The effect of baduanjin exercise in rehabilitation for functional ankle instability: A study protocol for a ra ndomized controlled pilot trial
2021-06-29
ABSTRACT
Keywords: Baduanjin, balance function, functional ankle instability, surface electromyography
INTRODUCTION
Ankle sprains occur frequently in daily life and during physical exercise. There are 23,000 cases of ankle sprains per day in the USA[1]and about 1.5 million ankle sprains in UK emergency departments each year.[2]A study has shown that about 20%–40% of people develop chronic ankle instability (CAI) after an acute ankle sprain.[3]In general, CAI is divided into functional ankle instability (FAI)and “mechanical ankle instability (MAI),” Doctors usually certify FAI through reference to the history of the rates of recurrent ankle sprains, and a “giving-way” feeling occurring during physical movement, with or without mechanical instability.[4-6]Four-fifth of ankle osteoarthritis cases are reported to be caused by previous musculoskeletal injuries,and these patients are on average 10 years younger than those with primary ankle osteoarthritis.[7]Thus, FAI can develop into MAI.[8]If treatment is not timely, an ankle injury can lead to joint inflammation, degeneration, and other cartilage symptoms.
In patients with severe cases of FAI, there may be permanent inability to move the joint. Patients with FAI have problems with joint control, balance, and gait due to the impairment in neuromuscular control and sensorimotor problems. The functional changes mainly include reduced muscle strength,decreased ankle joint proprioception, and prolonged muscle response time. Furthermore, FAI can result in recurrent ankle sprains and chronic ankle joint pain. Patients with FAI often feel unable to risk relying on the affected joint and may have asymmetry in respect of their posture (which can include asymmetry related to sitting and standing) and asymmetry of motor function (which can include balancing and coordinating functions and can occur in both the limbs and the trunk).[9]Asymmetry of posture and of motor function can lead to problems with the lumbar spine, spinal muscles, and the knee joint that bears the increased body weight. These disorders can seriously affect a patient’s abilities and quality of life.
Nonsurgical treatment is ordinarily the primary approach taken for most patients, while the earlier surgical treatment is generally used in patients with MAI and in elite athletes.[10,11]Rehabilitation exercise plays a definitive role in conservative (nonsurgical) treatment and includes strength training of the muscles around the ankle, standing stability training, proprioceptive training under different conditions,and the use of external support methods such as bandages and Kinesio (elastic therapeutic) taping. Previous studies have shown that conservative treatment is effective in the prevention of ankle sprain recurrences and with regard to the stability of the ankle.[12-14]
Drawing on PubMed, China National Knowledge Infrastructure,and the Wanfang and VIP databases, our literature review concerning ankle sprains in both the sports and medical fields (in China and internationally) indicated that ankle sprains and CAI have been attracting increased scholarly attention in recent years. More than 100 articles are published in English every year that cover the diagnosis, biomechanical characteristics, and treatment of acute ankle sprains[9]and CAI.[15]The above-noted high rates of ankle sprains and sequelae impact patients’ health, which is one factor leading to the proliferation of studies pertaining to ankle sprains, CAI,and other related problems over the past 20 years.Baduanjin is a widely practiced form of exercise in China. Its history can be traced back to ancient times. In recent years,many studies[16-18]on the efficacy of Baduanjin exercise on psychosomatic function in different groups of patients have been or will be conducted. It has been found to be effective in improving adults’ physical flexibility, increasing mind–body coordination,[19]and modulating blood lipid metabolism.[20]Baduanjin improves pulmonary function in patients with chronic obstructive pulmonary disease[21]and increases self-efficacy in patients with cardiovascular diseases.[22]It can ameliorate the symptoms of depression effectively and safely in patients with depression, and it can also reduce these patients’ blood glucose levels.[23]Baduanjin is also a safe adjunctive rehabilitation method for patients who have suffered from a stroke, as it has been shown to have benefits regarding lower extremity sensorimotor function, depression,and activities of daily living (ADL).[24]Researchers have also identified the positive effects of Baduanjin on balance[24,25]and have shown that it could improve gait performance and functional mobility in patients with Parkinson’s disease.[26]
The assessment and treatment of patients with CAI, in China and internationally, still requires further study.[27]At present,rehabilitation exercise programs for patients with FAI tend to focus on a single joint or limb, and many of the exercises require supervision or direct assistance from therapists or other individuals. To our knowledge, there are presently no effective or convenient methods for the other associated challenges, such as those related to weight-bearing on both lower limbs, symmetry of bilateral limb movements, and postural symmetry.
All Baduanjin movements are bilateral and bilateral limb movement is conducive to the improvement of bilateral limb coordination, balance function, and symmetry of the body.Considering all the potential as well as proven benefits of Baduanjin, a randomized controlled trial (RCT) will be used to explore the efficacy and safety of this form of exercise in individuals with FAI. The hypothesis is that the patients in the Baduanjin group will show better outcomes in comparison to those in conventional treatment group.
METHODS
Design and setting
An assessor-blinded, parallel-controlled RCT will be implemented to observe the efficacy and safety of Baduanjin in patients with FAI. The trial will be carried out in Dongzhimen Hospital, the First Affiliated Hospital of Beijing University of Chinese Medicine. A total of 72 patients with FAI who are eligible according to the inclusion and exclusion criteria will be recruited and randomly assigned (using a 1:1 ratio) to the Baduanjin or the conventional treatment groups. Outcomes will be evaluated at three time points: baseline, 2 weeks, and 4 weeks [Figure 1].
Ethics
The trial has been approved by the Research Ethical Committee of Dongzhimen Hospital, the First Affiliated Hospital of Beijing University of Chinese Medicine (no.DZMEC-KY-2019-18), and it will follow the principles of the Consolidated Standards of Reporting Trials statements as well as the Declaration of Helsinki. The trial is registered with the Chinese Clinical Trial Registry (ChiCTR1900021939).Any changes of the protocol will be reported to the Research Ethical Committee, which will determine whether the change of the protocol is necessary. The members of the study team will supervise the safety of participants during the trial.
Participants and recruitment
Participant recruitment will be conducted in the outpatient clinics of the Rehabilitation Department and the Orthopedics Department of Dongzhimen Hospital, the First Affiliated Hospital of Beijing University of Chinese Medicine. An advertisement will be posted on the hospital’s bulletin board. The advert will provide the contact information of the eligibility screener (who will not be involved in other study tasks).
Inclusion criteria
The inclusion criteria based on those originally proposed by the International Ankle Consortium in 2014[28]are as follows:
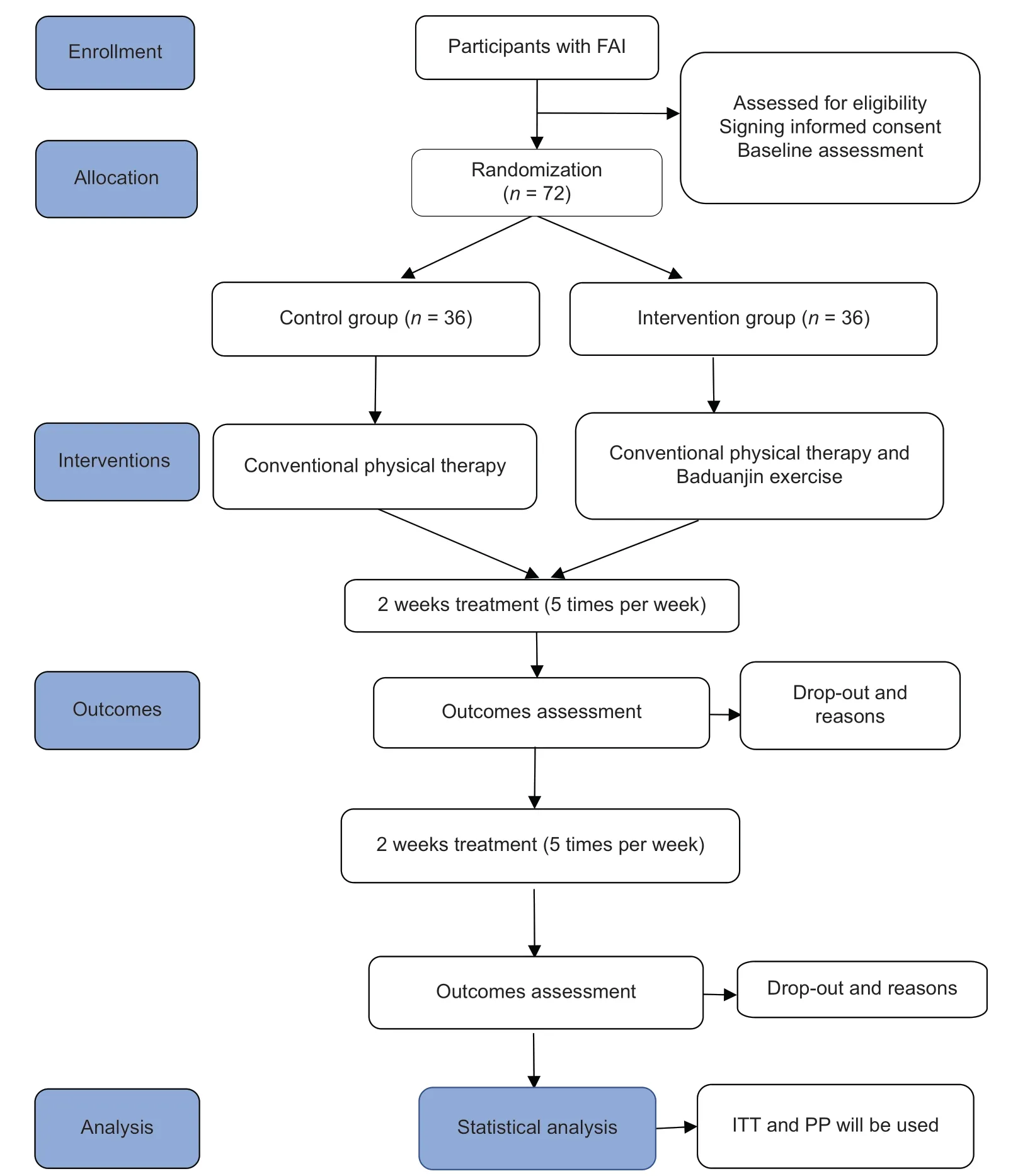
Figure 1: Flowchart of the trial. FAI: Funcほonal ankle instability; ITT: Intenほon‑to‑treat analysis; PP: Perprotocol analysis
1. Aged 15–50 years
2. A history of at least one significant ankle sprain The initial sprain must have occurred at least 12 months prior to study enrollment, was associated with inflammatory symptoms (pain, swelling, etc.), and led to at least one interrupted day of desired physical activity.The most recent injury must have occurred more than 3 months prior to study enrollment. An ankle sprain is defined as “an acute traumatic injury to the lateral ligament complex of the ankle joint as a result of excessive inversion of the rear foot or a combined plantar flexion and adduction of the foot. This usually results in some initial deficits of function and disability”[8]
3. A history of the previously injured ankle joint“giving-way” (with at least two episodes of “giving-way”in the 6 months prior to study enrollment) and/or“recurrent sprain” and/or “feelings of instability”The phrase “giving-way” is defined as “The regular occurrence of uncontrolled and unpredictable episodes of excessive inversion of the rear foot (usually experienced during initial contact while walking or running), which do not result in an acute lateral ankle sprain.”[28]The phrase“recurrent sprain” is defined as two or more sprains to the same ankle.[8]The phrase “feeling of ankle joint instability” is defined as “The situation whereby during ADL and sporting activities, the participant feels that the ankle joint is unstable, and is usually associated with the fear of sustaining an acute ligament sprain”[8]
4. Cumberland Ankle Instability Tool (CAIT)[29]score <24
5. Written informed consent.
Exclusion criteria
The exclusion criteria which again follow those of the International Ankle Consortium in 2014[28]are as follows:
1. A history of previous surgeries to the musculoskeletal structures (i.e., bones, joint structures, and nerves) in either lower-limb extremity It is understood and accepted in clinical and research practice that surgery to repair insufficient joint structures is designed to restore structural integrity but creates residual changes in the central and peripheral portions of the nervous system. Even with appropriate rehabilitation and follow-up management, there are concomitant neuromuscular and structural alterations after surgery that would confound the ability to isolate the effects of CAI
2. A history of a fracture in either lower-limb extremity requiring realignment Similar to the first exclusion criterion, significant compromise to skeletal tissue threatens the internal validity of the selection of participants with isolated CAI
3. Acute injury (i.e., sprains or fractures) to musculoskeletal structures of other lower extremity joints in the previous 3 months, which affected joint integrity and function resulting in at least 1 interrupted day of desired physical activity
4. Central nervous system disease, ear disease history,cognitive dysfunction, or other diseases which make the participants unable to cooperate with the rehabilitation treatment and assessment.
Informed consent
The patients will be notified about the study method and processes and will be informed of their rights, the benefits of taking part in the trial, and the things the patients will do if the patients consent to take part. Participation will be voluntary and participants will be able to withdraw from the trial at any point for any reason. If there are any adverse events (including any accidents), participants will be asked if they want to withdraw. If participants withdraw from the trial, their data will be saved and analyzed at the end of the trial. Participants will sign an informed consent form when they agree to take part in the trial.
Sample size
Since there are no available data from prior studies, this trial will entail a preliminary pilot study (based on the availability of resources); therefore, the sample size will be small. Following several rounds of discussion and demonstration, the experts of the present study group decided to include a sample size of 30 patients in each group. Allowing for a potential 20% loss rate,36 patients should therefore be included in each group.[30,31]
It is anticipated that this research will provide some useful data regarding implementing a sample size calculation of large-scale RCTs in future.
Randomization and allocation concealment
A random number list has been generated using the computer by the study statistician, and the random opaque envelope method was used for grouping concealment. Patients who are eligible according to the inclusion and exclusion criteria and who consent to take part in the trial will be assigned to the Baduanjin group or the conventional treatment group.The group allocation outcomes will be sealed in opaque envelopes; the group allocation manager will open each envelope and inform the eligible participants of their group assignments.
Blinding
The eligibility screener, outcome assessor, data analysts, and data collectors will be blinded to each participant’s group allocation. The eligibility screener will be responsible for collecting the baseline data. The group allocation manager will inform the Chinese medicine rehabilitation specialist about participants’ information in the Baduanjin group. However,during the trial, it will be impossible to blind participants regarding group allocation. Accordingly, before the trial, the differences between the two study groups will be explained to the participants. The participants will know that they must do the exercise in accordance with the trial plan if they consent to participate. The rehabilitation specialist and Chinese medicine rehabilitation specialist will monitor and instruct the participants’ exercise programs. The eligibility screener,group allocation manager, and outcome assessors will not be responsible for patient treatment or data analysis. The rehabilitation specialists and Chinese medicine rehabilitation specialist will not be responsible for data analysis or any other study work. Before the data analysis stage, all the data will be saved by the researcher who is responsible for the data analysis and the blinding will last until the data analysis ends.All the researchers have been trained with regard to the trial plan and schedule. All the researchers will not communicate details pertaining to group allocation or data analysis privately and will not violate the trial schedule.
Interventions
Conventional treatment group
Participants allocated to the conventional treatment group will receive conventional rehabilitation therapy[32-34]including muscle strengthening, balance function training, gait training,and so on. The qualified and experienced rehabilitation specialists will arrange suitable therapy for the participants.The specialists have been trained before the start of the trial and will select appropriate treatments according to the participants’ symptoms. All patients did rehabilitation training for 30 min every day, 5 days/week, 4 weeks in total.
Baduanjin group
Participants allocated to the Baduanjin group will be trained in this form of exercise by a Chinese medicine rehabilitation specialist who is qualified and experienced in teaching Baduanjin in addition to the conventional treatment therapy. The Chinese medicine rehabilitation specialist will first present the official and standard teaching video of Baduanjin (which was made by the General Administration of Sport of China) and then assign printed materials to the participants that will further help them to understand the principles and standards of exercising through Baduanjin.The Baduanjin group will undertake additional Baduanjin exercises after completing conventional treatment therapy,with the Baduanjin training consistently performed for 30 min every time and last for 4 weeks. The Chinese medicine rehabilitation specialist will instruct the participants about the Baduanjin exercise and ensure their safety.
Outcome measures
Several objective evaluation parameters and a patient-reported quantitative evaluation outcome measure will be selected to observe and investigate our study’s outcomes. The primary outcome measurement will include surface electromyography (sEMG) results pertaining to the bilateral erector spinae between the fifth lumbar and the first sacral (because there are more problems in this section),tibialis anterior, and peroneus longus extracted from graphic information generated by the FlexComp Infiniti System (Canada).The secondary outcome measurement will comprise balance function outcomes generated by the NeuroCom Balance Manager system (46 mm × 46 mm; Basic Balance Master,USA) and CAIT scores. All primary and secondary outcome measurements will be assessed at baseline, 2-week, and 4-week time points by an experienced and qualified outcome assessor.
The outcome assessor who has been trained about the schedule of the trial assessment will be blinded to each participant’s group allocation until the end of the trial.
Basic demographic characteristic
The two groups’ participant demographic information,including gender, age, ethnicity, occupation, medical history, and other detailed information, will be recorded and collected at the baseline time point to compare their statuses and features. The rehabilitation specialists will monitor participants’ statuses and measure their vital signs, such as blood pressure, respiration rate, and pulse.
Primary outcome measures SURFACE ELECTROMYOGRAPHY RESULTS
The peroneal muscles play an important role in supporting the ankle joint, and its function is regarded as one of the primary outcome measures. The tibialis anterior muscle’s function mostly influences the gait performance, while the bilateral erector spinae is important with respect to the core muscles and so the sEMG results will be set as the primary outcome measurements. The sEMG of every muscle will be tested for three times while it is static or dynamic. The sEMG oscillation’s mean root-mean-square and median frequency will be selected as the observed parameter. The differences in all of these bilateral muscle function results will be compared before and after treatment, and the differences between the Baduanjin and conventional treatment groups will be analyzed.
Secondary outcome measures Balance function
The evaluation equipment of the Neurocom Balance Manager system includes a 46 mm × 46 mm fixed force plate, a 46 mm × 46 mm foam surface, two data processors, and a screen. The evaluation outcomes are shown in a chart,with the center-of-gravity (COG) track presented in each evaluation condition, and a comprehensive score that shows patients’ COG control ability also featured in the chart. The evaluation items include a Modified Clinical Test of Sensory Interaction on Balance, a weight-bearing squat (WBS), limits of stability, unilateral stance (US), and rhythmic weight shift.The characteristics of balance function will change under different conditions. Two parameters will be focused: WBS and US. The differences in balance function between the groups, and the trend in balance function over time in the two groups, will be assessed. The WBS and the US can show the difference in participants’ bilateral weight-bearing and balance function.
Cumberland ankle instability tool questionnaire
The CAIT questionnaire will be used for the secondary outcome measures. The questionnaire includes nine self-reported questions for participants to answer. The contents relate to pain around the ankle joint, balance function, ankle sensorimotor function, ankle sprains, and severity of ankle instability. The higher the questionnaire score, the better the ankle function.[35]
Adverse events
Should an adverse event occur during the trial, the researcher will report it to the Research Ethical Committee and record it on the case report form (CRF). Furthermore, the researchers will protect the study’s participants and arrange adaptive exercises for them according to the study schedule. If the participants experience any adverse events (e.g., recurrent ankle sprain, fatigue, etc.), the researchers will take first aid treatment and the clinician in the study group will come to participate in the treatment. Moreover, the participants will be able to withdraw from the trial if they need to suspend the exercise arises [Figure 2]. This protocol has been planned in accordance with the Standard Protocol Items:Recommendations for Interventional Trials 2013 Checklist.[36]
Data management and monitoring
When the assessor completes a CRF, the data collectors will save the contents of the form, which include the baseline data and outcome measurements data, on a computer. The two data collectors will check the contents for each other to make sure all of their data are correct. All the materials and data including the electronic data and paper forms will be saved in the database at the Clinical Research Center of Dongzhimen Hospital. Only the members of the trial group will have access to and the right to check the data. The participants’ personal information will not be revealed.
Statistical analysis
The researchers blinded to group allocation will analyze the data using Statistical Product and Service Solutions (SPSS)software version 20.0 (IBM, USA) for Windows (Chicago, IL,USA). The results’ change (including the sEMG of bilateral muscles, bilateral weight-bearing capacity) of the Baduanjin and conventional treatment groups will be assessed. The data between the Baduanjin and conventional treatment groups over time at three time points (baseline, 2 weeks, and four weeks) will be compared to further explore the functional recovery over time and the mechanism of action of Baduanjin in patients with FAI. For normally distributed continuous variables, the t-test will be used, and for nonnormally distributed continuous variables, the Mann–Whitney U-test will be used. The continuous variables are shown with means and standard deviations. Count data will be described according to the number of cases and percentages, and the Chi-squared test will be applied to analyze the count data.The intention-to-treat analysis and perprotocol analysiswill be applied during our analysis of the results, and all the results after carrying out will be analyzed by the two methods. All statistical analyses will use two-tailed tests; the statistical significance threshold will be set at 0.05, with 95%confidence intervals.
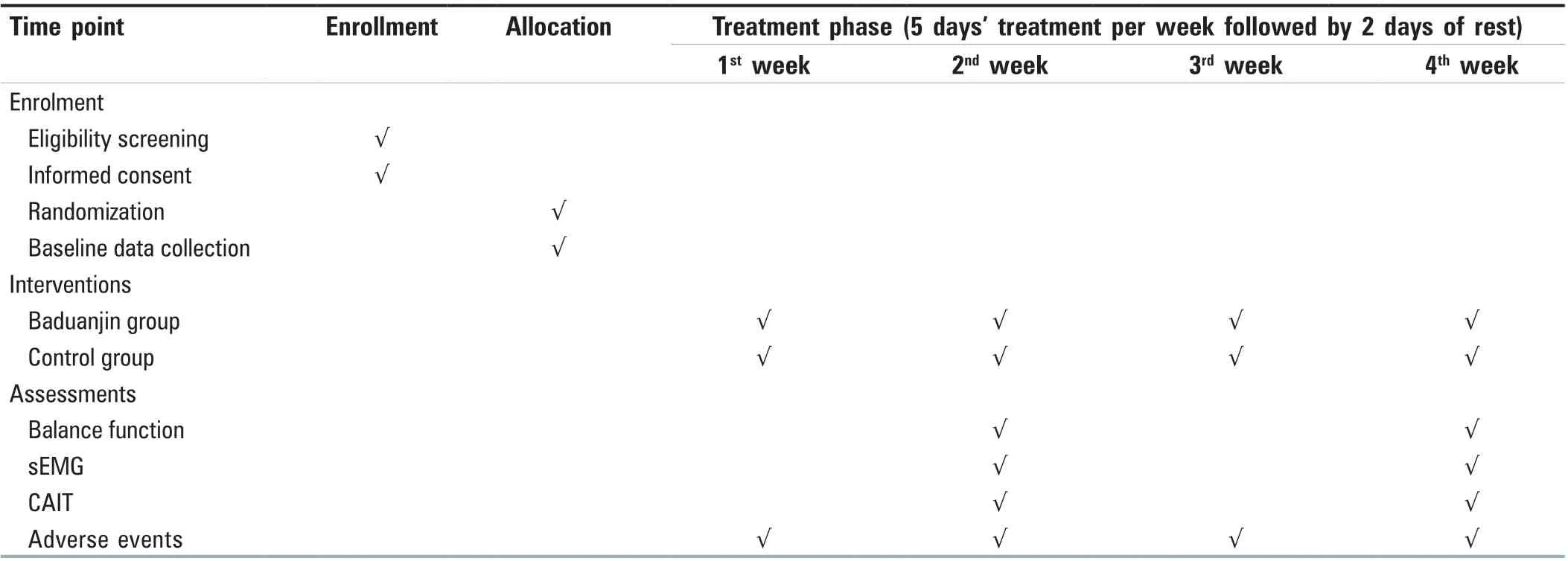
Figure 2: Planned visit schedule of enrollment, interventions, and assessments
Trial status
The trial is currently in the recruitment phase. This trial started on April 1, 2019, and will end on December 31, 2020.
DICUSSION
Baduanjin involves easy to learn bilateral movements that patients with unstable ankle joints can perform by themselves. Bilateral limb movements are helpful for improving coordination, postural symmetry, and balance function. In addition, during the practice of Baduanjin, the knee joints are slightly bent, which helps to increase lower limb strength. Closed-chain motion (in which the distal segment, such as the foot, is fixed against resistance) is a safer weight-bearing motion than open-chain motion (where in the distal segment is not fixed). Thus, Baduanjin is suitable for patients with an unstable ankle joint. Baduanjin movements are simple to learn and can be easily adhered to.
During the practice of Baduanjin, respiratory movements and respiratory rhythm promote the contraction and stability of the core muscles, which improves trunk stability,postural control, and postural symmetry. The requirements regarding limb movement coordination can also promote the contraction and stability of core muscles. The practice of Baduanjin requires stable posture and a focus on the exercise, and individuals must maintain control of their body as much as possible, in order that, the more muscles will be mobilized and the greater strength will be used. In this trial,the hypothesis on the efficacy of Baduanjin will be studied more deeply.
This trial will be the first to study the efficacy of Baduanjin on patients with FAI. The results will provide data about the effects of Baduanjin on patients with FAI, in terms of whether it can provide easy, safe, and effective relief for such patients, and with reference to its effects on postural symmetry and erector spinae functional status. The study’s outcome assessor will test the bilateral erector spinae,tibialis anterior, and peroneus longus muscles to observe whether the bilateral muscles become more symmetrical after Baduanjin. If Baduanjin can play an important role in improving balance and postural symmetry, patients with FAI could routinely practice the exercise by themselves, which would reduce medical costs. The study’s findings could
highlight the importance of Baduanjin in promoting the bilateral symmetry of motor function. Additionally, it may give rise to more effective methods with which to reduce the rates of acute and chronic ankle injury, particularly among more physically active individuals. The effect of Baduanjin on ankle injuries has not been previously studied. The results of this study will provide clinical data on the problems associated with ankle injuries, and may determine whether Baduanjin has an effect on the bilateral symmetry of motor function and balance function in individuals with FAI. The results may suggest positive outcomes in favor of the Baduanjin, such that a much larger RCT is required in the future to further test the clinical effectiveness of this traditional Chinese form of exercise.
Ethics approval and consent to participate
This trial has been approved by the Research Ethical Committee of Dongzhimen Hospital, the First Affiliated Hospital of Beijing University of Chinese Medicine (no.DZMEC-KY-2019-18). Each participant will sign an informed consent form before he or she enters into the trial and each consent form will be saved in the corresponding CRF.
Financial support and sponsorship
The study is supported by the Fundamental Research Funds for the Central Universities (grant no. 2019-JYB-JS-053).
Conflicts of interest
There are no conflicts of interest.
杂志排行
Journal of Integrative Nursing的其它文章
- Operations of school health program assessment at selected secondary schools in Egor Local Government Area, Benin City, Nigeria
- Control effect of structured skin care plan of integrated Chinese and Western medicine in elderly patients with incontinence‑associated dermatitis
- Analysis of risk factors for lymphedema of the lower limbs after endometrial cancer surgery and suggestions for prevention and treatment
- Effect of structured education program on physiological and psychosocial outcomes in type 2 diabetes patients:A randomized controlled trial
