Potential aquatic environmental risks of trifloxystrobin:Enhancement of virus susceptibility in zebrafish through initiation of autophagy
2021-06-17HuanWangTianXiuQiuJianFeiLuHanWeiLiuLingHuLeiLiuJiongChen
Huan Wang, Tian-Xiu Qiu, Jian-Fei Lu, Han-Wei Liu, Ling Hu, Lei Liu,*, Jiong Chen,*
1 State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo,Zhejiang 315211, China
2 Laboratory of Biochemistry and Molecular Biology, School of Marine Sciences, Meishan Campus, Ningbo University, Ningbo, Zhejiang 315832, China
3 Key Laboratory of Applied Marine Biotechnology of Ministry of Education, Meishan Campus, Ningbo University, Ningbo, Zhejiang315832, China
4 Ningbo Customs District Technology Center, Ningbo, Zhejiang 315832, China
ABSTRACT Chronic pollution in aquatic ecosystems can lead to many adverse effects, including a greater susceptibility to pathogens among resident biota.Trifloxystrobin (TFS) is a strobilurin fungicide widely used in Asia to control soybean rust. However, it has the potential to enter aquatic ecosystems, where it may impair fish resistance to viral infections. To explore the potential environmental risks of TFS, we characterized the antiviral capacities of fish chronically exposed to TFS and subsequently infected with spring viraemia of carp virus (SVCV).Although TFS exhibited no significant cytotoxicity at the tested environmental concentrations during viral challenge, SVCV replication increased significantly in a time-dependent manner within epithelioma papulosum cyprini (EPC) cells and zebrafish exposed to 25 μg/L TFS. Results showed that the highest viral load was more than 100-fold that of the controls. Intracellular biochemical assays indicated that autophagy was induced by TFS, and associated changes included an increase in autophagosomes,conversion of LC3-II, accumulation of Beclin-1, and degradation of P62 in EPC cells and zebrafish. In addition, TFS markedly decreased the expression and phosphorylation of mTOR, indicating that activation of TFS may be associated with the mTORmediated autophagy pathway. This study provides new insights into the mechanism of the immunosuppressive effects of TFS on non-target aquatic hosts and suggests that the existence of TFS in aquatic environments may contribute to outbreaks of viral diseases.
Keywords: Trifloxystrobin; Autophagy; SVCV;Chronic toxicity; Susceptibility
lNTRODUCTlON
Strobilurins are a type of agricultural fungicide developed from natural β-methoxyacrylate derivatives (Bartlett et al., 2002).Over the last 20 years, strobilurins have been increasingly applied to crop production systems as the most important component of crop disease management (Deb et al., 2010;Feng et al., 2020). Because strobilurins impart broadspectrum, rapid, and highly efficient germicidal activities, and because these fungicides are cost effective and degrade rapidly during plant metabolism, their large-scale use has increased considerably; indeed, this class represents the most important fungicide group, accounting for 20% of the total fungicide market in 2016 (Wang et al., 2020).
Trifloxystrobin (TFS, methyl-a-methoxyimino-2-((1-(3-(trifluoromethyl) phenyl) ethylidene ((amino) oxymethyl)-benzeneacetate)), a systemic broad-spectrum foliar fungicide synthesized from the natural antibiotic strobilurin with an oximether side chain, is widely used to control fungi in greenhouses, nurseries, orchards, vegetable gardens, and paddy fields (Zhu et al., 2015b). Due to its rapid degradation in plants, soil, and water, as well as its long-lasting effects and resistance to rain, it occupies an important position among strobilurins (Luo et al., 2020). However, the widespread application of TFS has raised serious public health concerns,in particular, that the potential off-site transport of environmental residues could have non-target toxic effects on humans and wildlife (Liu et al., 2020b).
TFS exhibits relatively high water solubility and has a halflife of 1.9 to 9.8 d in soil (Wang et al., 2015). These properties promote its entry into water directly following aerial spraying or indirectly through leaching and drainage flow, after which it accumulates in water and sediment (Komárek et al., 2010;Wang et al., 2015). TFS has been frequently detected in aquatic systems, e.g., at concentrations of 0.73 μg/L in horticultural catchments of southeastern Australia and of 170 μg/L in Chinese paddy field water (Wightwick et al., 2012; Cao et al., 2015). Although the European Food Safety Authority(EFSA, 2017) states that TFS exhibits low acute and chronic toxicity to humans, birds, mammals, and bees, several studies have indicated that TFS can affect various biotic components of freshwater ecosystems and can be acutely toxic to some non-target freshwater organisms (Cui et al., 2017; Junges et al., 2012; Li et al., 2018; Liu et al., 2013; Zhu et al., 2015a).Thus, it is important to investigate the potential adverse effects of TFS on aquatic organisms.
Although viruses are essential intracellular agents, they can cause devastating diseases in aquatic animals because of limited effective treatments and prophylactics (Xiong et al.,2019). Given the increasing demand for fish and shellfish in contemporary societies, losses caused by viral diseases must be reduced (Balmer et al., 2017). According to the World Organisation for Animal Health (OIE), spring viraemia of carp(SVC) is a notifiable disease responsible for significant mortality in carp species, including the common carp(Cyprinus carpio), koi carp (C. carpio koi), crucian carp(Carassius carassius), grass carp (Ctenopharyngodon idella),and goldfish (Carassius auratus) (Ahne et al., 2002; Taylor et al., 2013). Since its first detection in Yugoslavia, the SVC virus(SVCV) has spread to Europe, South America, North America,and Asia (Dikkeboom et al., 2004; Garver et al., 2007). At present, all aquatic organisms infected by SVCV are destroyed to eradicate the virus in affected ponds (Ahne et al.,2002), but viral outbreak remains a crucial issue to ecological security.
Depending on their physicochemical properties, pollutants may accumulate in aquatic organisms at low environmental concentrations and interact with tissues and cells, potentially leading to a series of chronic poisoning characteristics,including cell cycle arrest and endoplasmic reticulum stress.These events can, in turn, transduce messages to stress sensors related to oxidative stress and immune system damage (Ma et al., 2019; Xing et al., 2015). These physiological disorders can increase the virulence of pathogens and reduce host resistance. TFS can induce embryonic lesions, decrease hatching success, and increase oxidative stress in zebrafish embryos by increasing reactive oxygen species (ROS) and malonaldehyde (MDA) content,and inhibiting superoxide dismutase (SOD), glutathione(GSH), catalase (CAT) and antioxidant system-related mRNA genes (i.e.,Mn-SOD,Cu/Zn-SOD,CAT,Nrf2, andBcl2) (Li et al., 2018; Zhu et al., 2015a). These cellular components are crucial for maintaining antiviral resistance in fish against the acutely contagious and hemorrhagic SVC disease (Liu et al.,2020a; Yuan et al., 2014). Therefore, the above studies raise concern that chronic exposure to TFS at environmental concentrations may affect the resistance of aquatic organisms to pathogens.
In the present study, we characterized the defensive capacities of epithelioma papulosum cyprini (EPC) cells and zebrafish chronically exposed to TFS and subsequently challenged with SVCV infection. To provide comprehensive mechanistic information useful for evaluating the ecological risks of TFS in aquatic ecosystems, the related responses involved in the initiation of autophagy in hosts were assessed at different levels of organization, from individual organisms to gene expression.
MATERlALS AND METHODS
Fungicide formulation
TFS in analytical standard (CAS Registry Number: 141517-21-7) was purchased from Sigma-Aldrich (USA) with 100% purity.The TFS solution was mixed with dimethyl sulfoxide (DMSO;Aladdin, China) to a stock concentration of 50 mg/mL, then stored at -20 °C until further use. To obtain the final concentration and ensure that TFS was fully dissolved and mixed in the dilution water, an appropriate amount of the applied solution was sonicated for approximately 10 min.
Cell culture, virus propagation, and zebrafish husbandry
The EPC SVCV-susceptible cell line was provided by Prof.Ling-Bing Zeng (Yangtze River Fisheries Research Institute,China) and cultured in medium 199 (M199, Hyclone, USA)supplemented with 10% fetal bovine serum (FBS; Every Green, China), penicillin (100 IU/mL), and streptomycin(0.1 mg/mL) at 25 °C in a 5% CO2incubator for 48 h.
The SVCV strain (0504) provided by Prof. Qiang Li (Key Laboratory of Mariculture, Agriculture Ministry, Dalian Ocean University, Dalian, China) was isolated from common carp and propagated in EPC cells until a terminal cytopathic effect(>75% of observed CPE) occurred (Chen et al., 2006). The virus in diseased cells was harvested and stored at -80 °C until further use. After SVCV titration into cell-cultured 96-well plates for 48 h, analysis of viral titer with a 50% tissue culture infective dose (TCID50) was performed according to the Reed-Muench method (Pizzi, 1950).
Zebrafish (n=2 500, total length of 1.98±0.22 cm, body weight of 49.89±14.10 mg, mean±standard deviation (SD))were purchased from the Qing-Feng Zebrafish Breeding Center (Shanghai, China), and maintained in five static water systems consisting of six 200 L aquarium ponds at 20 °C.Standard dilution water (ISO7346/3-1998) was obtained from a sterile circulating water system. Photoperiod was maintained on a constant 12:12 h (light: dark) cycle. Prior to the experiments, the fish were transferred into new containers and acclimatized at 15 °C under laboratory conditions for two weeks and fed to apparent satiation three times a day (from 0800h to 2200h) with a diet of commercial dry pellets (0.1%body weight). Ten zebrafish were randomly selected to verify SVCV-free status, as described previously (Koutná et al.,2003), with no virus found in the experimental zebrafish. All procedures were performed according to the Experimental Animal Management Law of China and approved by the Animal Ethics Committee of Ningbo University (No.10300).
Antibodies
The antibody LC3B (#3868) was purchased from Cell Signaling Technology, Inc (USA). The antibodies p62(ab109012), Beclin-1 (ab210498), mTOR (ab32028), and pmTOR (S2448) (ab109268) were purchased from Abcam(USA). The antibody α-tubulin (AF0001 and AT819) and secondary antibodies, including horse radish peroxidase(HRP)-labeled goat anti-mouse IgG (H+L) (A0216), HRPlabeled goat anti-rabbit IgG (H+L) (A0208), Alexa Fluor 647-labeled goat anti-rabbit IgG (H+L) (A0468), and Alexa Fluor 488-labeled goat anti-mouse IgG (H+L) (A0428), were purchased from Beyotime (China).
Cytotoxicity of TFS to EPC cells
Cytotoxicity analyses were performed using Cell Counting Kit-8 (CCK-8) (Beyotime, China) based on a water-soluble tetrazolium dye (WST-8), which is more sensitive than the conventional 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium(MTS)-based assay. Briefly, serial dilutions of TFS at concentrations of 500/2nμg/L (n=0-6) in triplicate were added to cells cultured on a 96-well plate in M199 supplemented with 5% FBS. At each 24 h period, cell viability following TFS exposure was tested as per the manufacturer’s protocols for CCK-8. Colorimetric absorbance after the mitochondrial enzyme substrate reaction was measured at 450 nm using a microplate reader (ELX800, Gene, Hong Kong, China). As DMSO exhibits no cytotoxicity at doses of up to 0.1% (v/v)(Liu et al., 2020a), TFS-exposed signals were compared to solvent-control signals (DMSO up to 0.001%, v/v).
Virus susceptibility in EPC cells
To explore the negative influence of TFS pre-exposure on virus susceptibility, prior to SVCV infection, the EPC cells seeded on 12-well plates were exposed to TFS at 2.5 μg/L and 25 μg/L for 1, 3, 5, and 7 d. Afterwards, the EPC cells were washed with 0.1 mol/L phosphate-buffered saline (PBS,pH=7.4; Beyotime, China) and infected with SVCV at 103×TCID50/mL over 48 h. The expression of SVCV nucleoprotein was detected by quantitative real-time polymerase chain reaction (qRT-PCR). The solution was replaced with fresh TFS diluent every 24 h to ensure that actual concentrations deviated little from initial target concentrations.
Simultaneously, the antiviral effects of TFS were monitored as described previously (Liu et al., 2017). Briefly, EPC cells were seeded on 12-well plates to a monolayer and infected with SVCV at 103×TCID50/mL at 25 °C, followed by the addition of TFS at 2.5 μg/L and 25 μg/L. After 48 h of infection,the antiviral effects of TFS on SVCV replication in EPC cells were tested by qRT-PCR.
Cell ultrastructural observations
The sampled cells were harvested and maintained in 2.5%glutaraldehyde at 4 °C overnight. The sampled cells were prepared for transmission electron microscopy as described in an earlier study (Zhang et al., 2011). Ultrathin cell sections were observed under a 1200EX transmission electron microscope (TEM) (JEOL, Japan).
Virus susceptibility in zebrafish
A total of 225 healthy zebrafish were randomly chosen and distributed into three groups (DMSO-control and TFS at 2.5 μg/L and 25 μg/L (75 zebrafish in each) containing ultraviolet (UV)-sterilized water). To overcome the lack of samples due to the accidental death of zebrafish, there were 25 fish per tank (20 zebrafish sampled) and three tanks per treatment. The rearing temperature of each aquarium was kept at 15 °C. The experimental fish were exposed to TFS at 2.5 μg/L and 25 μg/L for 1, 3, 5, 7, and 14 d, followed by immersion with SVCV at 103×TCID50/mL (M199 served as the control). At each sampling time, four zebrafish in each replicate were euthanized using a washrag soaked with 40 μg/mL MS-222 (3-aminobenzoic acid ethyl ester methanesulfonate, CAS Registry Number: 886-86-2; Sigma,USA), with samples frozen for later analysis. Subsequently,samples were thawed and homogenized with liquid nitrogen to extract total RNA for detection of SVCV nucleoprotein expression. During exposure, the solutions were replaced with fresh TFS diluent every 24 h to ensure that actual concentrations deviated little from initial target concentrations and to maintain dissolved oxygen concentrations. The same process of TFS exposure was repeated without SVCV infection. Total RNA and protein in the sampled zebrafish were isolated to determine the expression of autophagyrelated genes and analyze antioxidant enzyme activity,respectively.
Total RNA extraction, viral load quantification, and qRTPCR
The samples of EPC cells and zebrafish were immediately frozen in liquid nitrogen for subsequent RNA isolation. Total RNA was extracted using TRIZOL reagent (Takara, Japan)following the manufacturer’s instructions, and eluted in 10-40 μL nuclease-free water for storage at -80 °C. Nucleic acid concentrations were quantified using a NanoDrop spectrophotometer (ThermoFisher, USA). Extracted RNA purity was determined using the A260 nm/A280 nm ratio, with absorbance at 260 and 280 nm between 1.8 and 2.0. In total,500 ng/μL of purified RNA was used for cDNA generation per reaction, which was reverse transcribed using HiScript Q Select RT SuperMix for qPCR (Vazyme, China). qRT-PCR was performed using ChamQTMSYBR®qPCR Green Master Mix (Vazyme, China) in an ABI StepOnePlusTMReal-Time PCR Detection System (ThermoFisher, USA). PCR analysis was amplified as follows: 95 °C for 30 s, denaturation for 40 cycles at 95 °C for 15 s, and annealing at 60 °C for 60 s. To assess the specificity of each amplicon, melt curve analysis was also performed at the end of each thermal profile. The sequences of primers are listed in Table 1 (Bello-Perez et al.,2020; Gotesman et al., 2015; Varela et al., 2014). Each sample was run in triplicate and theβ-actingene was used as an internal control for normalization. Relative expression was determined as fold induction relative to the expression level in the positive control (without TFS exposure) (set to 1). Relative mRNA expression levels were calculated using the 2-ΔΔCtmethod with the formula ΔΔCt=(Ct,targetgene-Ct,referencegene)-(Ct,targetgene-Ct,referencegene)control(Livak & Schmittgen, 2001).
Western blot assays
Total protein in the EPC cells and zebrafish was extracted using RIPA Lysis Buffer (Beyotime, China) under ice-bath cooling according to the manufacturer’s instructions. The homogenate was centrifuged at 8 000gfor 15 min at 4 °C to obtain the protein supernatant. For western blot assays, total protein (isolated using a Nuclear and Cytoplasmic Protein Extraction Kit, Beyotime, China) was separated via 12%sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE) and then transferred to polyvinylidene fluoride(PVDF) membranes (0.22 μm). The blots were blocked for 2 h at room temperature with 5% bovine serum albumin (BSA) in tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with primary antibodies (1:1 000) at 4 °C overnight.After TBST washing, the blots were incubated with secondary antibodies (1:1 000) at room temperature for 1 h.
lmmunofluorescence
The EPC cells were exposed to TFS for 1, 3, 5, and 7 d, as described above. The TFS-exposed cells were fixed with 4%paraformaldehyde at 4 °C overnight and permeabilized with Triton X100 (Beyotime, China) for 30 min at room temperature. Subsequently, the cells were incubated with specific primary antibodies (LC3B, 1:250; α-tubulin, 1:500) at 4 °C overnight. After incubation with Alexa Fluor 488/647-labeled secondary antibody (1:1 000) at 37 °C for 1 h,fluorescence signals were examined using an upright fluorescence microscope (Nikon, Japan).
Statistical analysis
Analysis of variance (ANOVA) and Tukey’spost hoctests were used to compare TFS-exposed samples with DMSOcontrols to determine significance (SPSS 18.0, USA). Results are presented as mean±SD andP<0.05 was considered statistically significant.
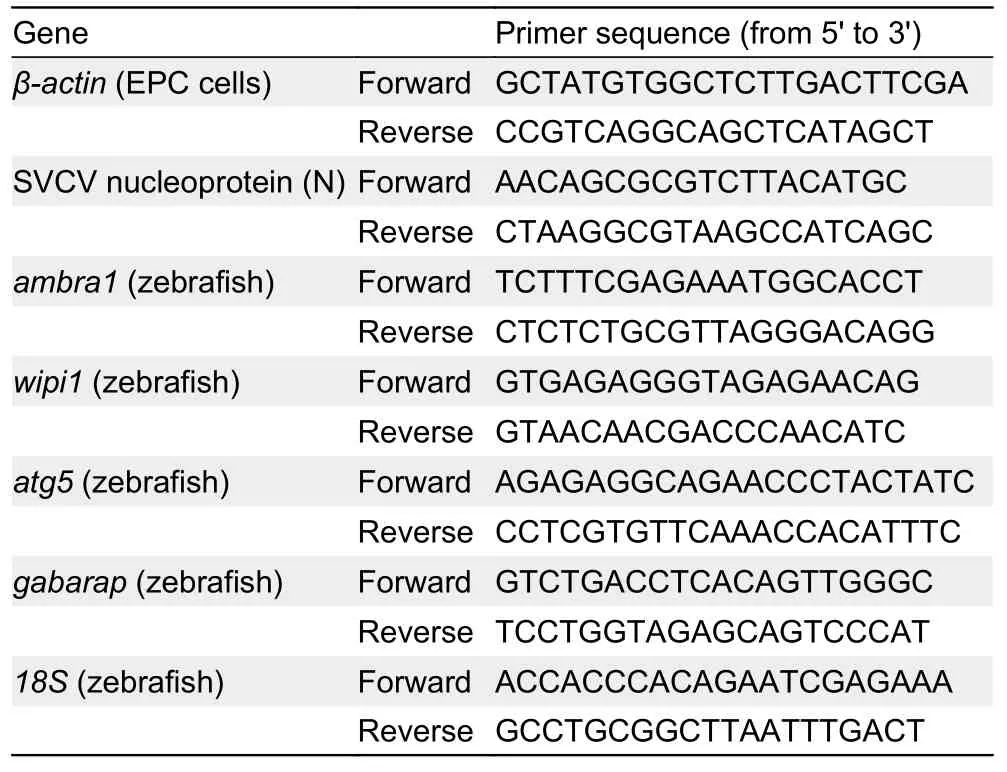
Table 1 Sequences of primer pairs used for analysis of gene expression by qRT-PCR
RESULTS
Cytotoxicity of TFS to EPC cells
The potential cytotoxicity of TFS was quantitatively assessed.As shown in Figure 1, the data indicated no evident cytotoxicity in EPC cells exposed to concentrations of TFS up to 125 μg/L for 4 d, whereas cytotoxicity was observed after exposure to 250 μg/L for 4 d and 500 μg/L for 1-4 d. It should be noted that TFS was not renewed in the 96-well plates during exposure. These findings suggest that TFS at concentrations (2.5 μg/L and 25 μg/L) used in subsequent experiments did not affect EPC cell growth.
Effects of TFS on SVCV replication in EPC cells
To determine whether environmental concentrations of TFS impact viral replication, we evaluated the effects of TFS at 2.5 μg/L and 25 μg/L on SVCV replication. As shown in Figure 2A, TFS at 2.5 μg/L and 25 μg/L (without renewal during exposure) significantly up-regulated (3-fold) the expression of the SVCV nucleoprotein at 48 h post-infection(hpi), suggesting that TFS may enhance viral infection in fish cells. TFS affected SVCV infectivity without acting on the virions as there was no obvious change in SVCV replication during co-incubation of TFS and SVCV (Figure 2B). Based on these data, we analyzed the longer-term effects of TFS on SVCV replication. As shown in Figure 2C-F, up-regulation occurred at 1, 3, 5, and 7 d pre-exposure, with a nearly 40-fold increase in the highest dose group. These results suggest that TFS may have an adverse effect on the resistance of fish cells to SVCV infection.
TFS induced autophagy in EPC cells
Based on earlier studies showing that TFS induces mitophagy in human skin keratinocytes and SVCV induces autophagy necessary for viral replication (Jang et al., 2016; Liu et al.,2015), we hypothesized that TFS may regulate SVCV replication via autophagy in fish. Autophagy is characterized by the formation of double-membrane vesicles(autophagosomes) that engulf cellular cargo and degrade internal contents upon fusion with endolysosomes (Liu et al.,2015). Results obtained via TEM showed that the number of autophagic vesicles with cytosolic contents increased in the cytoplasm of TFS-exposed EPC cells, whereas vesicles were rarely observed in the control cells (Figure 3). To further investigate the effects of TFS on autophagy in EPC cells, we examined LC3 lipidation, which is regarded as a significant indicator of autophagy activation, using western blot and immunofluorescence techniques. After TFS exposure, the conversion of LC3-I to LC3-II increased significantly, thus reflecting an increase in the lipidated form compared to that in the DMSO control (Figure 4A). Furthermore, a similar effect was observed during immunocytochemical testing, in which a specific antibody against LC3 was observed in untreated and viral-infected cells. A decrease in puncta number was found in the DMSO-exposed cells compared with TFS exposure,indicating that TFS activated autophagy in EPC cells at 1, 3, 5,and 7 d (Figure 4B-E).
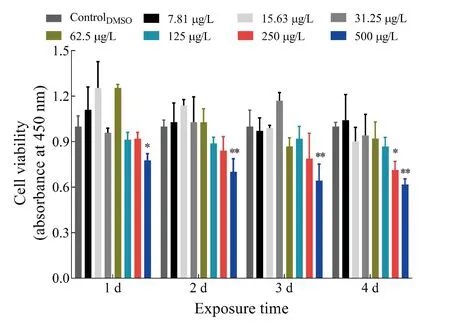
Figure 1 Cytotoxicity of TFS in EPC cells
Figure 2 Antiviral effects of TFS in vitro
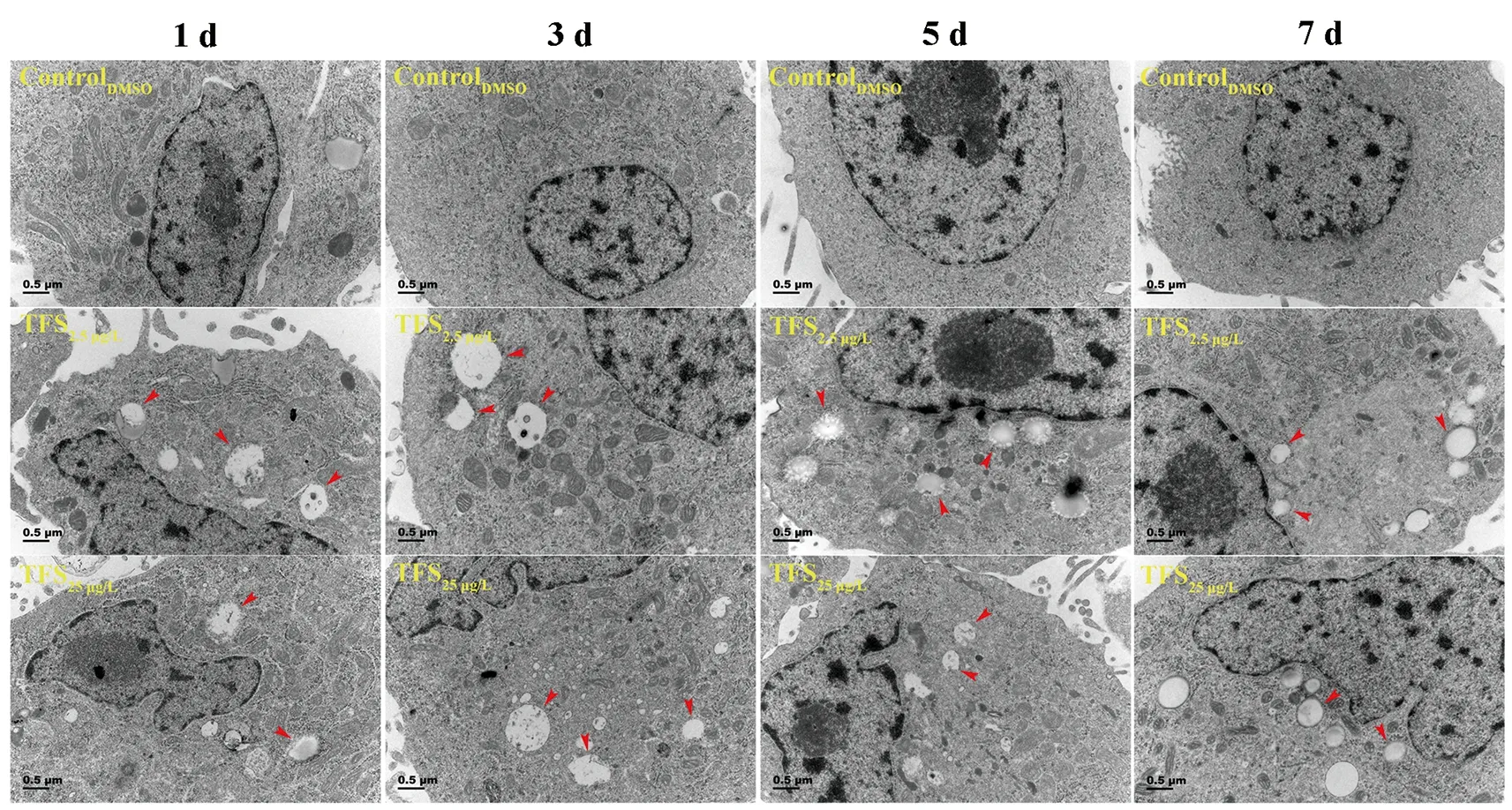
Figure 3 Ultrastructural features of TFS-exposed cells observed via transmission electron microscopy (TEM)
Autophagy-related representative protein expression in TFS-exposed cells
Autophagy induced by TFS in EPC cells was further assessed and confirmed by western blot analysis of the protein levels of several essential components. TFS altered these protein levels to some extent (Figure 5), and autophagy substrate P62/SQSTM1 decreased upon autophagy activation by TFS.Of note, TFS reduced Beclin-1 content at 1 d, but there were no obvious changes at 3, 5, or 7 d. Additionally, to investigate the mechanism by which autophagy was induced by TFS exposure, we investigated the involvement of the mTOR protein. Results showed that the expression of mTOR was inhibited by TFS, and the inhibition effect intensified at longer exposure times. As mTOR protein activation is dependent on its phosphorylation level, we also assessed pmTOR levels in EPC cells. As expected, TFS reduced mTOR phosphorylation at Ser2448 at 5 and 7 d. The change trend of mTOR-pmTOR corresponded to SVCV replication in the TFS pre-exposed cells. These results suggest that TFS facilitated autophagy in EPC cells, which increased SVCV replication.
Promotion of SVCV replication in zebrafish by TFSinduced autophagy
Based onin vitropre-exposure data, we explored the ability of TFS to enhance SVCV infectionin vivo. Zebrafish were exposed to TFS for 1, 3, 5, 7, and 14 d, followed by immersion infection with SVCV. Except for day 1, the SVCV-infected fish from the TFS-exposed groups (3 d and 5 d) exhibited significantly higher viral replication than the DMSO-exposed SVCV-infected fish (Figure 6A). Moreover, the virus increased dramatically (more than a hundred-fold) following TFS exposure for 7 and 14 d (Figure 6B, C). These results provide further evidence that TFS promotes SVCV proliferation in zebrafish.
Autophagy-related gene and protein expression levels in TFS-exposed zebrafish
To determine whether TFS-exposed zebrafish exhibited abnormal autophagy activation, TFS-induced changes in the expression of four autophagy-related genes were analyzed.As expected, the expression levels ofgabarap,atg5,wipi5,andambra1were significantly up-regulated in the TFS preexposed fish (no virus infection) (Figure 7A). This finding is consistent with the data from thein vitrostudy showing that autophagy-related protein levels at 25 μg/L were obviously elevated compared with those in the negative control(Figure 7B). TFS reversed the up-regulation of LC3-II/LC3-I and down-regulation of P62 in zebrafish. Furthermore, TFS exposure resulted in a decrease in mTOR levels, suggesting that TFS may enhance SVCV infection in zebrafish via excessive autophagy.
DlSCUSSlON
Increased food production under pesticide use has led to the detection of high levels of pesticides in environmental matrices(Collotta et al., 2013), potentially resulting in the emergence and outbreak of different diseases in fish due to interactions among pathogen virulence, host resistance, and aquatic ecosystem stability. In fact, increasing evidence points to associations between pesticide exposure and a greater predisposition of aquatic organisms to circulating pathogens,as well as an increase in disease sensitivity and mortality in fish (Dietrich et al., 2014; Morley, 2010). For example,Chinook salmon exposed to malathion and challenged withAeromonas salmonicidashow an increase in mortality(Dietrich et al., 2014); exposure to polycyclic aromatic hydrocarbons alters resistance in Japanese flounder(Paralichthys olivaceus) to viral hemorrhagic septicemia virus(Song et al., 2011); and exposure of rainbow trout(Oncorhynchusmykiss) to pendimethalin suppresses immunological responses and enhances infectious hematopoietic necrosis virus infections (Dupuy et al., 2019).Therefore, the adverse effects of pesticides on fish should be continuously investigated to protect aquatic environments as well as human health (Dupuy et al., 2019). In this study, we investigated the effects of TFS on antiviral defenses in zebrafish subjected to chronicin vivoTFS at environmentally relevant concentrations followed by SVCV challenge. Results showed a significant increase in viral replication in TFSexposed fish cells and zebrafish. This could be attributed to autophagic benefits to SVCV replication by removing damaged mitochondrial DNA in SVCV-infected cells (Liu et al.,2015).
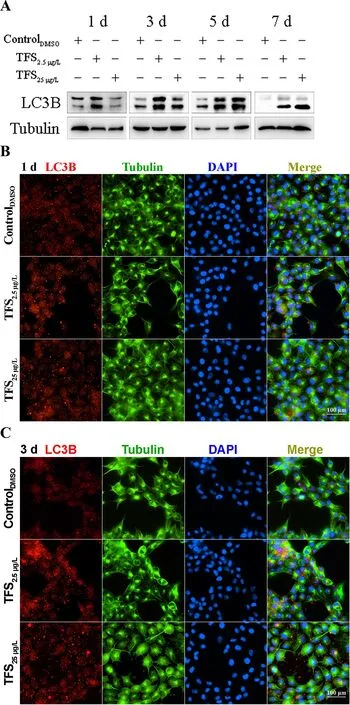
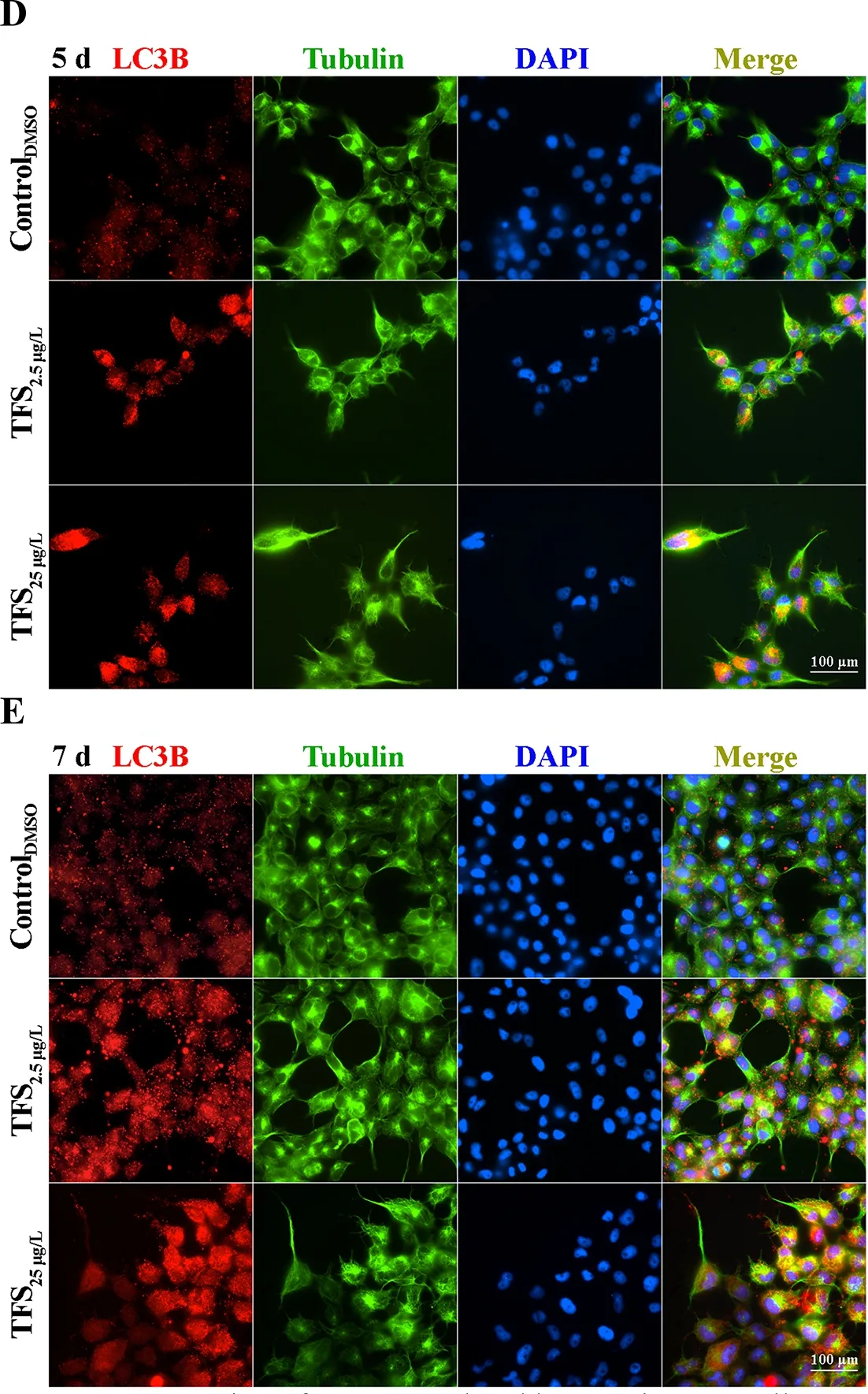
Figure 4 Expression of LC3B regulated by TFS in EPC cells
Autophagy is an evolutionarily conserved membranetrafficking process by which cells degrade old and damaged cytoplasmic components via lysosomes into basic biomolecules, thereby recycling cellular material. Autophagy therefore plays an important role in many crucial physiological processes such as cell differentiation and immunity in response to foreign substances and harmful influences (Dikic& Elazar, 2018; He & Klionsky, 2009). Autophagy is not only important for maintaining homeostasis in cells, but also in many pathogenic processes (Schmid et al., 2006; Zhang et al., 2011). The contrasting nature of autophagy has inspired much research. On the one hand, autophagy mediates both surveillance and effector functions involved in the detection and clearance of pathogens (Levine & Deretic, 2007; Shoji-Kawata & Levine, 2009) and is necessary for maintaining protein and organelle turnover. Conversely, some viruses,including SVCV, utilize the autophagy pathway to facilitate their own genomic replication and produce stronger infections(Liu et al., 2015; Orvedahl et al., 2007; Yoon et al., 2008).Numerous studies have shown that chemicals such as pesticides and metals can interfere with autophagy depending on cell type, exposure duration, and dose (Pesonen &Vähäkangas, 2019). Notably, elevated ROS levels can lead to mitochondrial dysfunction and activation of autophagy for the elimination of dysfunctional mitochondria (Cheng et al., 2009).Interestingly, TFS targets mitochondrial respiration by blocking the flow between cytochromeband cytochrome c1 at the ubiquinol oxidizing site (Qo) of complex III (Bartlett et al.,2002), which leads to damaged mitochondria. Evidence to date suggests that TFS can cause representative mitochondrial damage, such as altered membrane potential and oxidative stress, in zebrafish (Zhu et al., 2015a), and thus,there is a possible connection between autophagy and TFS exposure. In this study, SVCV replication was associated with increased autophagy in TFS-exposed cells and higher numbers of autophagic vesicles were observed by TEM.
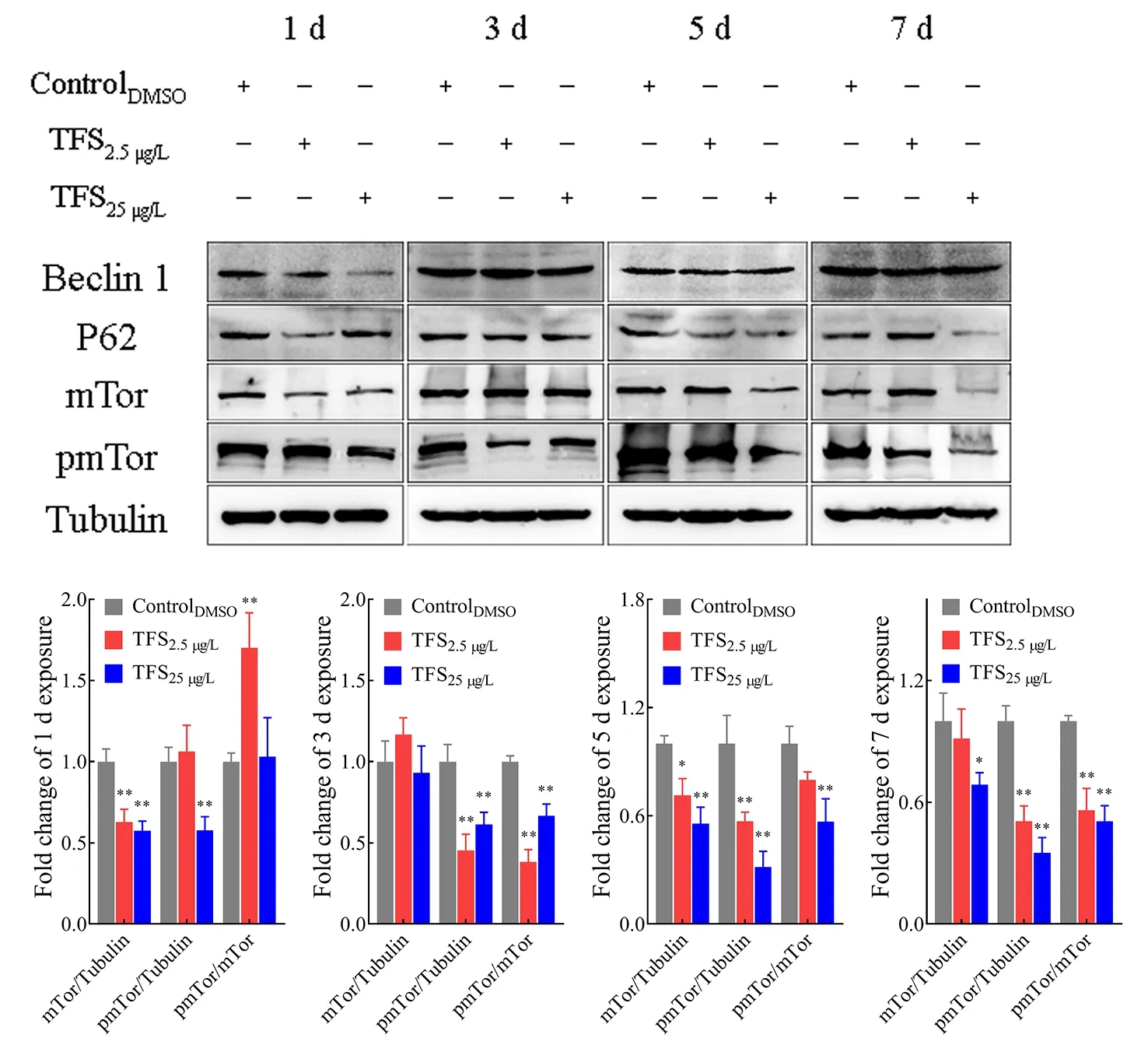
Figure 5 Total amounts of Beclin-1, P62, and mTOR, and phosphorylation level of pmTOR in EPC cells, as analyzed by western blotting
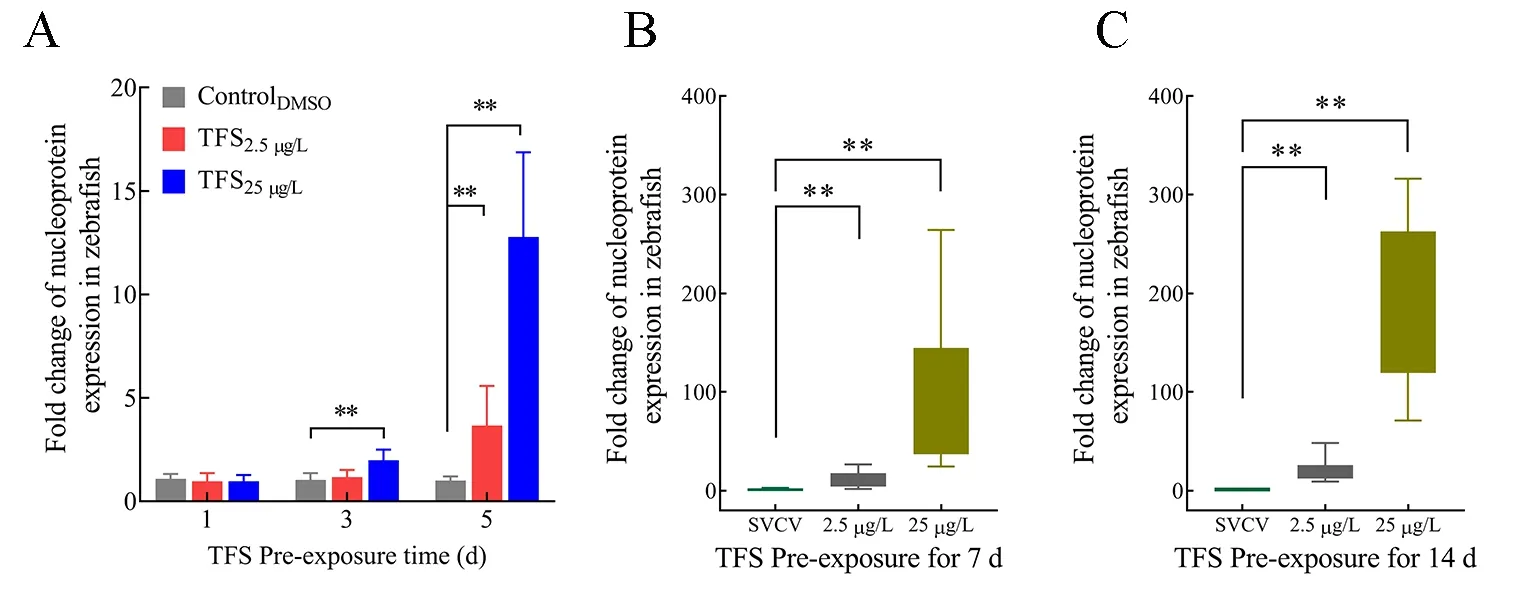
Figure 6 SVCV replication in TFS pre-exposed zebrafish at 1-5 d (A), 7 d (B), and 14 d (C)
Autophagosome formation and maturation are tightly controlled and coordinated by specialized proteins (He et al.,2020), e.g., the Atg8/LC3 conjugation system and Beclin-1,which are related to autophagosome formation (Zhang et al.,2011), and P62 (known as sequestosome 1, SQSTM1), which is correlated with protein degradation in autophagy (Liu et al.,2015). Our western blot assays confirmed the activation of autophagy by TFS, as indicated by increased Beclin-1 and LC3I/LC3II along with decreased P62 after EPC cells and zebrafish were exposed to TFS. mTOR is one of the bestknown cytoplasmic sensors and regulators of the autophagy pathway (Qin et al., 2010), and belongs to the Ser/Thr kinases, including rapamycin-sensitive mTORC1 and nonsensitive mTORC2. Specifically, mTORC1 negatively regulates autophagosome formation by regulating the phosphorylation level of the ULK1-Atg13-RB1CC1-C12ORF44/Atg101 complex (Wan et al., 2014), while mTORC2 inhibits autophagy by activating the Atg1 protein(Cheng et al., 2013). The activation of mTOR can block the expression and function of autophagy-related (ATG) proteins.Liu et al. (2015) determined that SVCV causes a significant reduction in pmTOR, which facilitates the replication of mTOR signals in fish. Our work suggests that exposure to TFS can result in the activation of autophagy in fish by inhibition of mTOR signaling, which enhances SVCV susceptibility in zebrafish.
In conclusion, we explored the aquatic environmental risks of TFS by investigating the cellular and molecular responses of zebrafish following exposure to TFS, with subsequent SVCV challenge. Our findings demonstrated non-significant cytotoxicity of TFS up to 125 μg/L in EPC cells. The significant enhancement of SVCV replication in TFS-exposed EPC cells and zebrafish was associated with activation of autophagy.This suggests that TFS in aquatic environments may contribute to SVCV outbreaks and endanger ecological safety.At present, however, we are unable to determine the upstream regulatory mechanism of autophagy in mTOR signaling and whether there is permanent damage in fish following TFS exposure. This topic will be the focus of future research.
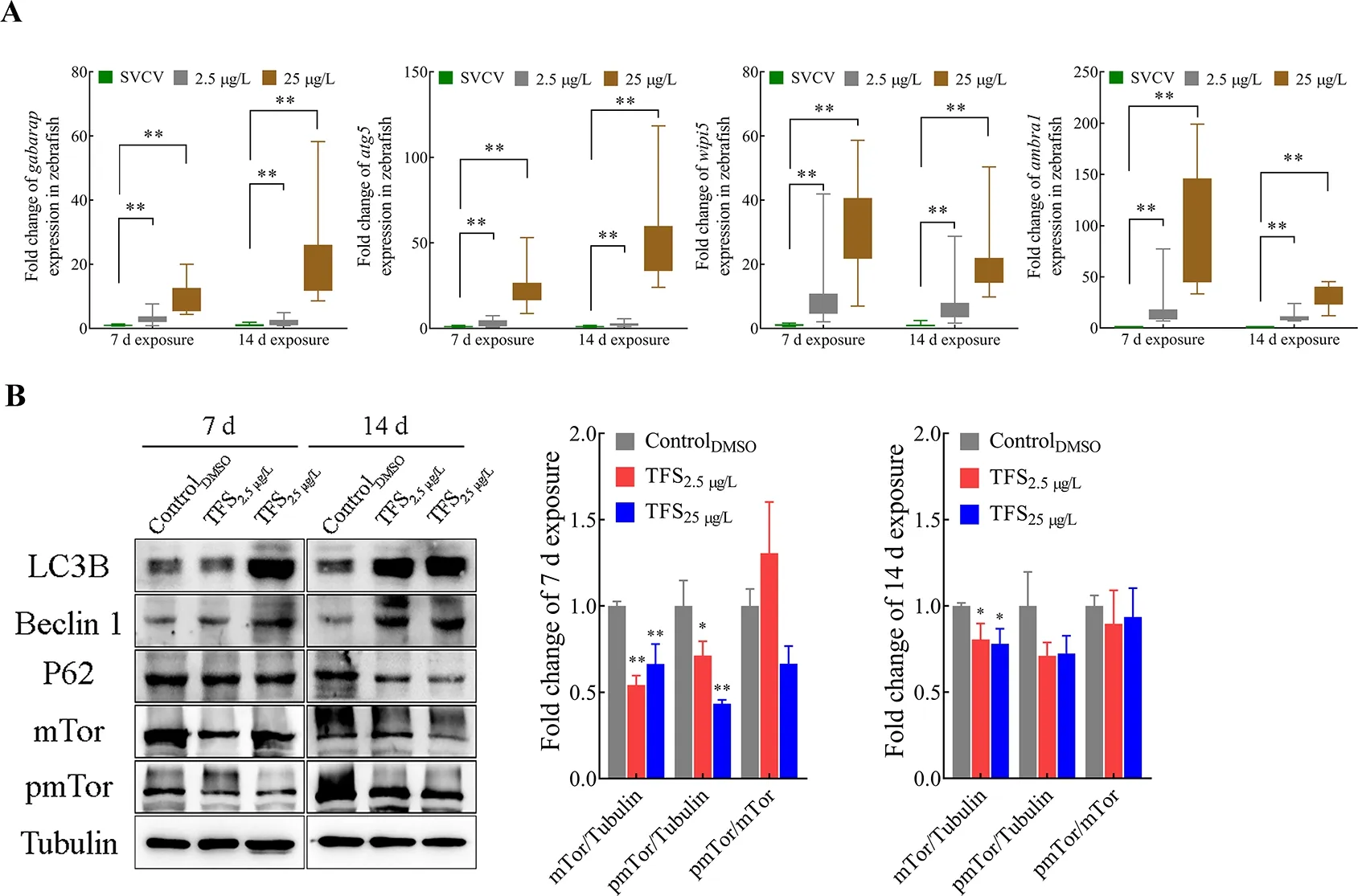
Figure 7 Alteration in autophagy-related gene and protein expression levels in TFS-exposed zebrafish
COMPETlNG lNTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRlBUTlONS
J.C. and L.L. drafted the experiments; H.W., H.W.L, L.H., and J.F.L. performed the experiments. T.X.Q. and L.L. analyzed the data and wrote the paper. All authors read and approved the final version of the manuscript.
杂志排行
Zoological Research的其它文章
- Species bias and spillover effects in scientific research on Carnivora in China
- A review of the Cypriniform tribe Yunnanilini Prokofiev,2010 from China, with an emphasis on five genera based on morphologies and complete mitochondrial genomes of some species
- Particulate matter exposure exacerbates susceptibility to SARS-CoV-2 infection in humanized ACE2 mice
- Northern pig-tailed macaques (Macaca leonina)infected with SARS-CoV-2 show rapid viral clearance and persistent immune response
- Cataract-causing allele in CRYAA (Y118D) proceeds through endoplasmic reticulum stress in mouse model
- Molecular mechanisms of intermuscular bone development in fish: a review
