Ulcerative colitis triggered by pegylated interferon alpha-2b in a patient with chronic hepatitis B:A case report and literature review☆
2021-07-02ZhishuoMoJinTngZeqinWuDioChenDongyingXiePeipeiWng
Zhishuo Mo,Jin Tng,Zeqin Wu,Dio Chen,Dongying Xie,Peipei Wng,*
a Department of Infectious Diseases,The Third Affiliated Hospital of Sun Yat-sen University,Guangzhou,China
b Department of Gastroenterology,The Sixth Affiliated Hospital of Sun Yat-sen University,Guangzhou,China
Keywords:Interferon (IFN)Hepatitis Chronic hepatitis B (CHB)Chronic hepatitis C (CHC)Ulcerative colitis (UC)
ABSTRACT Interferon (IFN) is a multifaceted immunomodulator that is effective against many diseases,including chronic hepatitis B and chronic hepatitis C infection.IFN defends against viral infection,but may also cause various side effects,such as ulcerative colitis (UC).Herein,we present a case of UC triggered by pegylated interferon alpha-2b (PEG-IFN-α-2b) therapy in a patient with concurrent chronic hepatitis B(CHB) infection.The diagnosis was based on typical clinical symptoms,colonoscopy findings,colonic mucosal biopsy,and histopathology.Accordingly,treatment with mesalazine was initiated without stopping PEG-IFN-α-2b.Fortunately,UC relieved gradually without compromising the effects of treatment.Simultaneously,we conducted a literature review of previously published case reports on the side effect of UC in patients with underlying chronic hepatitis.Various reactions have been reported,including induction,exacerbation,and no change.This is the first report of UC triggered by PEG-IFN-α-2b in a CHB patient.We recommend that physicians pay attention to the rare side effect of UC during administration of PEG-IFN-α-2b.Mesalazine can relieve UC with sustained use of PEG-IFN-α-2b.
1.Introduction
Chronic hepatitis B (CHB) and chronic hepatitis C (CHC) infection are severe global health problems that pose a significant socioeconomic burden worldwide.1Interferon(IFN)and nucleos(t)ide analogues are both first-line therapies for CHB.At present,major authoritative clinical guidelines for treatment of CHB recommend aiming for a functional cure(i.e.,hepatitis B surface antigen(HBsAg)loss with/without positive hepatitis B surface antibody (anti-HBs)seroconversion) in eligible patients.2-4INF boasts significant advantages in terms of HBsAg clearance as shown in an increasing number of studies.5-8IFN combined with ribavirin was previously applied as a standard regimen for CHC.In recent years,with the emergence of direct antiviral agents for CHC,the use of IFNcontaining regimens has decreased worldwide.However,given the cost and shortage of the supply of direct antiviral agents,IFNcontaining regimens continue to be used in some areas.
IFN exerts antiviral,antiangiogenic,and immunomodulatory effects.The most frequent side effects are flu-like symptoms,myelosuppression,nausea,diarrhea,and weight loss.9Because of its potent immunomodulatory effects,IFN may induce various autoimmune diseases during long-term treatment.10-12There are few published reports of newly developed ulcerative colitis (UC)during IFN treatment.Herein,we describe a case of UC triggered by pegylated interferon alpha-2b (PEG-IFN-α-2b) in a patient with CHB.To our knowledge,this is the first report of such an occurrence.
2.Case report
The study was approved by the Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University.Written informed consent was obtained from the patient.A 39-year-old man with CHB was treated with tenofovir disoproxil fumarate (TDF) for 3 years.He presented with low HBsAg level (273.7 IU/mL).After providing consent to IFN treatment (which aims to achieve functional cure of CHB,and is recommended by authoritative clinical practice guidelines),the patient discontinued TDF and underwent treatment with PEG-IFN-α-2b (180 μg/0.5 mL per week,hypodermic injection) beginning in September 2018.
Two and a half months after initiation of PEG-IFN-α-2b therapy,he began experiencing five to six mucous/bloody diarrheal defecations per day,accompanied by an increase in bowel movement pain and intermittent lower abdominal pain,beginning in December 2018,with weight loss of 5.5 kg.Gastrointestinal history and clinical physical examination were unrevealing.Stool examination for ova(including ova of Clonorchis sinensis),parasites,and cultures,and serologic tests for human immunodeficiency virus(HIV) infection were negative.Colonoscopy revealed diffuse hyperemia and edematous mucosa in the colon,obscured vessel pattern,increased fragility,and easy bleeding on contact.UC was therefore suspected (Fig.1A).Biopsies of the mucosa described above revealed interstitial edema,fibrosis,hyaline degeneration,and crypt epithelial cell apoptosis (Fig.1B),which confirmed the diagnosis of UC.
The patient was referred to the Centre for Inflammatory Bowel Disease of The Sixth Affiliated Hospital of Sun Yat-sen University in January 2019.Treatment with mesalazine(5-aminosalicylic acid(5-ASA),3.0 g orally per day) and enemas (0.5 g,twice per day) was initiated.With reference to published literature and with the consent of the patient,PEG-IFN-α-2b was continued.One month later,the patient reported improvement in diarrhea to one to two mucous defecations per day,and abdominal pain disappeared.Beginning in March 2019,the dose of 5-ASA was changed (2.25 g orally per day and 0.5 g suppository per day).Hypomyxia was observed within the next 3 months without other abdominal discomforts.Weight loss remained at 7 kg over the next 9 months.PEG-IFN-α-2b was discontinued in August 2019 because the expected efficacy was not met:HBsAg was 301.1 IU/mL at week 48(48W) (453.6 IU/mL at 12W;406.5 IU/mL at 24W;346.4 IU/mL at 36W).Clinical data of the patient during PEG-IFN-α-2b treatment are shown in Table 1.Moreover,the patient and his spouse planned to have their second child.TDF was restarted and mesalazine was simultaneously discontinued.Colonoscopy revealed marked improvement.The mucosa was intact and no corrosive focus or ulcer was observed(Fig.1C).
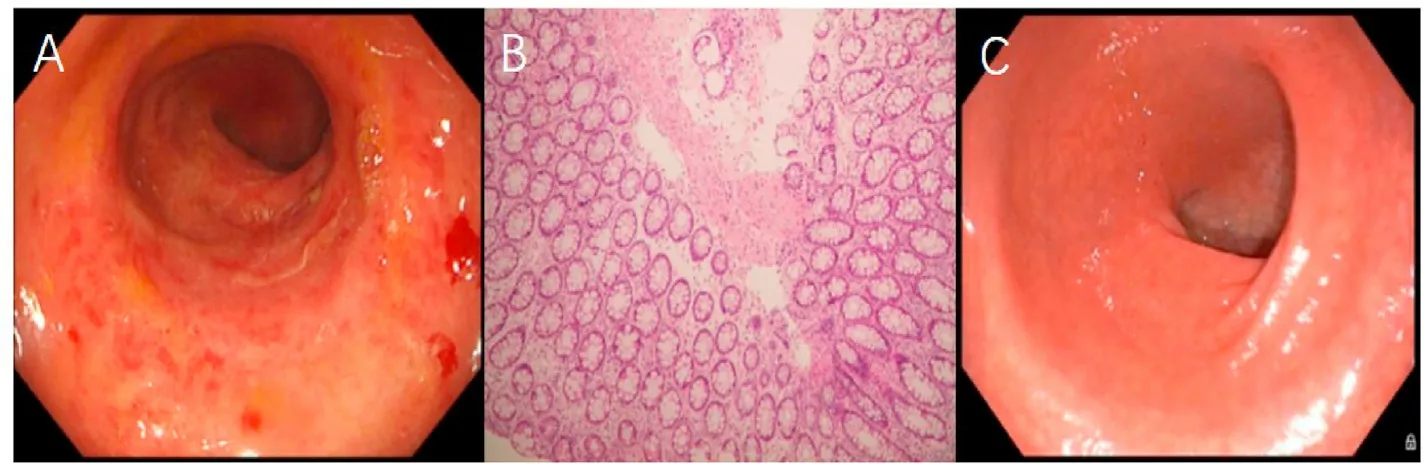
Fig.1.Colonoscopy and biopsy findings of the patient.
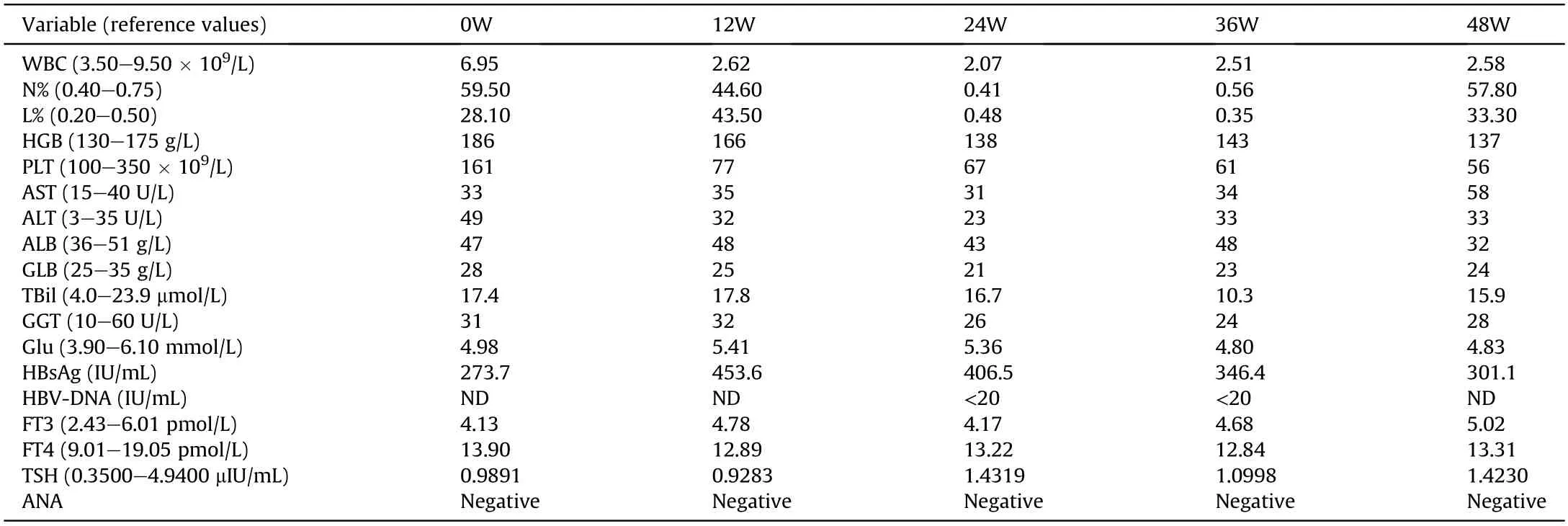
Table 1 Clinical data of the patient during PEG-IFN-α-2b treatment.
The patient was followed every 3 months over the next year.He regained weight (10 kg) by the first follow-up and an additional 2 kg by the second follow-up.His body weight remained stable after a half-year of follow-up.Regular bowel movements reappeared during the first week following discontinuation of PEG-IFNα-2b.
3.Discussion
In the present case,UC was apparently triggered by PEG-IFN-α-2b monotherapy for CHB.We have not previously encountered such an occurrence.A literature search using the China National Knowledge Infrastructure and WANFANG database (keywords:interferon,hepatitis,ulcerative colitis;retrieval period:1990-2020) identified zero such cases in China.Conversely,literature search using PubMed(retrieval period:1990-2020)revealed 19 reports,including 36 cases(Table 2):nine from Japan,four from Italy,two from Spain,and one each from Turkey,Austria,Portugal,and Greece.13-31Some of the above studies only have English titles or abstracts,14-18,20,22,29and detailed information is lacking.Analysis of the remaining available data revealed that most of these reports were of patients with underlying CHC (26/28),whereas only two cases were of CHB.Most patients were male,with a male:female ratio of 18:5.All 36 patients were diagnosed with chronic hepatitis,27 of whom had concurrent UC before IFN treatment.Following administration of IFN treatment,the 27 patients with UC experienced different responses,including exacerbation in five patients(interval period:18 days to 4.5 months),remission in three patients,and no change in 19 patients.During IFN treatment,UC developed in eight of nine patients with chronic hepatitis,while one patient developed UC 7 days after IFN treatment was stopped.According to available data from the above studies,both newly developed or aggravated symptoms of UC can be alleviated by steroids,mesalazine,or salicylazosulfapyridine(SASP),either alone or in combination.
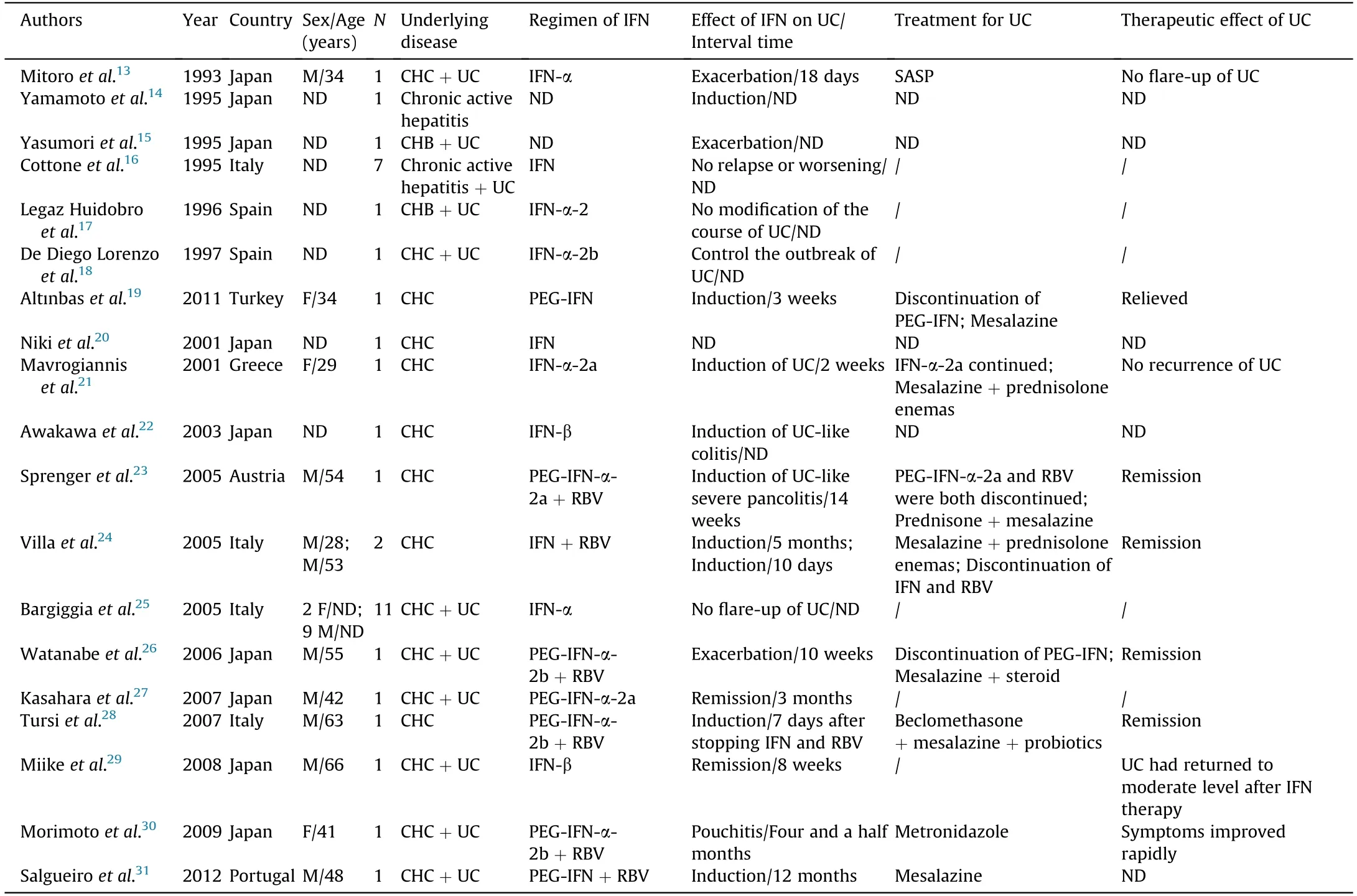
Table 2 Reported cases of UC during IFN treatment of chronic active hepatitis patients.
UC is an idiopathic inflammatory disease,with relapsing and remitting mucosal inflammation of the colon and rectum.32The exact cause of UC remains unclear,although it is universally acknowledged that it results from an intestinal immune response induced by intraluminal microbiota or other toxic pathogens under the setting of a host genetic predisposition.33There is evidence that interleukin(IL)-4 and IL-13 mRNA levels are significantly increased in rectal biopsies from patients with UC.34IL-13 was shown to be a key T-helper (Th) 2 cytokine involved in the pathogenesis of UC.35,36Additionally,Th2-polarized T cells producing IL-5 were found in patients with UC.37Most pertinent studies have shown that UC is a modified Th2-related disease,and the Th1/Th2 imbalance may be the primary factor influencing development of IFN-induced UC.38
Studies on the effects of IFN on UC are contradictory.IFN-α was shown to be an important factor in T cell differentiation toward the Th1-type of immune response by downregulating Th2 cytokines.39-41This observation revealed the potential of IFN as treatment for UC.Furthermore,Sümer et al.42reported on 28 patients with chronic active UC who did not respond to salicylic acid or steroid therapy and were treated with IFN-α-2a.Twenty-three of them were relieved of symptoms thereafter.In another study,Musch et al.43showed that 88%of 25 patients with steroid-resistant UC achieved remission with IFN-β-1b treatment.Recently,a randomized study also proved the beneficial effect of IFN-β-1a for treatment of UC.44There are three case reports of active UC patients with concurrent hepatitis C virus infection who achieved remission with IFN treatment.18,27,29In contrast,as a proinflammatory cytokine,IFN can theoretically also induce relapse of inflammatory bowel disease.18The case reports described above support this point of view.13,15,26Finally,the phenomenon whereby there is no effect of IFN on UC appears to represent the largest proportion of cases.Some randomized controlled trials found no significant difference between IFN and placebo in the effective remission rate of UC,although good safety was observed during treatment.45-47
4.Conclusions
Herein,we present a previously unreported case of UC triggered by PEG-IFN-α-2b in a CHB patient.Although current studies show that UC is a rare side effect of IFN in chronic hepatitis patients,based on the present report,physicians should pay attention to this side effect during IFN treatment.
Authors’ contributions
Z.Mo and J.Tang contributed equally to this work.Study conception and design:P.Wang and Z.Mo;Acquisition of data:Z.Wu and D.Chen;Drafting of the manuscript:Z.Mo;Critical revision:J.Tang;Acquisition of funding:D.Xie.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study was supported by National Science and Technology Major Project of China:No.2017ZX10202202-005-009.
杂志排行
Liver Research的其它文章
- Expert consensus on perioperative management of liver transplantation in adults with acute-on-chronic liver failure☆,☆☆,☆☆☆
- Hsa-miR-637 inhibits human hepatocyte proliferation by targeting Med1-interacting proteins☆
- Liver-specific deletion of mechanistic target of rapamycin does not protect against acetaminophen-induced liver injury in mice☆
- Novel organoid model in drug screening:Past,present,and future☆
- Vitamins and non-alcoholic fatty liver disease:A molecular insight☆
- Mesenchymal stem cells therapy for acute liver failure:Recent advances and future perspectives☆