Carbon and nitrogen budget in fish-polychaete integrated aquaculture system*
2021-06-15FawenHUMingSUNJinghuiFANGGuodongWANGLiLIFengxiangGAOYuxiaJIANXueWANGGuangbinLIUYanZOUWenGUO
Fawen HU , Ming SUN , Jinghui FANG , Guodong WANG, Li LI, Fengxiang GAO,Yuxia JIAN, Xue WANG , Guangbin LIU, Yan ZOU, Wen GUO
1 Marine Biology Institute of Shandong Province, Qingdao 266104, China
2 Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
3 Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266071, China
4 Marine Science and Engineering College, Qingdao Agricultural University, Qingdao 266109, China
Abstract Integrated multi-tropic aquaculture (IMTA) systems have been used in China for many years and have achieved significant economic, social, and ecological benefits. However, there is still a lack of benthic bioremediation species that can eff ectively utilize the aquaculture particulate organic waste in the system. Polychaete Perinereis aibuhitensis Grube is used as an environmental remediation species for large-scale aquaculture to reduce particulate organic waste, which is of great significance to environmental protection. To improve bio-elements utilization effi ciency, P. aibuhitensis was applied for IMTA indoor fish ( Hexagrammos otakii) farming. Results showed that in the system, production of 1 kg of the fish discharged 2 141–2 338 mg of carbon and 529–532 mg of nitrogen, while in the monoculture of the fish, the figures were 3 033–3 390 mg and 764–794 mg, or 24.84%–35.26% and 30.35%–33.32% less, respectively.This approach promoted IMTA technology that could utilize the particulate organic waste from intensive aquaculture and reduce the adverse environmental eff ects.
Keyword: Perinereis aibuhitensis Grube; Hexagrammos otakii; integrated multi-tropic aquaculture (IMTA);carbon and nitrogen budget; sediment remediation
1 INTRODUCTION
There has been a sharp increase in the global demand for seafood products coinciding with rapid population growth. As a result, aquaculture production has undergone unprecedented growth and has become the fastest-growing food-producing sector in the world. Fish farming supports major aquaculture across the world, including fish cage farming and indoor fish farming (FAO, 2018). However, increasing concern had been posed regarding its environmental impact (Read and Fernandes, 2003; Mente et al.,2006; Wen et al., 2007). For example, the discharged particle waste and dissolved compounds from fish cage farming adversely aff ected the surrounding environment (Wu, 1995; Gillibrand et al., 1996; Wen et al., 2007; Á Norði et al., 2011). The discharged nitrogen could account for 75% and 80% of the nitrogen input in pond and cage aquaculture system,respectively (Chang et al., 2006; Zhang, 2012). An excess amount of these compounds caused environmental problems, such as eutrophication,changes in the function and structure of the macrofauna community, and biochemical functions (Mazzola et al., 2000; Sanz-Lázaro and Marin, 2006; Jiang et al.,2013), which attracted researchers’ interests.
Integrated multi-tropic aquaculture (IMTA) is based on the principle of bioelements recycling,where excess nutrients released by animals (such as fed species) are recaptured and reused by lower trophic species (including animals and plants) to reduce negative environmental impacts and produce additional value (Chopin et al., 2010; Fang et al.,2019). Excess nutrients in IMTA systems are used to diversify the system’s products; thus, providing more effi cient handling of resources. A basic mode combines an intensively raised organism (e.g., fish or shrimp) with a species that extracts organic matter(e.g., shellfish) and/or a species that extracts inorganic matter (e.g., macroalgae). As a result, feces and inorganic waste of one resource consumer become the resource for the others. The particulate organic as well as dissolved inorganic nutrients are taken up and converted into biomass, which is resembling a natural,balanced ecosystem (Chopin et al., 2001; Neori et al.,2004; Reid et al., 2007). Several successful combinations have been proposed, such as fishseaweed IMTA (Stenton-Dozey, 2007; Abreu et al.,2009), finfish-shellfish IMTA (Mazzola and Sarà,2001; Langan, 2004), abalone-kelp IMTA, finfishbivalve-kelp IMTA, abalone-sea cucumber-clamseaweed IMTA (Fang et al., 2016a), and shellfishseaweed IMTA (Chopin et al., 2008). Seaweed absorbs dissolved bioelements, such as dissolved inorganic carbon, dissolved inorganic nitrogen and phosphorous, and produces oxygen at the same time.Suspension-feeding shellfish species consume suspended organic particulates and produce ammonia and carbon dioxide. At the same time, shellfish imbeds a significant quantity of carbon in the shells in the form of CaCO3during the calcification process. Both the cultured animals and seaweed benefit each other in an IMTA system. However, there is lack of benthic animals that utilize the settling organic matter from fish farm in current IMTA systems, because sea cucumber cannot endure organic-enriched sediment with low oxygen condition (Li, 2016) and their hibernation and aestivation limit their ability to recover the sediment. A suitable benthic species is the key factor to expand and improve IMTA technology.
Polychaetes are important species in seashore areas. They feed on organic debris and sediment, and can endure sediment environments with high organic content, which makes them very suitable for an IMTA system (Fang et al., 2016b). The polychaetes were often found underneath the fish farm (Mazzola et al.,2000). Moreover, polychaetes dominated the meiofauna in some fish farming areas (Gao et al.,2003; Huang et al., 2005; Yang et al., 2007), which were known to play an important role in the recovery of sediments beneath fish farms (Mazzola et al., 2000;Huang et al., 2005; Porrello et al., 2005; Brennan,2018) and can significantly stimulate mineralization of organic matter (Heilskov and Holmer, 2001).
Hexagrammosotakiimainly distributed in the off shore regions of Korea, Japan, and China (Ren et al., 2013). It is an important fish species for economy in East Asia. In China,H.otakiiis cultured in cage or indoor area. The waste from the cages or indoor factories were discharged into the surrounding environment, which is lack of technology for waste treatment and utilization.PerinereisaibuhitensisGrube is an important cultured polychaete species in China and is an important fresh feed for aquaculture due to its high nutritional value (Gu et al., 2002).P.aibuhitensiscould consume particulate waste discharged by fish and shrimp, such asParalichthysolivaceus(Fang et al., 2014, 2017; Yang et al., 2015)andFenneropenaeuschinensis(Deng et al., 2006). A fish-polychaete IMTA system not only reduces the adverse eff ects from fed species, but also increases the production. In this study, we quantified the nutrient(N and C) balances in IMTA mode ofH.otakiiandP.aibuhitensis, aiming to reducing C and N discharges and compared those of theH.otakiimonoculture. It demonstrates technology for utilizing waste and improving water quality. Furthermore, it will improve the economic and ecological profits of the fish farming.
2 MATERIAL AND METHOD
2.1 Experimental design and management
This study was conducted at the National Oceanographic Center, Qingdao, China between July 14 and August 13, 2018. TheH.otakii(7.84±1.90 g)were provided by the Marine Biology Institute of Shandong Province.P.aibuhitensis(1.89±0.38 g)were obtained from Rongcheng Chudao Aquatic Products Co., Ltd. The experimental cement ponds were transformed into a “1+3” continuous culture system (Fig.1). The experiment was conducted in three cement fish ponds (4 m×4 m×1 m, 16 m2), and each fish pond corresponded to three rectangle polychaete ponds (0.6 m×1.5 m×0.5 m, 0.9 m2). TheP.aibuhitensiswere stocked at a density of 200 ind./m2(D1), 400 ind./m2(D2), and 600 ind./m2(D3) in the three corresponding polychaete ponds, respectively.The bottom of polychaete ponds were covered with a 15-cm thick sediment taken from the natural habitat ofP.aibuhitensis. The sediments were allowed to soak for 11 days and were naturally deposited. The amount of sediment was calculated by sampling 10 cm×10 cm area at three site of each polychaete pond (middle and both ends) in the beginning and end of the experiment. Prior to the experiment, the fish were acclimated in the cement fish ponds for 10 days.The artificially propagated fish used in the experiment were from the same batch to ensure they were of similar sizes and biological characteristics.
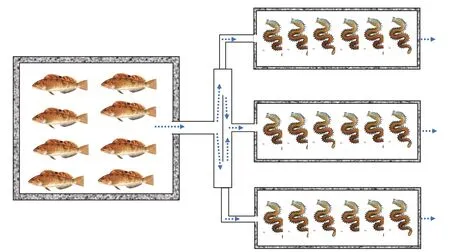
Fig.1 Experimental system schematic map
HealthyH.otakiiof similar size were selected and 900 fish were stocked in each cement fish pond. The fish were fed a commercial fish feed ad libitum twice daily at 7:30 and 18:00. Feeding stopped when the fish did not grab the feed, and the amount of the feed in each pond was recorded (1%–2% daily). The carbon and nitrogen discharge (reduced ratio) and utilization (production/input) in the fish pond would be compared with those in the integrated system. The compositions of the feed were 50.0% protein, 10.0%fat, 8.0% fiber, 17.0% ash, 0.8% phosphorus, 12.0%moisture, 8.6% nitrogen, 46.6% carbon (Surgreen,Qingdao). Water temperature was 18–20 °C from underground. In addition, the water flow rate was 4 000–5 000 L/d, which was recorded by a flowmeter at each water inlet.
2.2 Sampling method
Water was sampled five times from inlet and outlet every day at 6:00, 10:00, 14:00, 16:00, and 20:00,then mixed and filtered through a glass microporous membrane (pore size 0.45 μm) to determine the particle weight, particle carbon and nitrogen values.Saturated mercury chloride was added to the filtered water, and it was stored at -20 °C. Total nitrogen in the filtered water samples were determined every week. The diff erence of nitrogen contents between the inlet and outlet of the system would be the output of dissolved nitrogen. Sediment samples for elements analysis taken from six locations in each pond at the beginning and end of the experiment were thoroughly mixed into a composite sample for further analysis.
At the beginning of the experiment, 20 fish and 20P.aibuhitensiswere sampled and starved for 24 h to determine initial average body weight, then stored at -20 °C. All fish were starved for 24 h at the end of the experiment. All polychaetes were dug out by hands and starved in a cement pond. Then, all animals were weighed, and 20 fish in each fish pond and 20 polychaetes in each polychaete pond were sampled.All samples except for the water were dried in an oven at 70 °C to constant weight. The dried feed,sediment, fish, andP.aibuhitensiswere homogenized to analyze carbon and nitrogen contents.
2.3 Chemical analysis
The moisture content of the feed and fish wasdetermined after the samples were oven-dried to constant weight at 70 °C. Carbon and nitrogen in the solid samples were determined using a Vario Elemental Analyzer (Elementar, Langenselbold,Germany), while dissolved organic carbon was determined in the water samples using a total organic carbon analyzer (TOC-5000A). Total nitrogen was determined by referring to the People’s Republic of China Environmental Protection Standards (HJ 636-2012). Briefly, the water samples were digested using alkaline potassium persulfate and analyzed by ultraviolet spectrophotometry.
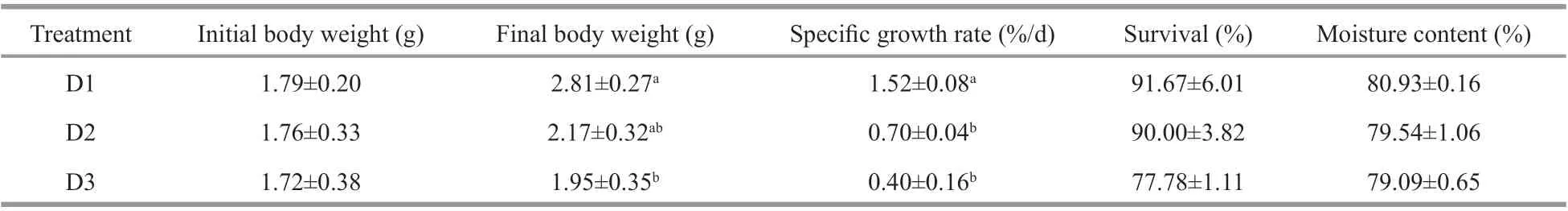
Table 1 Growth and survival of P. aibuhitensis at diff erent densities (means±S.E.)
2.4 Determination of the carbon and nitrogen budgets
The carbon and nitrogen budgets in each pond were estimated with the following equations:
CIn=CF+CS0+CP0,
NIn=NF+NS0+NP0,
CD=CIn−CPt−CSt−CSh−CWo−CSm,
NOut=NPt+NSt+NSh+NWo+NSm,
COr=100×(CPt+CSt+CSh)/CIn,
NOr=100×(NPt+NSt+NSh)/NIn,
whereCInandNInare carbon and nitrogen input (g);CFandNFare the carbon and nitrogen content in feed(g);CS0andNS0are the initial carbon and nitrogen content ofP.aibuhitensis(g);CP0andNP0are initial carbon and nitrogen content ofH.otakii(g);CDis the dissolved inorganic carbon lost in the water (g);Noutis nitrogen output (g);CPtandNPtare the final carbon and nitrogen ofH.otakii(g);CStandNStare the final carbon and nitrogen ofP.aibuhitensis(g);CShandNShare the carbon and nitrogen content of heteronereis(g);CWois the output of organic carbon andNWois the nitrogen in water (g);CSmandNSmare sediment accumulation of carbon and nitrogen (g); andCOrandNOrare the production/input of carbon and nitrogen(%), respectively.
2.5 Calculation of specific growth rate
Specific growth rate (SGR, %/d) was calculated as(Fang et al., 2016b):
SGR=100×(lnWt−lnW0)/t,
whereWtandW0are the final and initial wet body weight (mg) of polychaetes, respectively; andtis feeding duration (days).
2.6 Statistical analysis
One-way analysis of variance and Duncan’s test were used to determine significant diff erences between variables of polychaete ponds.P-value <0.05 was considered significant. The SPSS 16.0 for Windows software package (SPSS Inc., Chicago, IL,USA) was used for all analyses.
3 RESULT
3.1 Growth and survival
No significant diff erence was observed in the initial body weight ofP.aibuhitensisat diff erent densities(P>0.05). The body weight ofP.aibuhitensisin group D1 was significantly higher than that in group D3 at the end of the experiment (P<0.05). The specific growth rate (SGR) ofP.aibuhitensisin D1 was significantly higher than that in D2 and D3 (P<0.05).The survival ofP.aibuhitensisdecreased with increasing densities, but was not significant among the groups (P>0.05). No significant diff erence in moisture content was observed among the three groups at the end of the experiment (P>0.05, Table 1).
As the data shown in Table 2, the parameters including body weight, SGR, survival, feed consumption and moisture content of fish were similar among diff erent treatments in the end of the experiment.
3.2 Carbon and nitrogen contents
The initial carbon and nitrogen contents inH.otakii,P.aibuhitensisand sediment were 42.59%,48.29%, 0.33% and 9.83%, 9.76%, 0.03%,respectively. The carbon and nitrogen contents in animals were similar among treatments at the end of the experiment (P>0.05). The carbon and nitrogen content in sediment increased with the increasingpolychaete densities, but no significant diff erence was found (P>0.05, Table 3).
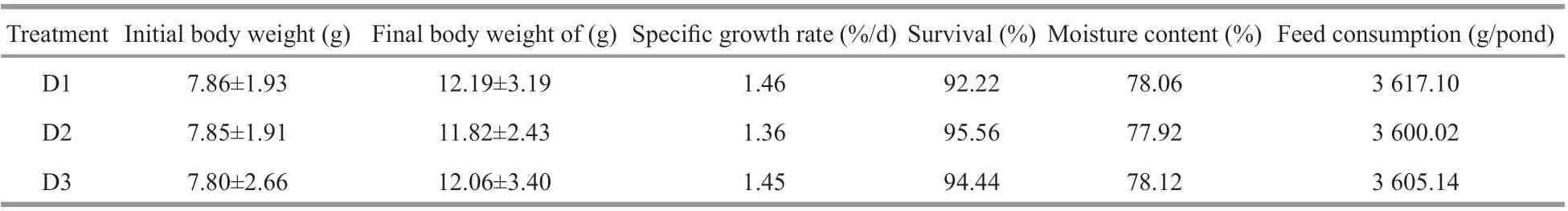
Table 2 Growth and survival of fish in the diff erent treatments (means±S.E.)

Table 3 Carbon and nitrogen contents in animals and sediment in diff erent treatments at the end of the experiment (%,means±S.E.)
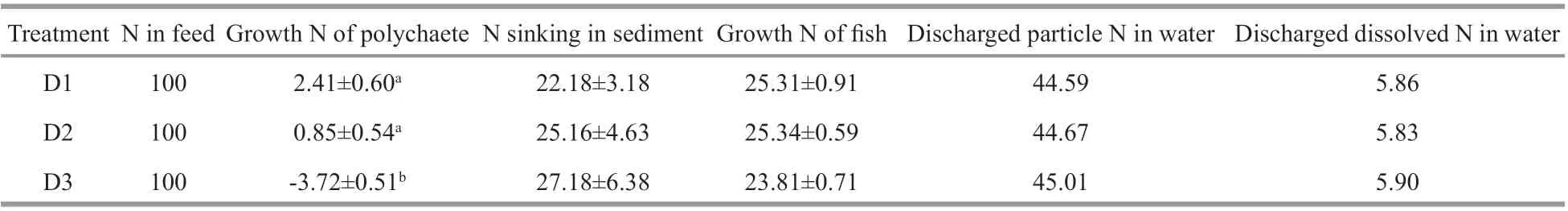
Table 4 Percentages ofinputs and outputs of nitrogen in the diff erent treatments of the integrated culture mode (%)
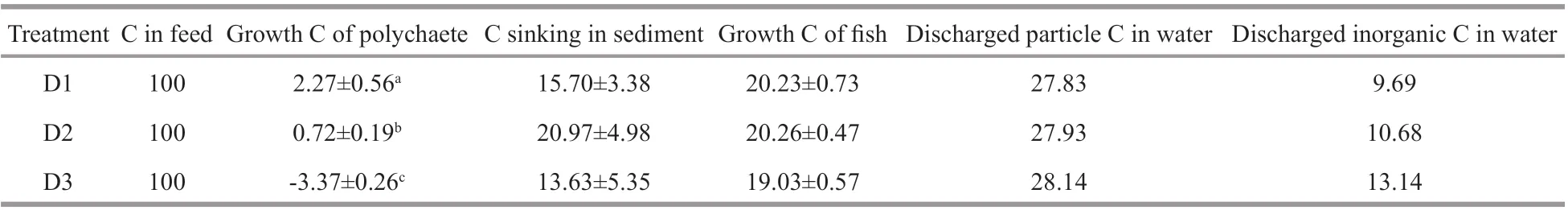
Table 5 Percentages ofinputs and outputs of carbon in the diff erent treatments of the integrated culture mode (%)
3.3 Nitrogen budget
The nitrogen inputs of commercial feed in the fish integrated polychaete system were mostly discharged in the form of particles in water, the nitrogen discharge ranged from 44.59% to 45.01%. The remaining proportions of nitrogen found in growth N of fish ranged from 23.81% to 25.34%. The nitrogen sinking in the sediment ranged from 22.18% to 27.18%.Discharged nitrogen (NO3ˉ-N, NO2ˉ-N, and NH4+-N)dissolved in the water accounted for 5.83%–5.90%.The proportion of nitrogen found inP.aibuhitensiswas very low (2.41%) in group D1, but was higher than the other two groups. Group D3 showed a higher proportion of nitrogen deposition in sediment due to high mortality (Table 4).
3.4 Carbon budget
Discharged particulate C accounted for 27.83%–28.14% of the carbon in feed input flushed out with the water, followed by growth carbon for fish and carbon sinking into the sediment, which accounted for 19.03%–20.26% and 13.63%–20.97%,respectively. Discharged inorganic carbon in the water accounted for 9.69%–13.14% of the carbon in feed input (Table 5). The proportion of growth carbon inP.aibuhitensiswas very low, but was highest in group D1 (2.27%). Negative carbon growth was observed in D3 due to high mortality.
3.5 Carbon and nitrogen discharge comparison
The nitrogen and carbon discharged from the water were equivalent when 1-kg fish was cultured one day.In the present integrated culture system, 1 kg of fish discharged 2 141–2 338 mg of carbon and 529–532 mg of nitrogen daily. In the fish monoculture system, 1 kg of fish discharged 3 033–3 390 mg of carbon and 764–794 mg of nitrogen (Table 6).Nitrogen and carbon discharge decreased by 30.35%–33.32% and 24.84%–35.26%, respectively after adding polychaetes to the system (Fig.2). Nitrogen and carbon discharge decreased more in the D2 group than the other two groups. All the three polychaete treatments showed higher production/input of carbon and nitrogen than fish monoculture (Fig.3).
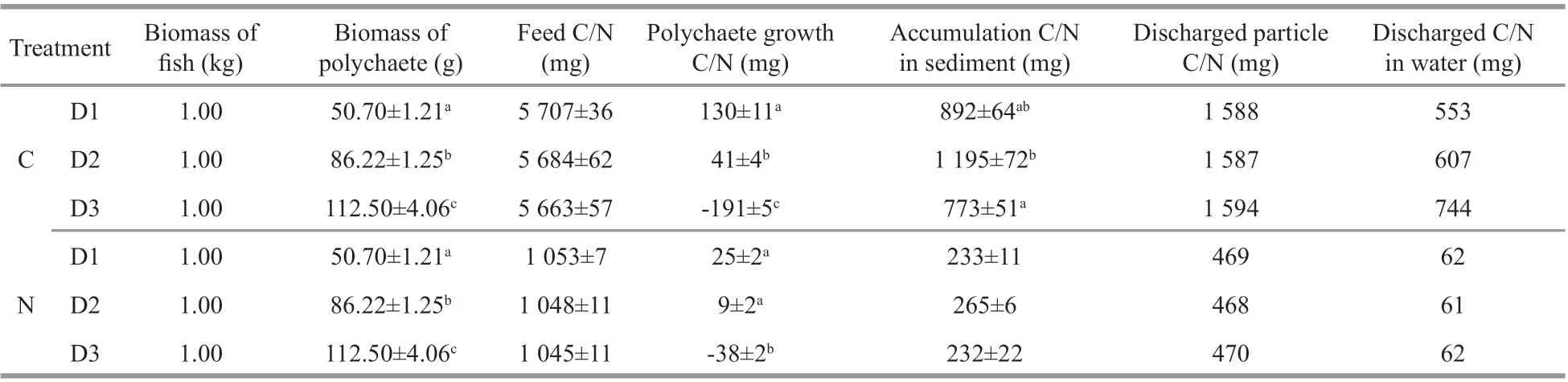
Table 6 Relative daily amounts of diff erent elements of the integrated culture system (based on 1 kg of H. otakii)
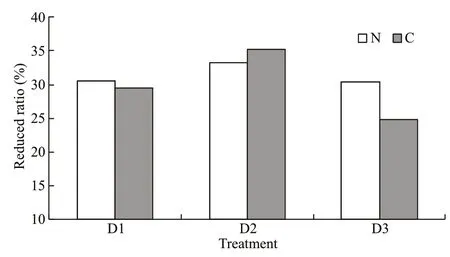
Fig.2 Reduced ratios of carbon (C) and nitrogen (N)discharge in the integrated fish-polychaete system compared with fish monoculture
4 DISCUSSION
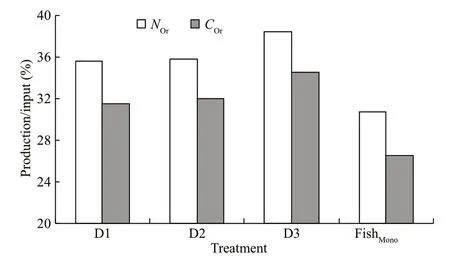
Fig.3 Production/input of carbon (C Or) and nitrogen (N Or)in the integrated fish-polychaete system compared and fish monoculture (Fish Mono) pond
The results show that there were more nitrogen and carbon released into water other than in sediment, and this process played a major role in the nutrient budgets of the current IMTA system. Such findings were in accordance with the literature (Fang et al., 2014), as nutrients trapped in the net output of N and C in water played an important role in the nutrient balance of an IMTA system. Fang et al. (2014) reported that growth N and C of fish and the net output of C and N in water account for most of the N and C input, while the accumulated N and C in sediments account for a lower proportion (7.91%–16.44% of N and 9.22%–14.96% of C). Both of the IMTA systems were flowthrough systems. The flowing water took most of the particulate nutrients away. However, Pouil et al.(2019) reported that accumulated sediments played a major role in nutrient budgets because a large proportion of the N input settles on the sediment directly, which was mainly enriched by dead organisms and particle waste produced by the cultured organisms, such as fishes and shellfishes. Such findings were similar to other reports. For example,Nhan et al. (2008) reported that nutrient trapping in sediments dominates the nutrient budget, while high water-exchange ponds discharged more nutrients than low water-exchange ponds. This finding meant that water exchange (flow rate) was an important factor for nutrient discharge in the water due to high particulate content. However, the particulate organic matter settled when water flow was slow. Thus, if the area of the polychaete pond was enlarged to reduce the flow rate, nutrient discharge would be reduced significantly and the system could support more polychaetes.
Perinereisaibuhitensissignificantly reduced carbon and nitrogen contents in the sediment by consuming organic matters under fish cages in an indoor experiment (Fang et al., 2014).P.aibuhitensisalso had the potential function of environmental recovery in farming areas because they consume feces and uneaten feed from fish cages (Fang et al.,2017). The proportions of N and C that accumulated in sediments in our study were low for at least two reasons: 1) theP.aibuhitensisingested fish feces and uneaten feed for growth and metabolism; 2)bioirrigation accelerated the decomposition of organic matter in the sediment. Both of these activities reduced N and C accumulation in sediments. Deng et al. (2006) reported that introducingP.aibuhitensisto shrimp ponds significantly improved sediment quality. Another study found that the deposit-feeding polychaete (Capitellasp. I) significantly enhanced the decomposition rate of organic matter in the sediment underneath a fish farm (Kinoshita et al.,2008).
In the present study, the D2 (400 ind./m2) group achieved the highest reduced ratio of discharged carbon and nitrogen as well as polychaete growth in the integrated system. The carbon and nitrogen emissions decreased by 19.96% and 14.28% after addingP.aibuhitensisto the system. However, as mentioned above, the polychaete pond could be enlarged allowing more particulate organic matter to settle. The additional food resources would support a higher polychaete biomass. The density ofP.aibuhitensiscould reach about 300 ind./m2in natural ecosystems (Zhao et al.,1993), but might increase to 500–1 000 ind./m2in aquaculture settings supplying ample food (Chen et al.,2007). The environmental remediation potential ofP.aibuhitensiswas not fully developed in the present study. More combinations should be tried in future studies. For example, an integrated polychaete pond(cement or earth) could be set outdoors with a larger area, in which more species, such as sea cucumber and shellfish, could be cultured.
IMTA was often adapted as a practice that increases environmental sustainability of aquaculture and income (Neori et al., 2004; Troell et al., 2009). Fang et al. (2014) established a mode ofP.olivaceuscage culture integrated withP.albuhitensisunderneath the cage. In this mode, theP.albuhitensisfed on residual feed and feces of the fish. The accumulation of carbon and nitrogen in the substrate decreased and the ratio of production to investment increased. Lu et al. (2011)constructed three kinds of compound farms: fish +algae, fish + P. aibuhitensis, and fish + algae +P.aibuhitensis. In the mode, total nitrogen, total phosphorus, and inorganic phosphorus in sediment decreased by 8.9%–9.2%, 6.1%–6.3%, and 8.0%–8.1% respectively after addingP.aibuhitensiscompared to the fish monoculture, which eff ectively alleviated the accumulation of nitrogen and phosphorus in the sediments. Co-culturing fish with polychaete in this study had a significant eff ect on reducing the negative impact ofintegrated culture on the environment. Furthermore,P.aibuhitensisis a very good fresh feed for aquaculture (Fish and Fish,1989; Gu et al., 2002). This is indeed a cycle of bioelement utilization. The fish integrated withP.aibuhitensisaquaculture system is theoretically feasible and useful.
5 CONCLUSION
The rank order of the N or C was discharged particulate N (C) in the water>growth N (C) of fish>N(C) sinking in the sediment>discharged NO3ˉ-N,NO2ˉ-N, and NH4+-N (inorganic C) in the water>growth N of polychaetes. The integrated fish-polychaete system would reduce the carbon and nitrogen discharge. Enlarging the polychaete pond would reduce flow velocity and increase the remediation eff ect of the polychaetes.P.aibuhitensishas the potential to play an important role in the environmental recovery of sediment in an integrated culture system.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
