Synchronous Variations in Abundance and Distribution of Ommastrephes bartramii and Dosidicus gigas in the Pacific Ocean
2021-06-25YUWeiCHENXinjunandLIULianwei
YU Wei, CHEN Xinjun, and LIU Lianwei
Synchronous Variations in Abundance and Distribution ofandin the Pacific Ocean
YU Wei1), 3,) 4), 5), 6), CHEN Xinjun1), 2), 3), 4), 5), 6), *, and LIU Lianwei7)
1),,201306,2),,266237,3),,201306,4),,,201306,5),,201306,6),,201306,7),316021,
An analysis was performed in this study to investigate synchronous fluctuations in abundance and distribution ofin the Northwest Pacific Ocean andin the Southeast Pacific Ocean. The impacts of two Niño indices and regional water surface temperature on the two squids during 2006–2015 were evaluated, which possibly can explain the observed synchronicity. Catch per unit effort (CPUE) and the latitudinal gravity centers (LATG) of fishing effort were used to indicate squid abundance and distribution, respectively. The results indicated that both the CPUE and LATG showed highly interannual variations and synchronous fluctuation with significant negative associations between the two squid species from September to November. Strong positive cross-correlations with 2-month lag was found between sea surface temperature (SST) anomaly in the Niño 3.4 and Niño 1+2 regions, which have significant linkage with the SST on the fishing ground ofand, respectively. Moreover, the proportion of favorable-SST area (PFSST) and the latitudinal location of the optimal SST forandwere positively correlated with the CPUE and LATG, respectively. IncreasedPFSST clearly corresponded to decreasedPFSST in phase as well as the latitudinal location of the optimal SST from September to November over 2006– 2015. Our findings suggest that synchronous changes in abundance and distribution of the two squids were due to simultaneous variations in the PFSST and the latitudinal location of the optimal SST front which were affected by the SSTA changes in the Niño 3.4 and Niño 1+2 regions.
;; distribution and abundance; synchronous variability; environmental effects
1 Introduction
Ommastrephid squidsare characterized by short lifecycle, rapid growth, early maturation, high migratory ca- pacity, and complicated recruitment patterns (Boyle, 1990; Dunning and Wormuth, 1998; Rodhouse, 2008). Most squids inhabit in the waters of the shelf, slope, and open oceans, with the depths from the surface to 2000m (Anderson and Rodhouse, 2001). Squids play a critical role in the marine food weds, serving as prey for large-size marine animals and predator for small-size fish and zooplankton (Cherel and Weimerskirch, 1995; Parry, 2006). Many squids are economically important and considered as the crucial com- mercial fishery target among global distant-water fisher- ies (Arkhipkin., 2015). Annual catches of Ommas- trephid squids in the recent decade are over two million tons, accounting for about 50% of the cephalopod catches in the world (Chen., 2008). Among the Ommastrephid squids, the oceanic squid such as Japanese flying squidin the Sea of Japan, the East China Sea and the westerns Pacific Ocean (Kang., 2002; Lee., 2019), thein New Zealand waters (Jackson., 2000), the neon flying squidin the North Pacific Ocean (Bower and Ichii, 2005) and the jumbo flying squidin the Eastern Pacific Ocean (Nigmatullin., 2001) are the most important species in terms of catches and economic values. The two latter squid specie are the main fishing targets by Chinese squid-jigging fisheries.Mainland China started to exploitin 1993 andin 2001. Both catches accounted for a large amount of the total catches in China (Chen., 2008).
is an abundant squid species widely distributed in the North Pacific Ocean (Bower and Ichii, 2005). Thepopulation includes two seasonal spawning cohorts: the winter-spring cohort and the autumn cohort. Each cohort has different geographical stocks (Chen and Chiu, 2003). The former cohort comprises the western and central-eastern stocks, and the latter cohort comprises the central and eastern stocks (Ma., 2011). Both cohorts perform south-to-north migration from the spawning ground in the subtropical front to the feeding ground in the subarctic domain. At present,is mainly captured by China (including Chinese Taipei) and Japan (Ichii., 2006). Mainland China tar- geted the western winter-spring cohort on the fishing ground between 35˚–50˚N and 150˚–175˚E (Chen., 2009; Yu., 2015). The annual catch in China accounts for more than 80% of the total catches ofin the North Pacific Ocean (Chen., 2008).
For, it is a large-size squid species widely distributed in the Eastern Pacific Ocean (Argüelles., 2001). This squid is largely utilized by hundreds of international squid-jigging fishing vessels from Asia-Pacific (., China and Japan) and South America-Pacific countries (., Peru and Chile) (Taipe., 2001; Chen., 2008). Most fishing operations occur at night, using powerful lamps attracting the squids. At present, four major fishing grounds are largely exploited in the Gulf of California, the offshore waters of the Costa Rica Dome, the high seas at the equator between 5˚N–5˚S and 130˚– 90˚W, and the coastal and oceanic regions off Peru andChile (Hernández-Herrera., 1998; Waluda., 2004; Zeidberg and Robison, 2007; Morales-Bojórquez and Pa- checo-Bedoya, 2016). In the Southern Hemisphere, the most abundant fishing grounds are located in the oceanic regions off Peru, which are mainly exploited by Chinese fishing vessels (Hu., 2019). The total catches offrom China are the highest and accounted for about 50% of the total catches in the world (Chen., 2008).
The yearly catch ofandfrom Chi-na is high; however, it tends to demonstrably fluctuate across years (Paulino., 2016; Igarashi., 2017). According to previous studies, one important reason cau- sing the fluctuation is the climate-driven regional environ- mental changes on the fishing ground, which can strongly affect squid distribution and abundance (Igarashi.,2018; Frawley., 2019). Pelagic fishery enterprises from China have assigned hundreds of squid-jigging vessels into the Pacific Ocean to exploitand. Without understanding the impacts of local environmental variability on squid abundance and distribution,or the cause of synchronous fluctuations inand, the enterprises are difficult to decide where to fish and what the number of the fishing vessels should be assigned in the Northwest Pacific Ocean and in the Southeast Pacific Ocean. Thus, for more effective fishe- ries management, it is essential to examine the associations between the two squid species, especially the synchronous fluctuations in abundance and distribution in re- lation to the climatic and environmental factors.
In this study, catch per unit effort (CPUE) and the latitudinal gravity centers (LATG) of fishing effort were used to indicate squid abundance and distribution, respectively. The synchronous fluctuations in abundance and distribution ofin the Northwest Pacific Ocean andin the Southeast Pacific Ocean were investigated.The impacts of two Niño indices (., Niño 3.4 and 1+2 region) and regional water surface temperature on the two squid species were further assessed. The purposes of this study were to 1) examine the synchronous fluctuations in CPUE and LATG betweenand; 2) evaluate the impacts of environmental factors on variabi- lity of squid abundance and distribution; and 3) explore the possible cause that responsible for the observed synchronicity and provide some important implications for Chinese squid-jigging fisheries.
2 Materials and Methods
2.1 Fisheries Data Collection
Commercial logbook data for Chineseandsquid-jigging fisheries grouped by 0.5˚×0.5˚ grid cell and by month were obtained from the National Data Center for Distant-water fisheries of China, Shanghai Ocean University. The fishing months from September to November for both squids were the most important fishing seasons due to the extremely high squid abundance and catches. Thus, data from September to November during 2006–2015 were used in the analysis. The data contained fishing effort (days fished), the location of fishing ground (latitude and longitude in degrees) and catch (unit: tonnes). Fishing locations for theandfisheries were primarily bounded by 36˚–48˚N and 150˚– 170˚E in the Northwest Pacific Ocean and by 8˚–20˚S and 95˚–75˚W in the Southeast Pacific Ocean, respectively (Fig.1).

Fig.1 The geographical distribution of fishing ground (FG) for Ommastrephes bartramii in the high seas of Northwest Pacific Ocean and Dosidicus gigas outside of the exclusive economic zone off Peru in the Southeast Pacific Ocean. TheNiño 3.4 and Niño 1+2 regions are also shown on the map.
In this study, we examined the variability in the abundance and distribution ofandfrom September to November during 2006–2015. For short- lived squid species, abundance and distribution can be effectively indicated by CPUE and the latitudinal gravity centers (LATG) of fishing effort (Cao., 2009; Yu., 2016). The CPUE (catch per unit effort) within a 0.5˚×0.5˚ fishing grid for the two squid species were calculated by the following equation (Cao., 2009):
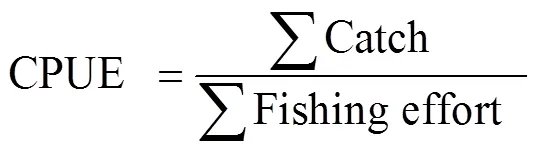
where ∑Catchis the sum of catch for all the fishing vessels within a fishing grid; and ∑Fishing effortis the sum of fishing days for all the fishing vessels within a fishing unit. For Chinese squid-jigging fishing vessels in the west- ern and southeastern Pacific Ocean, they were equipped with almost same fishing powers with similar engines, lamps. And fishing activities were all performed at night without bycatch (Chen., 2008). Thus, CPUE was used as a proxy to indicate squid abundance for the two squids.
The monthly LATG for the two squid fishery was calculated using the following equation (Li., 2014; Yu., 2016):

where Latitude(i, m)is the latitude within theth fishing unit in month;Fishing effort(i, m)is the total fishing efforts within theth fishing unit in month. In addition, correlations between annual CPUE and LATG for each squid fishery were examined statistically using Pearson’scorrelation analysis.
2.2 Oceanographic Variables and Climatic Index
Sea surface temperature (SST) was considered as a cri- tical environmental driver for the distribution and abun- dance of squid species (Yu., 2015). In this study, the monthly SST was from the National Oceanic and Atmospheric Administration (NOAA) Optimum Interpolation (OI) SST Version2 with spatial resolution of 0.25˚×0.25˚. The SST data were grouped on a 0.5˚×0.5˚ latitude/longi- tude grid to match with the spatial resolution of fishery data.
The monthly SST anomaly (SSTA) in the Niño 3.4 region (between 5˚N–5˚S and 120˚–170˚W, close tofishing ground) and Niño 1+2 region (between 0˚– 10˚S and 90˚–80˚W, close tofishing ground) were used as indicators to represent the climate variability in the Northwest and the Southeast Pacific Ocean, respectively. Many studies have proved that Niño 3.4 and 1+2 SST yielded significant impacts on environmental changes on the fishing ground ofand, res- pectively (Chen., 2007; Alabia., 2016; Yu., 2016). Therefore, the climate index data from Niño 3.4 and 1+2 regions were selected and obtained from the IRI/ LDEO Climate Data Library during the period from Ja- nuary 2005 to December 2015 (http://iridl.ldeo.columbia. edu/SOURCES/.Indices/).
2.3.Impacts of Environmental Changes on Squids
To explore the connection between the climate variability in the Northwest and the Southeast Pacific Ocean, the relationship between the SSTA in the Niño 3.4 and Niño 1+2 regions were initially evaluated by using cross- correlation functions (CCF). Moreover, in order to understand the impacts of large-scale climate variability on the local water thermal conditions, correlations between mon- thly Niño 3.4 SSTA/Niño 1+2 SSTA and monthly SST ano- maly on each fishing ground were also calculated based on spatial correlation analysis.
Histogram analysis was applied to define the suitable and optimal environmental range forandby relating fishing effort to the SST (Zainuddin., 2006). Through the analysis above, the proportion of favorable-SST area (PFSST) and the latitudinal location of the optimal SST were further determined and compared betweenandby month. The month- ly PFSST from September to November was calculated based on the percentage of suitable SST ranges favorable for the distribution ofandaccounting for the whole fishing ground. Moreover, the relationships between PFSST and the optimal SST latitude and the CPUE and LATG were examined to explore how squid abundance and distribution varied with different environ- mental conditions. Finally, the years of 2007 (a La Niña year), 2012 (a normal climate year) and 2015 (an El Niño year) were selected to compare the SST change on the fishing ground and its influence on the movement of LATG ofandunder different climate conditions.
3 Results
3.1 Temporal Variations in CPUE and LATG
The monthly CPUE from July to November forand from January to December forwere shown in Fig.2. Both squid fishery displayed a high degree of monthly variability. TheCPUE tend- ed to increase from July to August and then decreased in the following months. For theCPUE, it decreased from January to April and then increased in the subsequent fishing months. By comparing the two squid fisheries, opposite fluctuation trends in the monthly average CPUE were observed from September to November. DecreasedCPUE corresponded to increasedCPUE in each month.
The CPUE and LATG from September to November over 2006–2015 were chosen to compare the two squid fisheries (Fig.3). Interannual variability and synchronous fluctuation were observed in theandCPUEs with significant negative correlations between them (=−0.889,<0.001). With an opposing annually variability trend, statistically significant negative correlations were also found between the LATG ofand(=−0.820,<0.001), suggesting that the LATG ofperformed northward movement, while thealso generally shifted northward.

Fig.2 Monthly catch per unit effort (CPUE) of Ommastrephes bartramii from July to November and Dosidicus gigas from January to December during 2006–2015. The open circles indicate the monthly average CPUE.

Fig.3 Squid abundance (catch per unit effort, CPUE) and distribution (latitudinal gravity centers, LATG) ofOmmastrephes bartramii and Dosidicus gigasfrom September to November over 2006–2015.
3.2 Variability in the Environmental Conditions
Time series of SSTA in the Niño 3.4 and Niño 1+2 were examined from 2005 to 2015 (Fig.4). Results suggested that a basically consistent trend was observed between them. Furthermore, a significantly positive cross-correla-tion was found between the Niño 3.4 SSTA and Niño 1+2 SSTA at time lag of (−3)–7 months. With time-lag of 2 months, the highest correlation coefficient was 0.7135, implying that the variability trends of the SSTA within the two Niño regions were largely similar and synchronously occurred.

Fig.4 The time series of Niño 3.4 index and Niño 1+2 index from 2005 to 2015 (upper panel) and the cross-correlation coefficient between them (lower panel).
Because thefishing ground andfishing ground were close to the Niño 3.4 region and Niño 1+2 region, respectively (Fig.1),we then examined the influences of Niño 3.4 SSTA/Niño 1+2 SSTA on the SSTA in each adjacent fishing ground. Correlations between the Niño 3.4 SSTA and the SSTA on thefishing ground showed a significant negative relationship (Fig.5). However, in terms of the SSTA on thefishing ground, the Niño 1+2 SSTA was positively correlated with it (Fig.5).

Fig.5 Spatial distribution of correlation coefficient (A) be- tween the Niño 3.4 index and the sea surface temperature anomaly (SST) anomaly on the fishing ground of Om- mastrephes bartramii (upper panel); and (B) between the Niño 1+2 index and the sea surface temperature (SST) anomaly on the fishing ground of Dosidicus gigas (lower panel).
3.3 Influences of Environmental Conditions on Squid CPUE and LATG
Using the histogram analysis, the fishing efforts forfishery during September to November occurr- ed in areas where SST ranged from 4℃ to 25℃. However, most high fishing efforts were obtained in the waters where SST varied primarily between 11℃ and 20℃. The highest fishing effort in fishing ground tended to be centered at 15℃ SST (Fig.6). Distribution of fishing effort offishery in relation to SST indicated that thefishing ground occurred between 10℃ and 25℃ SST. The high fishing effort was mainly taken in fishing grounds where the SST ranged from 16℃ to 20℃. The highest fish- ing efforts most frequently occurred at 18℃ SST (Fig.6). Based on these results, the suitable SST ranges forandduring September to November were defined at 11–20℃ and 16–20℃, respectively. The opti- mal SST forandcorresponded to 15℃and 18℃, respectively. The suitable SST ranges for the two squids were then used to determine the PFSST.

Fig.6 Fishing efforts in relation to sea surface temperature (SST) for Ommastrephes bartramii (upper panel) and Do- sidicus gigas (lower panel) from September to November over 2006–2015.
The monthlyCPUEs were significantly in- creased with the PFSST (areas with SST between 11–20℃) on the fishing ground in the Northwest Pacific Ocean (Fig.7A) (<0.05). For the correlation betweenCPUEs and PFSST (areas with SST between 16–20℃) in the Southeast Pacific Ocean, no significant relationship was found; however, most high CPUEs occurred with enlarged PFSST (Fig.7B). Significant positive relationship were also found between the monthly latitudinal location of 15℃ isotherm andLATG (Fig.7C) (<0.001), as well as between the monthly latitudinal location of 18℃ isotherm andLATG (Fig.7D) (<0.05).
We compared the interannual variability in PFSST on thefishing ground andfishing ground month by month from September to November (Fig.8). During September,PFSST ranged from 45.1% in 2012 to 64% in 2015. For, the PFSST varied between 60.6%and 89.6%. Statistically significant negative correlations (=−0.714,<0.05) were found betweenPFSST andPFSST in September. In October, the range ofPFSST was from 42.2% in 2009 to 59.1% in 2006. The highestPFSST reached up to 89.6% in 2013,while the lowest value was observed in 2015. A significantly negative relationship (=−0.787,<0.01) was also found between them in October. However, for the PFSST in November, the relation- ship between the squid stocks was not significant (=−0.326,=0.179). It was observed that opposite fluctuation in the PFSST was found from 2006 to 2008 and from 2010 to 2014,while the variability trend was similar with other years.
The location of the most preferred SST isotherms forandwere drawn in Figs.9 and 10. It was found that the 15℃ isoline from September to November in 2007 and 2012 was distributed in the northern regions on the fishing ground ofrelative to the year in 2015. For, it was clear that the 18℃ isoline for each month was distributed in the northern, middle and southern regions on the fishing ground. Specifically, the 18℃ isoline moved out of the fishing ground in November 2015, as the SST on the fishing ground was higher than 18℃.
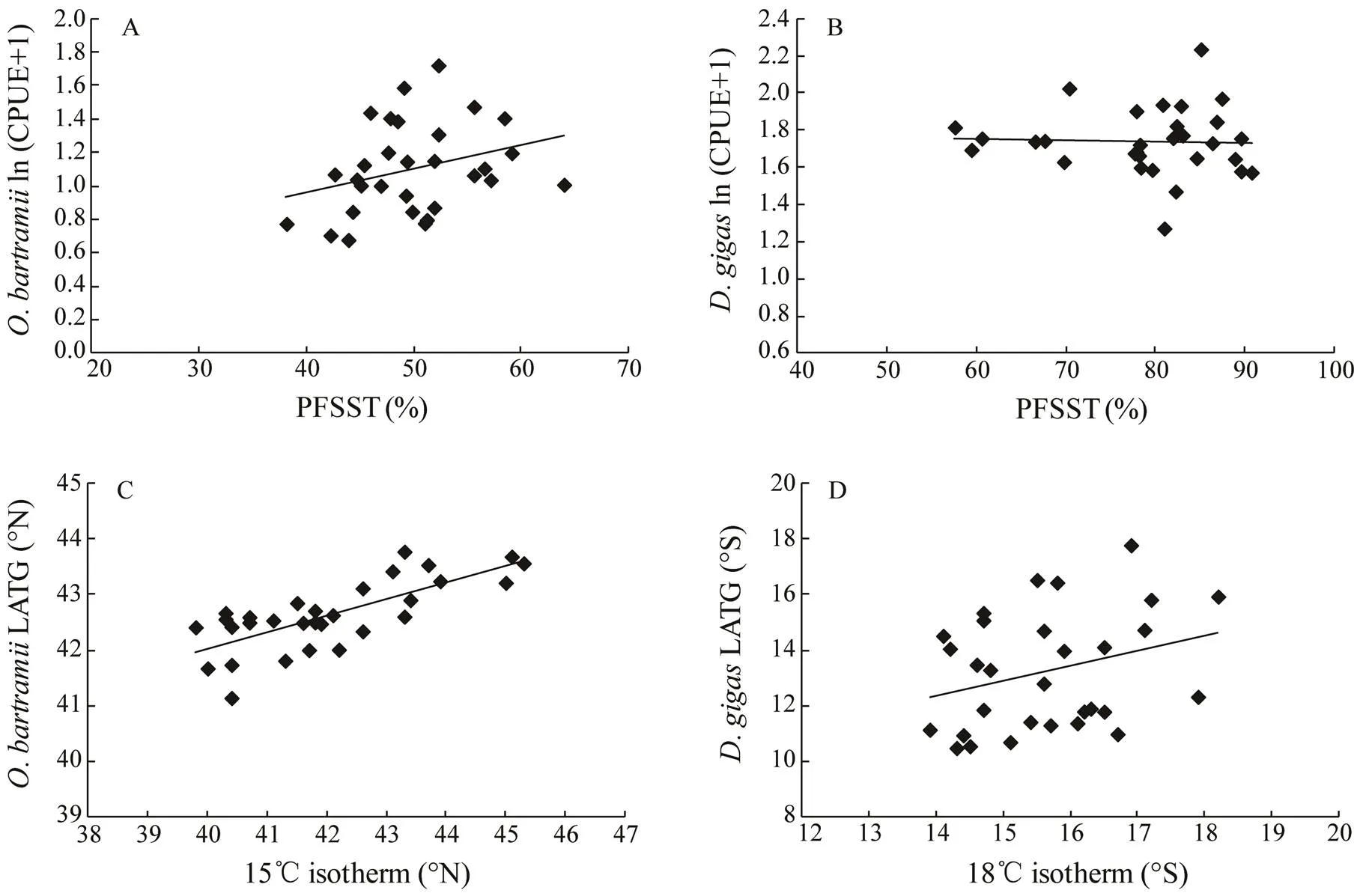
Fig.7 The relationship between the natural log-transformed catch per unit effort (CPUE) and proportion of favorable-SST area (PFSST) for (A) Ommastrephes bartramii and (B) Dosidicus gigas, and between the latitudinal gravity centers (LATG) and the average latitude of the optimal SST isotherm for (C) Ommastrephes bartramii and (D) Dosidicus gigas during September–November 2006–2015.

Fig.8 Monthly proportion of favorable-SST area (PFSST) for Ommastrephes bartramii and Dosidicus gigas from September to November, respectively.
4 Discussion
4.1 Monthly and Yearly Variations of O. bartramii and D. gigas Abundance and Distribution
Due to only 1-year life cycle, bothandare very sensitive to the climatic and environmental conditions on the spawning and fishing grounds at various spatio-temporal scales (Wang., 2017). Therefore, the catch, CPUE and LATG of the two squid species showed significant monthly and yearly variations (Ibánez., 2016; Yu., 2019). For, its abun- dance was low in July and relatively high from August to November. Such variability trends in CPUE ofwere consistent with its migration behavior (Yu., 2016). The western winter-springstock most- ly migrated into the fishing ground in August and back into the spawning ground in November (Bower and Ichii, 2005). Based on the characteristics of the life history, Chi- nese fishermen started to fishin July every year (Yu., 2015). Forfishery off Peru, the high CPUE occurred From July to December, it also corresponded to the migration route offrom the near- shore waters off Peru to the open sea outside the EEZ wa- ters off Peru (Xu., 2018). Our results were consistent with previous conclusions on seasonal changes of abundance ofand.

Fig.9 Contour maps of sea surface temperature (SST) with optimal SST isotherm (15℃) for Ommastrephes bartramii from September to November over 2007, 2012 and 2015.

Fig.10 Contour maps of sea surface temperature (SST) with the optimal SST isotherm (18℃) for Dosidicus gigas from September to November over 2007, 2012 and 2015.
Regarding the annual CPUE and LATG betweenand, our results showed the significantly negative relationship between them, while both CPUE and LATG changed synchronously for the two squids. To our knowledge, this is the first study to find such results. Interestingly, it was found that high CPUE ofgenerally corresponded to low CPUE of, and the movements of LATG of these two squid species were ba- sically similar from September to November during 2006–2015. Forstock, the CPUE was low in 2009 and 2015 and high in 2007 and 2008. Based on previous findings,was strongly affected by the El Niño and La Niña events (Chen., 2007). Generally, the El Niño events are not favorable for the formation of fishing ground with productive squid abundance (Yu., 2019). Therefore, the occurrence of the El Niño events in 2009 and 2015 led to the low CPUE of. On the con- trary, in 2007 and 2008, the La Niña event and the normal climate condition resulted in high abundance of. However, it was found that the CPUEs ofin 2009 and 2015 were high in this study. According to the findings from the previous studies, the El Niño events com- monly yielded enlarged poor habitats forand con- sequently led to relatively low abundance of it (Ichii., 2002; Waluda., 2006; Xu., 2012). The opposite results were likely due to the different data used in the analysis. This analysis only included the months with high CPUEs offrom September to November. The in- fluences of the El Niño and La Niña events onandare quite complicated, depending on the intensity and type of the anomalous climate conditions.
4.2 The Effect of SST onO. bartramii and D. gigasAbundance and Distribution
It was well known that various environmental factors such as SST, sea surface height (SSH), chlorophyll-(Chl-) concentration played crucial roles in regulating the abundance and distribution of squid species (Waluda and Rod- house, 2006; Robinson., 2013; Yu., 2015). How-ever, among these factors, SST was considered as the most important one during the whole life of ommastrephid squids (Yatsu., 2000; Ichii., 2009). SST directly affects squid spawning location, breeding time and behavior, mi- gration, feeding, growth, survival and physiological me- tabolism,(Pecl and Jackson, 2008; Rosa., 2011; Frawley., 2019). Squid species can quickly respond to SST changes.They will move to suitable habitat if the SST in the original region is not favorable for inhabiting (Xu., 2016; Yu and Chen, 2018). SST is frequently used in many studies to model the habitat formation and identify spatio-temporal changes of fishing ground for squid species (Chen., 2010; Yu., 2019).
In the Northwest Pacific Ocean, the SST is the most suitable index to explore fishing grounds of. For example, the fishing ground ofcan be identified by dense distribution of isotherm surface water layer, convergence of warm and cold waters and thermal layer based on the SST (Shen., 2004). The suitable SST range forvaried with seasons and fishing locations. The seasonal and spatial distributions ofwere largely explained by 7–17℃ SST in winter and 11–18℃ in summer (Alabia., 2015). The 17℃and 20℃ SST isothermsare regarded as the fishing ground index with highabundance in the west of 155˚E, and between 155˚ and 160˚E (Chen, 1997). These resultsare consistent with our findings. Moreover, the SST contributes the highest to the habitat model gain compar- ing to other factors, indicating that the habitat formation ofis highly vulnerable to drastic changes in SST (Alabia., 2015).
With regard to, there are four major fishing grounds in the Eastern Pacific Ocean, the suitable ranges of SST forvary with seasons and different geographical distributions (Medellín-Ortiz., 2016; Yu., 2018). Previous studies showed that jumbo squids off Peru usually live in waters with SST ranging from 17 to 22℃ (Waluda., 2006), which is in accordance with the results in this study. Habitat modeling method revealed that the weighing of SST relative to SSH and Chl-also contributed the highest to the suitable habitat model (Hu., 2010). Furthermore, strong relationship was found between SST and CPUE for the two squids. Interannual variability in squid abundance and distribution are closely linked to variations in SST on the spawning and fishing ground (Cao., 2009; Ichii., 2011). Given that SST can define the limits of the habitat of squids, this study employed SST as the only environmental variable to analyze the SST-related synchronous fluctuations in abundance and distribution ofand.
4.3 Possible Causes of Synchronous Fluctuations ofO. bartramii and D. gigas and Its Implications
A growing number of studies proved that large-scale climate changes have yielded strong impacts on local en- vironmental conditions on the fishing ground of pelagic fish (Tian., 2003; Zainuddin., 2006). In this study, it was found that the change trends of SSTA in the Niño 3.4 and Niño 1+2 regions showed the similar variability pattern during 2006–2015. The negative relationship was found between Niño 3.4 SSTA and the SSTA on the fishing ground of, while the positive one was found between the Niño 1+2 SSTA and the SSTA on the fishing ground of, suggesting that the SST on the fishing ground of these two squids was dramatically influenced by the large-scale synchronous SSTA variability in the Niño 3.4 and 1+2 regions. Considering all the information, the possible causes of synchronous fluctuations in abundance and distribution ofandcan be indicated.The fishing ground of the two squidsandare in the Northern He- misphere and Southern Hemisphere respectively. Synchro- ny in SST changes occurred in the Niño 3.4 and Niño 1+2 regions, leading to the synchronous opposite fluctuations in the SST on the fishing ground ofin the Northwest Pacific Ocean and on the fishing ground ofin the Southeast Pacific Ocean off Peru. Opposite changes in SST led to increasedPFSST corresponding to decreasedPFSST from September to November over 2006–2015, resulting in synchronous opposing changes in CPUE. At the same time, the movement of the latitudinal location of the optimal SST forwas in phase with the shift of optimal SST for, yielding similar movement pattern of the LATG. Our findings suggest that the potential mechanism behind synchronous opposing changes in abundance and similar movement in the latitudinal distribution of the two squid species was due to simultaneous variations in the PFSST and the latitudinal location of the optimal SST front, which were strongly influenced by the large-scale variability of the SSTA in the Niño 3.4 and Niño 1+2 regions. Understanding synchronous fluctuations in CPUE and LATG ofandhas important implications for Chinese squid fisheries management. Based on the present study, how the CPUE and LATG of the two squids syn- chronously fluctuated with the environmental conditions can be deduced. The fishermen then can make correct fish- ing decisions. For example, with the decreasing trends in CPUE ofand the concurrent increasing trends forCPUE, the fishermen can decrease the number of fishing vessels in the western Pacific and increase the number of fishing vessels in the southeastern Pacific off Peru.
Acknowledgements
This study was financially supported by the NationalKey R&D Program of China (No. 2019YFD0901405), the National Natural Science Foundation of China (No. 4190 6073), the Natural Science Foundation of Shanghai (No. 19ZR1423000), the Open Fund for Key Laboratory of Su- stainable Exploitation of Oceanic Fisheries Resources in Marine Fisheries Research Institute of Zhejiang (No. 2020 KF002), and the Shanghai Universities First-Class Disciplines Project (Fisheries A).
Alabia, I. D., Saitoh, S. I., Hirawake, T., Igarashi, H., Ishikawa, Y., Usui, N., Kamachi, M., Awaji, T., and Seito, M., 2016. Elu- cidating the potential squid habitat responses in the central North Pacific to the recent ENSO flavors., 772 (1): 215-227.
Alabia, I. D., Saitoh, S. I., Mugo, R., Igarashi, H., Ishikawa, Y., Usui, N., and Seito, M., 2015. Seasonal potential fishing ground prediction of neon flying squid () in the western and central North Pacific., 24 (2): 190-203.
Anderson, C. I., and Rodhouse, P. G., 2001. Life cycles, oceanography and variability: Ommastrephid squid in variable ocea- nographic environments., 54 (1): 133-143.
Argüelles, J., Rodhouse, P. G., Villegas, P., and Castillo, G., 2001.Age, growth and population structure of the jumbo flying squidin Peruvian waters., 54 (1): 51-61.
Arkhipkin, A. I., Rodhouse, P. G., Pierce, G. J., Sauer, W., Sakai, M., Allcock, L., Arguelles, J., Bower, J. R., Castillo, G., Ce- riola, L., Chen, C. S., Chen, X. J., Diaz-Santana, M., Dow- ney, N., Gonzalez, A. F., Granados A. J., Green, C. P., Guerra, A., Hendrickson, L. C., Ibanez, C., Ito, K., Jereb, P., Kato, Y., Katugin, O. N., Kawano, M., Kidokoro, H., and Kulik, V. V., 2015. World squid fisheries., 23 (2): 92-252.
Bower, J. R., and Ichii, T., 2005. The red flying squid (): A review of recent research and the fishery in Japan., 76 (1): 39-55.
Boyle, P. R., 1990. Cephalopod biology in the fisheries context., 8 (4): 303-321.
Cao, J., Chen, X., and Chen, Y., 2009. Influence of surface ocea- nographic variability on abundance of the western winter–spring cohort of neon flying squidin the NW Pacific Ocean., 381: 119- 127.
Chen, C. S., and Chiu, T. S., 2003. Variations of life history pa- rameters in two geographical groups of the neon flying squid,, from the North Pacific., 63 (3): 349-366.
Chen, X. J., 1997. An analysis on marine environment factors of fishing ground ofin Northwestern Pacific., 6 (4): 263- 267 (in Chinese with English abstract).
Chen, X. J., Liu, B. L., Tian, S. Q., Qian, W. G., and Li, G., 2009. Forecasting the fishing ground ofwith SST-based habitat suitability modeling in northwestern pacific., 40 (6): 707-713 (in Chinese with English abstract).
Chen, X. J., Zhao, X. H., and Chen, Y., 2007. Influence of El Niño/La Niña on the western winter–spring cohort of neon flying squid () in the northwestern Pacific Ocean., 64 (6): 1152- 1160.
Chen, X., Liu, B., and Chen, Y., 2008. A review of the development of Chinese distant-water squid jigging fisheries., 89 (3): 211-221.
Chen, X., Tian, S., Chen, Y., and Liu, B., 2010. A modeling ap- proach to identify optimal habitat and suitable fishing grounds for neon flying squid () in the North- west Pacific Ocean., 108 (1): 1-14.
Cherel, Y., and Weimerskirch, H., 1995. Seabirds as indicators of marine resources: Black-browed albatrosses feeding on ommastrephid squids in Kerguelen waters., 129 (1-3): 295-300.
Dunning, M. C., and Wormuth, J. H., 1998. The ommastrephid squid genus: A review of systematics, distribution, and biology (Cephalopoda: Teuthoidea)., 586 (2): 385-392.
Frawley, T. H., Briscoe, D. K., Daniel, P. C., Britten, G. L., Crow- der, L. B., Robinson, C. J., and Gilly, W. F., 2019. Impacts of a shift to a warm-water regime in the Gulf of California on jumbo squid ()., 76 (7): 2413-2426.
Hernández-Herrera, A., Morales-Bojórquez, E., Cisneros-Mata, M. A., Nevárez-Martínez, M. O., and Rivera-Parra, G. I., 1998. Ma- nagement strategy for the giant squid () fishery in the Gulf of California, Mexico., 39: 212-218.
Hu, G., Boenish, R., Gao, C., Li, B., Chen, X., Chen, Y., and Punt, A. E., 2019. Spatio-temporal variability in trophic ecology of jumbo squid () in the southeastern Pacific: Insights from isotopic signatures in beaks., 212: 56-62.
Hu, Z. M., Chen, X. J., Zhou, Y. Q., Qian, W. G., and Liu, B. L., 2010. Forecasting fishing ground ofbased on habitat suitability index off Peru.,32 (5): 67-75 (in Chinese with English abstract).
Ibánez, C. M., Argüelles, J., Yamashiro, C., Sepúlveda, R. D., Pardo-Gandarillas, M. C., and Keyl, F., 2016. Population dynamics of the squids(Oegopsida: Ommastre- phidae) and(Myopsida: Loliginidae) in nor- thern Peru., 173: 151-158.
Ichii, T., Mahapatra, K., Okamura, H., and Okada, Y., 2006. Stock assessment of the autumn cohort of neon flying squid () in the North Pacific based on past large- scale high seas driftnet fishery data., 78 (2-3): 286-297.
Ichii, T., Mahapatra, K., Sakai, M., and Okada, Y., 2009. Life his- tory of the neon flying squid: Effect of the oceanographic regime in the North Pacific Ocean., 378: 1-11.
Ichii, T., Mahapatra, K., Sakai, M., Wakabayashi, T., Okamura, H.,Igarashi, H., and Okada, Y., 2011. Changes in abundance of the neon flying squidin relation to climate change in the central North Pacific Ocean., 441: 151-164.
Ichii, T., Mahapatra, K., Watanabe, T., Yatsu, A., Inagake, D., and Okada, Y., 2002. Occurrence of jumbo flying squidaggregations associated with the countercurrent ridge off the Costa Rica Dome during 1997 El Niño and 1999 La Niña., 231: 151-166.
Igarashi, H., Ichii, T., Sakai, M., Ishikawa, Y., Toyoda, T., Masu- da, S., and Awaji, T., 2017. Possible link between interannual variation of neon flying squid () abun-dance in the North Pacific and the climate phase shift in 1998/ 1999., 150: 20-34.
Igarashi, H., Saitoh, S. I., Ishikawa, Y., Kamachi, M., Usui, N., Sakai, M., and Imamura, Y., 2018. Identifying potential habitat distribution of the neon flying squid () off the eastern coast of Japan in winter., 27 (1): 16-27.
Jackson, G. D., Shaw, A. G. P., and Lalas, C., 2000. Distribution and biomass of two squid species off southern New Zealand:and., 23 (10): 699-705.
Kang, Y. S., Kim, J. Y., Kim, H. G., and Park, J. H., 2002. Long- term changes in zooplankton and its relationship with squid,, catch in Japan/East Sea., 11 (6): 337-346.
Lee, D., Son, S. H., Lee, C. I., Kang, C. K., and Lee, S. H., 2019. Spatio-temporal variability of the habitat suitability index for the(Japanese common squid) around South Korea., 11 (23): 2720.
Li, G., Chen, X. J., Lei, L., and Guan, W. J., 2014. Distribution of hotspots of chub mackerel based on remote-sensing data in coastal waters of China., 35 (11-12): 4399-4421.
Ma, J., Chen, X. J., Liu, B. L., Tian, S. Q., Li, S. L., and Cao, J., 2011. Review of fisheries biology of neon flying squid () in the North Pacific Ocean., 20 (4): 563-570 (in Chinese with English abstract).
Medellín-Ortiz, A., Cadena-Cárdenas, L., and Santana-Morales, O., 2016. Environmental effects on the jumbo squid fishery along Baja California’s west coast., 82 (6): 851-861.
Morales-Bojórquez, E., and Pacheco-Bedoya, J. L., 2016. Jumbo squid: A new fishery in Ecuador., 24 (1): 98-110.
Nigmatullin, C. M., Nesis, K. N., and Arkhipkin, A. I., 2001. A review of the biology of the jumbo squid(Ce- phalopoda: Ommastrephidae)., 54 (1): 9- 19.
Parry, M., 2006. Feeding behavior of two ommastrephid squidsandoff Ha- waii., 318: 229-235.
Paulino, C., Segura, M., and Chacón, G., 2016. Spatial variability of jumbo flying squid () fishery related to remotely sensed SST and chlorophyll-concentration (2004– 2012)., 173 (2): 122-127.
Pecl, G. T., and Jackson, G. D., 2008. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries., 18 (4): 373-385.
Robinson, C. J., Gómez-Gutiérrez, J., and de León, D. A. S., 2013. Jumbo squid () landings in the Gulf of California related to remotely sensed SST and concentrations of chlorophyll(1998–2012)., 137: 97-103.
Rodhouse, P. G., 2008. Large-scale range expansion and varia- bility in ommastrephid squid populations: A review of environmental links., 49: 83-89.
Rosa, A. L., Yamamoto, J., and Sakurai, Y., 2011. Effects of en- vironmental variability on the spawning areas, catch, and recruitment of the Japanese common squid,(Cephalopoda: Ommastrephidae), from the 1970s to the 2000s., 68 (6): 1114-1121.
Shen, X., Fan, W., and Cui, X., 2004. Study on the relationship of fishing ground distribution ofand water temperature in the Northwest Pacific Ocean., 25 (3): 10-14 (in Chinese with English ab- stract).
Taipe, A., Yamashiro, C., Mariategui, L., Rojas, P., and Roque, C., 2001. Distribution and concentrations of jumbo flying squid () off the Peruvian coast between 1991 and 1999., 54 (1): 21-32.
Tian, Y., Akamine, T., and Suda, M., 2003. Variations in the abun- dance of Pacific saury () from the northwestern Pacific in relation to oceanic-climate changes., 60 (2-3): 439-454.
Waluda, C. M., and Rodhouse, P. G., 2006. Remotely sensed me- soscale oceanography of the Central Eastern Pacific and recruitment variability in., 310: 25-32.
Waluda, C. M., Yamashiro, C., and Rodhouse, P. G., 2006. Influ- ence of the ENSO cycle on the light-fishery forin the Peru Current: An analysis of remotely sensed data., 79 (1-2): 56-63.
Waluda, C. M., Yamashiro, C., Elvidge, C. D., Hobson, V. R., and Rodhouse, P. G., 2004. Quantifying light-fishing forin the eastern Pacific using satellite remote sensing., 91 (2): 129-133.
Wang, J., Chen, X., Tanaka, K., Cao, J., and Chen, Y., 2017. En- vironmental influences on commercial oceanic ommastrephid squids: A stock assessment perspective., 81 (1): 37-47.
Xu, B., Chen, X., Tian, S., Qian, W., and Liu, B., 2012. Effects of El Niño/La Niña on distribution of fishing ground ofoff Peru waters., 36 (5): 696-707 (in Chinese with English abstract).
Xu, J., Chen, X. J., Chen, Y., Ding, Q., and Tian, S. Q., 2016. The effect of sea surface temperature increase on the potential habitat ofin the Northwest Pacific Ocean., 35 (2): 109-116.
Xu, L., Chen, X., Guan, W., Tian, S., and Chen, Y., 2018. The im- pact of spatial autocorrelation on CPUE standardization be-tween two different fisheries., 36 (3): 973-980.
Yatsu, A., Watanabe, T., Mori, J., Nagasawa, K., Ishida, Y., Meguro, T., and Sakurai, Y., 2000. Interannual variability in stock abundance of the neon flying squid,, in the North Pacific Ocean during 1979–1998: Impact of drift- net fishing and oceanographic conditions., 9 (2): 163-170.
Yu, W., and Chen, X., 2018. Ocean warming-induced range- shifting of potential habitat for jumbo flying squidin the Southeast Pacific Ocean off Peru., 204: 137-146.
Yu, W., Chen, X., Yi, Q., and Chen, Y., 2016. Spatio-temporal distributions and habitat hotspots of the winter–spring cohort of neon flying squidin relation to oceanographic conditions in the Northwest Pacific Ocean., 175: 103-115.
Yu, W., Chen, X., Yi, Q., and Tian, S., 2015. A review of interaction between neon flying squid () and oceanographic variability in the North Pacific Ocean., 14 (4): 739-748.
Yu, W., Chen, X., Zhang, Y., and Yi, Q., 2019. Habitat suitabi- lity modelling revealing environmental-driven abundance vari-ability and geographical distribution shift of winter-spring cohort of neon flying squidin the Northwest Pacific Ocean., 76(6): 1722-1735.
Yu, W., Yi, Q., Chen, X., and Chen, Y., 2016. Modelling the effects of climate variability on habitat suitability of jumbo flying squid,, in the Southeast Pacific Ocean off Peru., 73 (2): 239-249.
Zainuddin, M., Kiyofuji, H., Saitoh, K., and Saitoh, S. I., 2006. Using multi-sensor satellite remote sensing and catch data to detect ocean hot spots for albacore () in the northwestern North Pacific., 53 (3-4): 419-431.
Zeidberg, L. D., and Robison, B. H., 2007. Invasive range expansion by the Humboldt squid,, in the eastern North Pacific., 104 (31): 12948-12950.
June 22, 2020;
July 27, 2020;
October 23, 2020
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. Tel: 0086-21-61900306 E-mail: xjchen@shou.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Case Study of a Short-Term Wave Energy Forecasting Scheme:North Indian Ocean
- Temporal and Spatial Characteristics of Wave Energy Resources in Sri Lankan Waters over the Past 30 Years
- Vibration Deformation Monitoring of Offshore Wind Turbines Based on GBIR
- Dependence of Estimating Whitecap Coverage on Currents and Swells
- The Variation of Microbial (Methanotroph) Communities in Marine Sediments Due to Aerobic Oxidation of Hydrocarbons
- 3-Aminopropyltriethoxysilane Complexation with Iron Ion Modified Anode in Marine Sediment Microbial Fuel Cells with Enhanced Electrochemical Performance
