide-tolerant trait could be transformed by conventional cross and backcross methods. In this study, the glyphosate-resistant gene was transferred into the Ningyou series of rape varieties bred in the ecological region of the lower reaches of the Yangtze River through conventional cross-breeding and backcross breeding methods, in order to lay a technical and material foundation for accelerating the cultivation of new herbicide-resistant rape varieties suitable for rapeseed production areas in the lower reaches of the Yangtze River.
References
[1]PU HM, QI CK, ZHANG JF, et al. Preliminary report on breeding of rapeseed (B. napus L.) hybrid with resistance to herbicide[J]. Jiangsu Agricultural Sciences, 2004(3): 27-29. (in Chinese)
[2]LIU H, ZHOU YM. Genetic transformation of rapeseed with the glyphosate-resistant gene mEPSPS [J]. Chinese Journal of Oil Crop Sciences, 2012, 34(6): 582-585. (in Chinese)
[3]SWANSON EB, COWNAUS MP, BROWN GL, et al. Chracterization of herbicide tolerant plants in Brassica napus L. after in vitro selection of microspores and protoplasts[J]. Plant Cell Reports, 1988, 7(2): 83-87
[4]QU GP, SUN YY, PANG HX, et al. EMS mutagenesis and ALS-inhibitor herbicide-resistant mutants of Brassica napus L.[J]. Chinese Journal of Oil Crop Sciences, 2014, 36(1): 025-031. (in Chinese)
[5]LI P, FU YF, XIAO G, et al. Selection and identification of herbicide resistant mutants in rapeseed[J]. Chinese Journal of Oil Crop Sciences, 2008, 30(3): 361-365. (in Chinese)
[6]MAGHA MI, GUERCHE P, BREGEON M, et al. Characterization of a spontaneous rapeseed mutant tolerant to sulfonylurea and imidazolinone herbicide[J]. Plant Breed, 1993(11): 132-141
[7]SUNDBY C, CHOW WS, ANDERSON JM. Effects on photosynthesis II function, photoinhibition, and plant performance of the spontaneous mutation of serine-264 in the photosystem II reaction center D1 protein in triazine-resistant Brassica napus L.[J]. Plant Physiol, 1993(103): 105-113.
[8]GIANESSI LP. Economic and herbicide use impacts of glyphosate-resistant crops[J]. Pest Manag Sci, 2005(61): 241-245.
[9]GRUYS KJ, WALKER MC, SIKORSKI JA. Substrate synergism and the steady-state kinetic reaction mechanism for EPSP synthase from E. coli[J]. Biochemistry, 1992(31): 5534-5544.
[10]GRUYS KJ, MARZABADI MR, PANSEGRAU PD, et al. Steady state kinetic evaluation of the reverse reaction for Escherichia coli 5-enolpyruvylshikimate-3-phosphate synthase[J]. Arch Biochem Biophys, 1993(304): 345-351.
[11]CERDEIRA AL, DUKE SO. The current status and environmental impacts of glyphosate-resistant crops: A review [J]. J Environ Qual, 2006(35): 1633-1658.
[12]VEIGA F, ZAPATA JM, FERNANDEZ MARCOS ML, et al. Dynamics of glyphosate and aminomethylphosphonic acid in a forest soil in Galicia, north-west Spain[J]. Sci Total Environ, 2001(27): 135-144.
[13]ZHONG R, ZHU F, LIU YL, et al. Oilseed rape transformation and the establishment of a bromoxynil-resistant transgenic oilseed rape[J]. Journal of Integrative Plant biology, 1997(1): 22-27. (in Chinese)
[14]ZHAO FY, XIE LX, TIAN YC, et al. Glyphosate-resistant and bollworm-resistant transgenic cotton plants with the aroAM12 and Bts1m genes[J]. Acta Agronomica Sinica, 2005, 31(1): 108-113. (in Chinese)
[15]WANG X, MA YB, WU X, et al. Molecular biology identification of transgenic cotton lines expressing exogenous G10aroA gene[J]. Scientia Agricultura Sinica, 2014, 47(6): 1051-1057. (in Chinese)
[16]LIU XJ, LIU YH, WANG ZX, et al. Generation of glyphosate-tolerant transgenic tobacco and cotton by transformation with a 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS) gene[J]. Journal of Agricultural Biotechnology, 2007, 15(6): 958-963.
[17]QIU LJ, GUO BF, GUO Y, et al. A glyphosate resistant transgenic soybean and preparation method and application thereof: WO 2017113573 A1[P]. 2017. (in Chinese)
[18]RONG F, WANG G, JI J, et al. Research of "minimal wound brush" to acquire genetically modified glyphosate-resistant soybean[J]. Soybean Science, 2015, 34(2): 217-223. (in Chinese)
[19]XIAO PY, LIU Y, CAO YP. Overexpression of G10-EPSPS in soybean provides high glyphosate tolerance[J]. Journal of Integrative Agriculture 2019, 18(8): 1851-1858
[20]CUI Y, HUANG SQ, LIU ZD, et al. Development of novel glyphosate-tolerant japonica rice lines: A step toward commercial release[J]. Front Plant Sci, 2016(7): 1218-1229
[21]SHI SW, ZHOU YM, WU JS. Studies on vernalization method of tube plantlets from semi-winter rapeseed (Brassica napus L.)[J]. Southwest China Journal of Agricultural Sciences, 2004, 17 (5): 584-587. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Review on Effects of Sunlight on the Internal Quality of Peach Fruit
- Research Progress on Genetic Breeding of Sweet Sorghum Related to Sugar Traits
- Screening of Red-flesh Small Watermelon Varieties for Substrate Cultivation in Spring Greenhouses
- Planting Techniques of Pennisetum giganteum in Huanghuai Area
- Bibliometric Analysis of Status Quo and Trend of the Research on Duck Based on the Web of Science Database
- Preparation and Insecticidal Activity of Sea Anemone Peptide AP-GI from Aiptasia pallida
