Isolation of a novel strain of Cyanobacterium sp. with good adaptation to extreme alkalinity and high polysaccharide yield*
2021-06-15ZishuoCHENTaoLIBingjieYANGXuejieJINHualianWUJiayiWUYanduLUWenzhouXIANG
Zishuo CHEN , Tao LI , , Bingjie YANG , Xuejie JIN ,, Hualian WU ,, Jiayi WU,,Yandu LU , Wenzhou XIANG,,**
1 CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, Guangdong Key Laboratory of Marine Materia Medica,Institution of South China Sea Ecology and Environmental Engineering, RNAM Center for Marine Microbiology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
2 State Key Laboratory of Marine Resource Utilization in South China Sea, College of Oceanology, Hainan University, Haikou 570228, China
3 Southern Marine Science and Engineering Guangdong Laboratory(Guangzhou), Guangzhou 511458, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract The use of high alkaline medium is a feasible way to provide carbon source and prevent biological contamination for the outdoor cultivation of alkaliphilic microalgae and cyanobacteria. A novel cyanobacterial strain was isolated from the open pond of a marine green alga ( Picochlorum sp. SCSIO-45015,Sanya, Hainan) and identified as Cyanobacterium sp. SCSIO-45682. The eff ects ofinitial sodium bicarbonate(NaHCO 3) concentrations on the growth and biochemical composition of Cyanobacterium sp. SCSIO-45682 were investigated. The results demonstrated that Cyanobacterium sp. SCSIO-45682 had good adaptation to 16.8-g/L NaHCO 3 (the same concentration of NaHCO 3 used in Zarrouk medium for Spirulina). Moreover,the yields of biomass, polysaccharide, chlorophyll a (chl a), and phycocyanin increased under high NaHCO 3 concentrations. The maximum final biomass concentration of 2.5 g/L was observed at 8.4-g/L NaHCO 3, while the highest intracellular total saccharide content of 49.2% of dry weight (DW) and exopolysaccharide (EPS)concentration of 93 mg/L were achieved at the NaHCO 3 concentration of 16.8 g/L. The crude protein content declined under high NaHCO 3 concentrations, which provide a possible explanation for the accumulation of polysaccharide. This study shows a good potential of alkaliphilic Cyanobacterium sp. SCSIO-45682 as a polysaccharide feedstock.
Keyword: alkaliphilic cyanobacterium; biochemical composition; Cyanobacterium sp. SCSIO-45682; high sodium bicarbonate (NaHCO 3) concentrations; polysaccharide
1 INTRODUCTION
Cyanobacteria, existing in diverse morphology, are a large group of prokaryotic organisms with promising biotechnological and commercial applications. These oldest photoautotrophic microbes on the earth colonize a diversity of habitats, from fresh or seawater to terrestrial environments (Lau et al., 2015). They can adapt to wide environmental stresses, such as high alkalinity, high salinity, and low irradiance.Some species can grow rapidly with excellent carbon capture and yield high-value metabolites (Khan et al.,2018). Thus, cyanobacteria are considered as suitable candidates to produce biologically active compounds,biodiesel, bioplastics, and so forth (Grossmann et al.,2019).
Polysaccharides produced by cyanobacteria have received increasing attention due to various biological activities, such as antiviral, antitumor, antioxidant,antidiabetic, and immunoregulation eff ects (Demay et al., 2019). The calcium sulfate polysaccharide isolated from hot water extract ofSpirulinashowed a pronounced eff ect on human immunodeficiency virus type 1, making itself a potential candidate for the treatment of Acquired Immune Deficiency Syndrome(Hayashi et al., 1996). Exopolysaccharides (EPSs)which can be easily collected from liquid cultures also have drawn extensive attention in recent years.Oral administration of EPS released byAphanothecehalophyticasignificantly inhibited influenza virus hemagglutinin type 1 and neuraminidase type 1-induced pneumonia in mice (Zheng et al., 2006).EPS fromNostoccommuneshowed marked hydroxyl radical and superoxide anion scavenging activity and moisture absorption and retention capacity, which may promote its commercial application in skin-care products (Morone et al., 2019).
Apart from polysaccharides, other cyanobacteriaderived molecules have applied to many fields. For example, phycobiliproteins, the light-harvesting soluble proteins with associated pigments, are well known for anti-oxidation, anti-tumor, and antidiabetes; phycocyanin has been approved by the Food and Drug Administration as a natural dye and commercialized as a raw food material in Europe(Pagels et al., 2019). Besides that, bioactive carotenoids and polyunsaturated fatty acids have been widely utilized in pharmaceutical, nutraceutical, and cosmeceutical industries (Meléndez-Martínez, 2019).
Therefore, the mass culture of economical algal species is of great significance. Nowadays, only several species such asSpirulinaplatensis,Chlorellavulgaris, andDunalielasalinahave been successfully cultivated on a large scale (Schipper et al., 2019),largely due to their extraordinary adaptation to outdoor open pond systems. The open pond system is exceedingly common because it is cost-eff ective,energy-effi cient, and environmentally-friendly(Grossmann et al., 2019). However, considering the almost inevitable biological contamination in open ponds, the isolation of promising algal species with good adaptation to environmental stresses such as high alkalinity is important. For example, high concentrations of NaHCO3were used in open pond cultures ofSpirulinato inhibit the contamination with other algae, and thus maintained an outdoor algal monoculture (Volkmann et al., 2008).
Alternative cyanobacterial species aside fromSpirulinathat have the potential to produce valueadded molecules and tolerate diff erent environmental stresses also need investigation, such asCyanobacteriumaponinum, which was isolated from diff erent thermal springs (Moro et al., 2007; Meng et al., 2018; Strunecký et al., 2019). Gris et al. (2017)reported thatC.aponinumwas able to accumulate released polysaccharides, phycocyanin, β-carotene,and zeaxanthin. The crude lipid content and C16 and C18 methyl ester yield ofC.aponinumwere also notably high compared with other previously reported cyanobacterial strains (Karatay and Dönmez, 2011).Also, the immunomodulatory calciferous exopolysaccharide (EPS-Ca) released fromC.aponinumwas beneficial for the psoriasis patients bathing in the Blue Lagoon (Gudmundsdottir et al.,2015, 2019). In virtue of skin conditioning functions,C.aponinumFerment was put on the list of International Nomenclature of Cosmetic Ingredients(Mourelle et al., 2017). Additionally,C.aponinumhad a broad range of tolerance to environmental stresses such as high temperature, high concentrations of carbon dioxide (CO2), and low pH (Meng et al.,2018). Despite all this, at present, the cultivation ofC.aponinumstill limits to a laboratory scale, except one pilot-scale culture in open ponds with detectable contaminations with green algae and diatoms(Winckelmann et al., 2016).
2 MATERIAL AND METHOD
2.1 Isolation of Cyanobacterium sp. SCSIO-45682
The cyanobacterial strain was isolated by streak plate method from water samples collected from the open pond of a marine oleaginous green alga(Picochlorumsp. SCSIO-45015) (109°19′38″E,18°18′31″N, Hainan, China) and was numbered as SCSIO-45682. The medium for the isolation of the algal strain was f/2 medium (with a salinity of 25)composed of: NaHCO3(0.50 g/L); NaNO3(0.40 g/L);NaH2PO4·2H2O (20 mg/L); Na2EDTA·2H2O(4.4 mg/L); FeCl3·6H2O (3.2 mg/L); MnCl2·4H2O(0.18 mg/L); ZnSO4·7H2O (22 μg/L); CoCl2·6H2O(10 μg/L); CuSO4·5H2O (9.8 μg/L); Na2MoO4·2H2O(6.3 μg/L). The water samples were streaked on the solid medium containing 1.5% agar and cultured at 25±1 ℃ and illuminated with fluorescent lamps at 60±5 μmol photons/(m2·s) on a 24 h:0 h (light:dark)photoperiod (Philips T8, Koninklijke Philips Electronics N.V., China). After cultivation for two
weeks, cyanobacterial colonies growing on the plates were partly picked out, and were observed under a light microscope (BX53, Olympus Co., Ltd., Japan,magnification up to 1 000×). Monoclonal colonies of cyanobacterial cells characterized by morphology were obtained after plate streaking for three times.Monoclonal colonies were picked and transferred to a 250-mL Erlenmeyer flask containing 150-mL f/2 medium. The culture was maintained under the same temperature and light conditions as mentioned above.
2.2 Identification of Cyanobacterium sp. SCSIO-45682
The light microscope (LM) observation was made with an optical microscope (BX53, Olympus Co.,Ltd., Japan) equipped with digital image acquisition(Leica Application Suite X, Leica Camera AG,Germany). Genomic DNA was extracted using a DNA kit (E.Z.N.A.TM HP Plant DNA Kit, OMEGA Bio-Tech Co., Ltd., Georgia). The 16S rRNA gene was amplified by polymerase chain reaction (PCR,2720, Thermo Fisher Scientific, China) using primers LZF (5′-AGAGTTTGATCCTGGCTCAG-3′) and LZR (5′-AAGGAGGTGATCCAGCCGCA-3′). The PCR reaction used in the following conditions: predenaturation at 94 ℃ for 6 min, followed by 36 cycles of denaturation at 94 ℃ for 45 s, annealing at 50 ℃for 45 s, and extension at 72 ℃ for 90 s with a final extension at 72 ℃ for 10 min. The internal transcribed spacer (ITS) rRNA gene was amplified using the primers 322F (5′-TGTACACACCGCCCGTC-3′)and 340R (5′-CTCTGTGTGCCTAGGTATCC-3′).The PCR conditions were pre-denaturation at 94 ℃for 3 min, followed by 10 cycles of denaturation at 94 ℃ for 45 s, annealing at 53 ℃ for 40 s, extension at 68 ℃ for 75 s, and 25 cycles of denaturation at 90 ℃ for 45 s, annealing at 53 ℃ for 40 s, extension at 68 ℃ for 75 s with a final extension at 68 ℃ for 7 min. Both the PCR products were purified and sequenced by Guangzhou Tianyi Huiyuan Gene Technology Co., Ltd., China. The identities of the sequences were checked using the BLAST program at the NCBI web server. Phylogenetic trees were built with MEGA 6.0 Software.
2.3 NaHCO 3 treatment experiment
The inoculum ofCyanobacteriumsp. SCSIO-45682 was grown in a 2 000-mL Erlenmeyer flask containing 1 200-mL f/2 medium under 150±5 μmol photons/(m2·s) with a 24 h:0 h (light:dark) photoperiod(Philips T8, Koninklijke Philips Electronics N.V.,China) at 25±1 ℃. For the NaHCO3treatment experiment, f/2 medium supplemented with the initial NaHCO3concentrations of 0, 1.0, 2.1, 4.2, 8.4, and 16.8 g/L, respectively, were prepared. The bicarbonate alkalinity of each medium was examined by Nordmann titration (Rieger and Weiland, 2006) and reached 111.00, 804.75, 1 387.50, 2 608.50, 4 939.49,and 10 489.48 mg/L (ormalized as CaCO3),respectively. The inoculum during exponential phase was centrifuged (3 000 r/min for 10 min). The pellets were gently washed with fresh NaHCO3-free f/2 medium and then transferred into f/2 medium with the diff erent initial NaHCO3concentrations for 14 days cultivation. The starting optical density at 750 nm(OD750) was 0.10 (the initial inoculum concentration was 0.030 g/L). The NaHCO3treatments were maintained at the same temperature and light conditions as that of the inoculum. The growth was measured on Days 0, 2, 4, 6, 8, 10, 12, and 14. The pH was concurrently determined by a pH meter (FE20,Mettler-Toledo Instruments Co., Ltd., China). After 14 days of cultivation, the cyanobacterial biomass was harvested by centrifugation (8 500 r/min for 10 min). The pellets were subsequently washed triple with deionized water, freeze-dried at -50 ℃ for 48 h(FD-1-50, Beijing Boyikang Laboratory Instrument Co., Ltd., China), and stored at -20 ℃ for biochemical composition determination. Each treatment had three biological replicates (n=3), and all the measurements were conducted in triplicate (n=3).
2.4 Growth measurement
2.4.1 Optical density at the wavelength of 750 nm
For each culture, optical density at the wavelength of 750 nm (OD750) was evaluated using a visible spectrophotometer (722S; Shanghai Shun Yu Heng Ping Scientific Instrument Co., Ltd., China).
2.4.2 Dry weight
A 5–100-mL (according to OD750) culture sample was filtered through pre-weighed 0.45-μm filters.After rinsing with deionized water for three times, the filters were dried at 80 ℃ until the weights were constant.
Specific growth rate (μ, /d) was calculated according to the following equation:

Biomass productivity (P, g/(L·d)) was calculated according to the following equation:

whereXiandX0are the dry weight (DW, g/L) at culture daystiandt0, respectively.
2.5 Biochemical composition determination
2.5.1 Intracellular total saccharide production
A 0.5-mol/L sulfuric acid solution was used to hydrolyze a 10-mg freeze-dried specimen at 80 ℃for 1 h. The process was repeated four times. The intracellular total saccharide content was measured by phenol-sulfuric acid method (Dubois et al., 1956),utilizing D-glucose as standard. The results were normalized on DW of the specimens.
2.5.2 Exopolysaccharide production
A 5–100-mL aliquot of the cultures was centrifuged at 8 500 r/min for 10 min. The supernatant was collected and dialyzed against deionized water for 72 h, cutting off polysaccharide with a molecular weight over 500 Da to avoid a possible disturbance of monosaccharide, oligosaccharide, and salts(MD77MM membrane, Viskase Co., Ltd., USA).EPS concentration was determined according to the phenol-sulfuric acid method (Dubois et al., 1956),using D-glucose as standard.
2.5.3 Crude protein production
The crude protein content of a 0.10-g specimen was measured using the Kieldahl method (Ma and Zuazaga, 1942), with a value of 6.25 as a conversion factor. The results were normalized on DW of the specimens.
2.5.4 Total lipid production
The total lipid content of an 80-mg specimen was measured using the method according to Khozin-Goldberg et al. (2005). The results were normalized on DW of the specimens.
2.5.5 Pigment and phycobiliprotein production
A 10-mg specimen was used to extract pigments by 100% acetone, stirring at 4 ℃ for 48 h (avoid light). Chlorophyllaand total carotenoids contents were measured by a spectrophotometer (TU-1810,Persee Instrument Co., Ltd., China) and calculated according to the equations proposed by Ehling-Schulz et al. (1997). Carotenoids profiles were determined by analyzing the same specimens using high performance lipid chromatography (HPLC,1525, Waters Corporation, USA), with a photodiodedetector (2996, Waters Corporation, USA). An aliquot of 20 μL of the extract was injected, and the mobile phase comprised 90% acetonitrile as solvent A and 100% ethyl acetate as solvent B. The retention time and absorption spectrum were used to identify single pigments. Phycobiliproteins were determined according to Bennett and Bogorad(1973). The results were normalized on DW of the specimens.
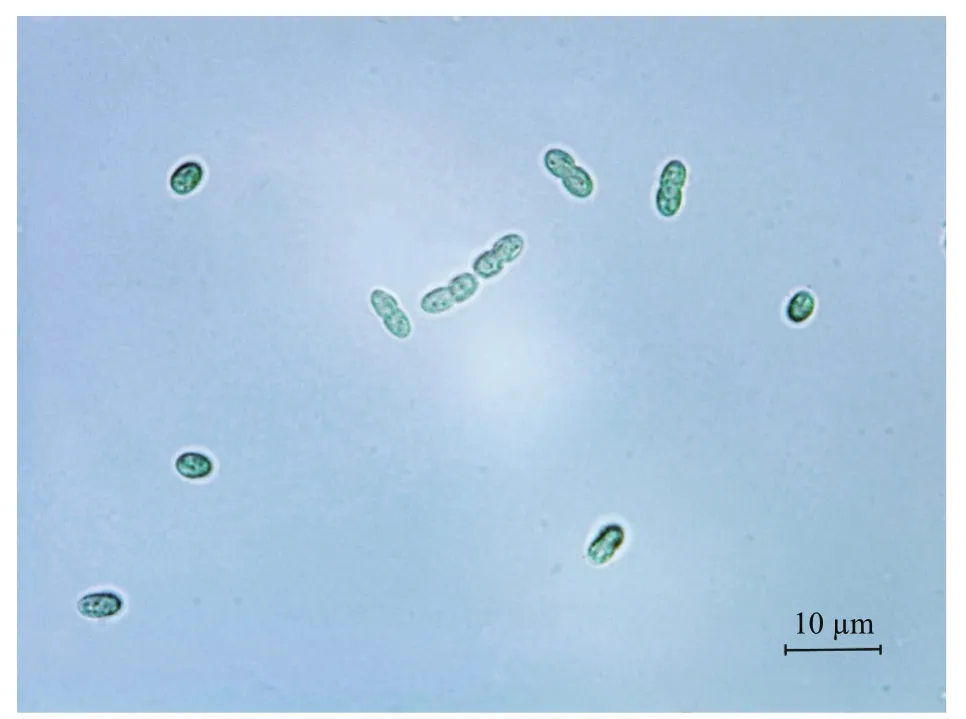
Fig.1 Optical microscopy graphs of Cyanobacterium sp.SCSIO-45682 grown in f/2 medium
2.6 Statistical analysis
The data were presented as mean±standard deviation of three independent biological replicates and three technical replicates. Diff erences between treatments were statistically analyzed by one-way analysis of variance (ANOVA) using SPSS 18.0(SPSS Inc., USA) (Pvalue less than 0.05 was considered to indicate significance). Graphing was performed using Origin Pro 8.5 software (OriginLab Corporation, USA).
3 RESULT
3.1 Identification of C yanobacterium sp. SCSIO-45682
The cells ofisolated strain numbered SCSIO-45682 were solitary or showed in pairs during binary fission under the optical microscope. They appeared bluegreen, oval, 2–3 μm in length, and 1–2 μm in diameter(Fig.1). The 16S rDNA sequence was 1 421 bp in length, and the ITS rDNA sequence was 479 bp long.BLAST analysis showed that SCSIO-45682 was closely related toC.aponinumPCC 10605 (with a high identity of 99.6% in 16S rDNA sequence and 98.7% in ITS rDNA sequence). The 16S rDNA phylogenetic tree revealed that SCSIO-45682 clustered withC.aponinumPCC 10605 (with a bootstrap value of 98%) (Fig.2). The ITS rDNA phylogenetic tree indicated that SCSIO-45682 clustered withC.aponinumPB1 (with a bootstrap value of 92%) (Fig.3). Thus, SCSIO-45682 was closely related toC.aponinumand was named as Cyanobacteriumsp. SCSIO-45682.
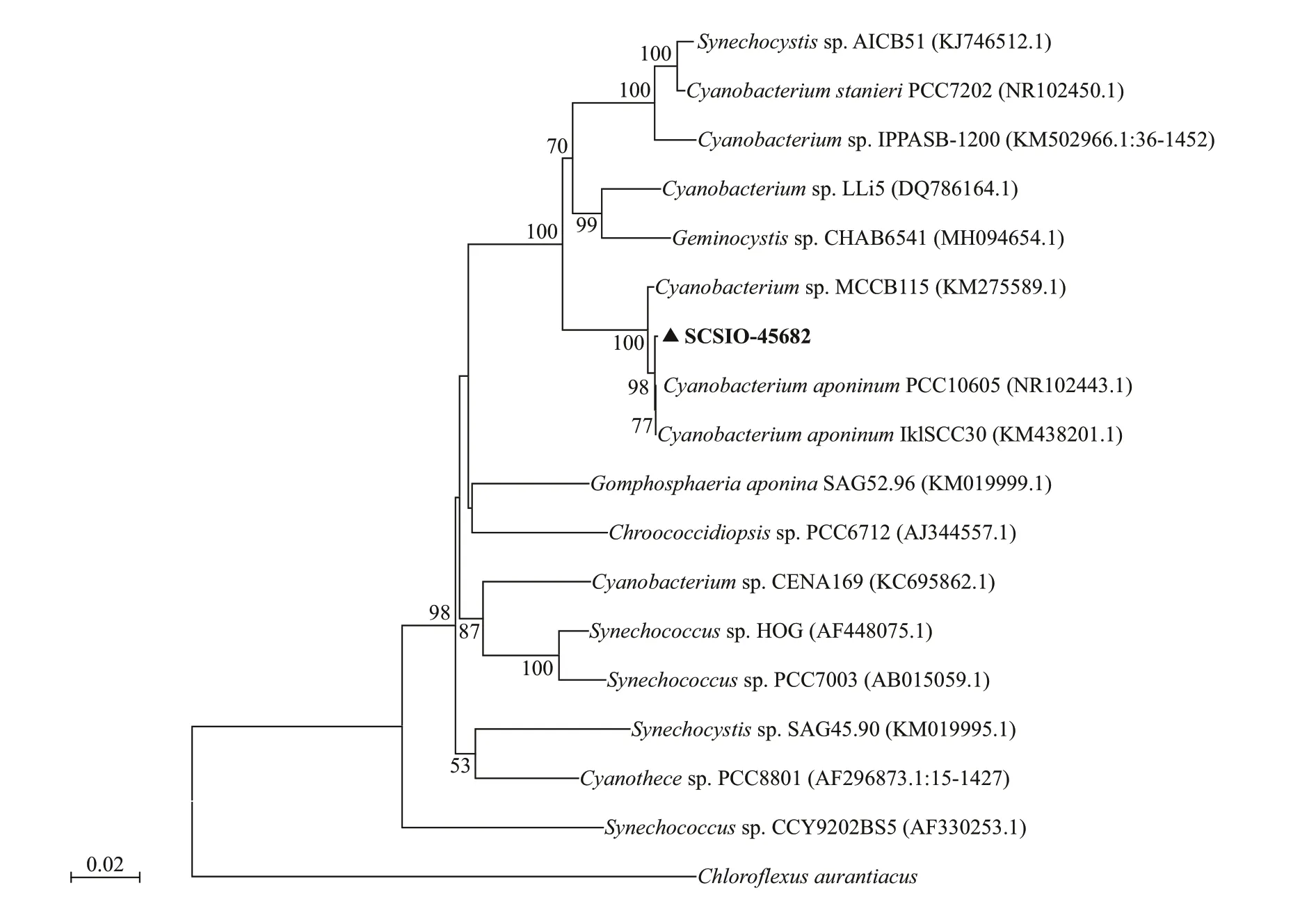
Fig.2 The Maximum-likelihood phylogenetic tree based on nearly complete 16S rDNA sequences of Cyanobacterium sp.SCSIO-45682
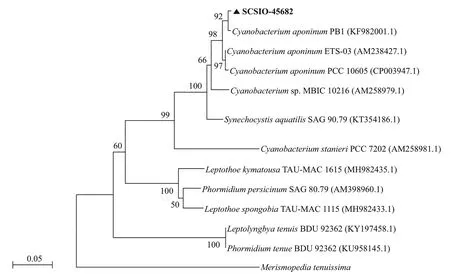
Fig.3 The Maximum-likelihood phylogenetic tree based on ITS rDNA sequences of Cyanobacterium sp. SCSIO-45682
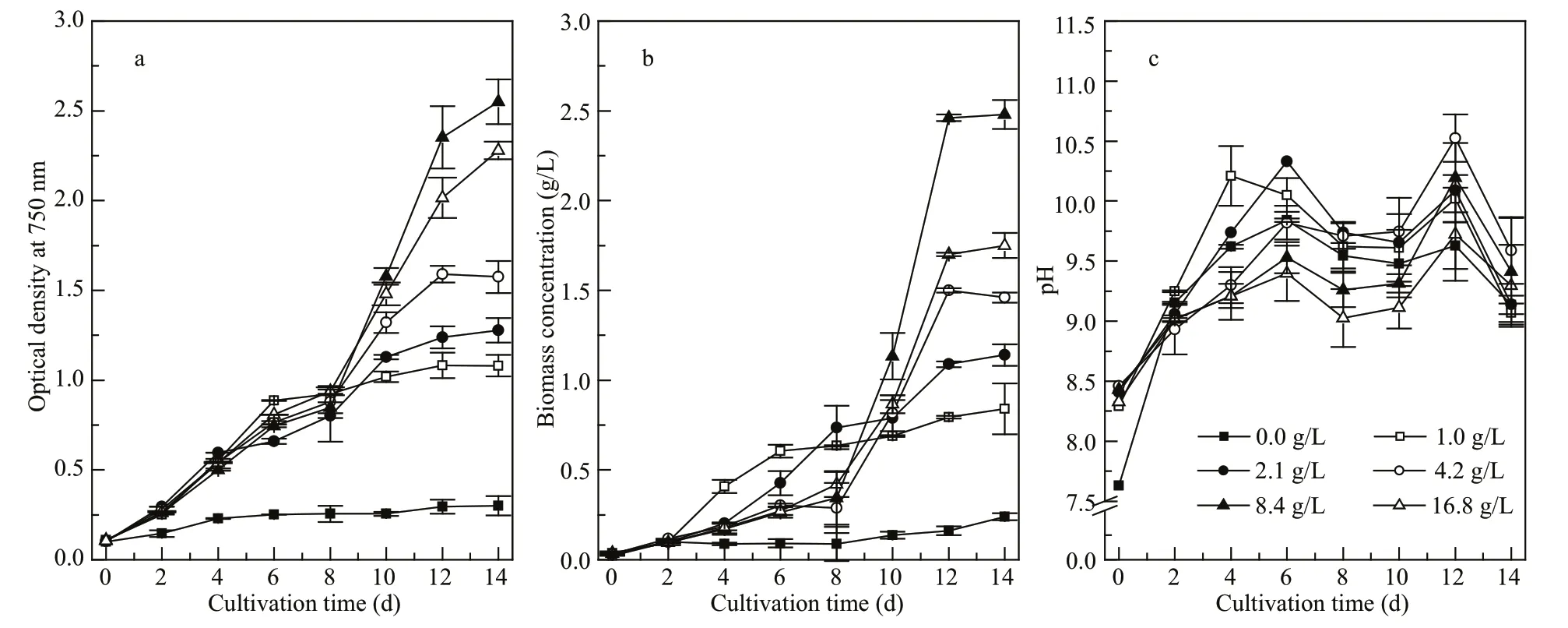
Fig.4 Growth of Cyanobacterium sp. SCSIO-45682 under diff erent initial NaHCO 3 concentrations
The values are presented as mean±standard deviation. a. optical density at 750 nm; b. biomass concentration; c. pH.
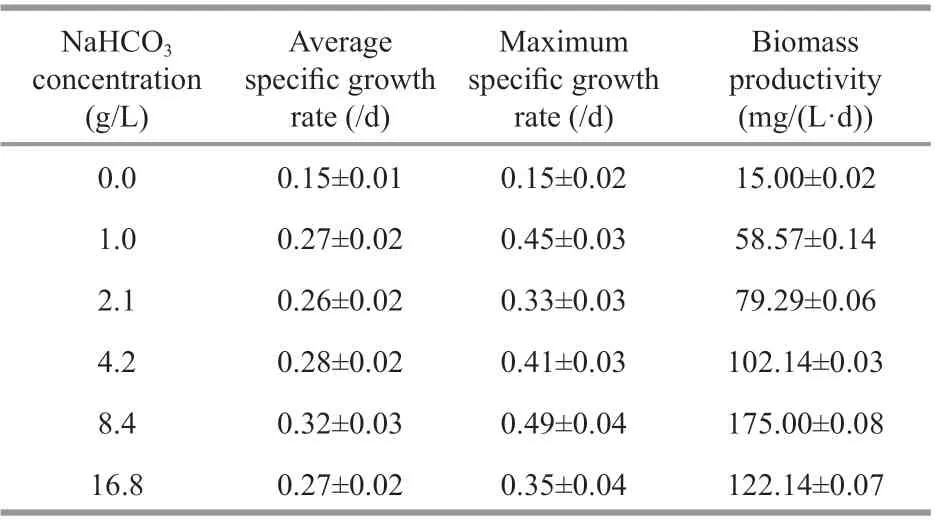
Table 1 Specific growth rate and biomass productivity of Cyanobacterium sp. SCSIO-45682 under diff erent initial NaHCO 3 concentrations
3.2 Eff ects of diff erent initial NaHCO 3 concentrations on the growth of Cyanobacterium sp. SCSIO-45682
Cyanobacteriumsp. SCSIO-45682 showed significant diff erences in growth under diff erent initial NaHCO3concentrations ranging from zero to 16.8 g/L. According to OD750(Fig.4a), the growth ofCyanobacteriumsp. SCSIO-45682 improved under high NaHCO3concentrations, and the group with no NaHCO3added revealed noticeably slow growth(OD750=0.3 on the final day of culture). After day 8,OD750of the 8.4-g/L NaHCO3group exceeded that of the 16.8-g/L NaHCO3group, and reached the highest OD750of 2.5 on Day 14.
Biomass concentrations ofCyanobacteriumsp.SCSIO-45682 under diff erent initial NaHCO3concentrations are shown in Fig.4b. No remarkable diff erences were observed between any of the NaHCO3concentrations in the first two days. The biomass concentrations of cultures with 4.2–16.8-g/L NaHCO3greatly increased from Day 8 to Day 12. On the last day of cultivation, the biomass concentration of the 8.4-g/L NaHCO3group reached 2.5 g/L, 41.7%higher than that of the 16.8-g/L NaHCO3group(P<0.05). Similarly, the highest average specific growth rate, maximum specific growth rate, and biomass productivity were obtained by the 8.4-g/L NaHCO3group, which reached 0.32/d, 0.49/d, and 0.18 g/(L·d) respectively (Table 1).
The pH variations of the culture medium were showed in Fig.4c. The highest pH value (10.5)among all groups was obtained by the 4.2-g/L NaHCO3group on Day 12. The 8.4-g/L and 16.8-g/L groups reached a pH value of 10.2 and 9.7,respectively, on Day 12, and reached 9.4 and 9.3,respectively, on Day 14.
3.3 Eff ects of diff erent initial NaHCO 3 concentrations on intracellular total saccharide production of Cyanobacterium sp. SCSIO-45682
The intracellular total saccharide content ofCyanobacteriumsp. SCSIO-45682 significantly increased with the increase ofinitial NaHCO3concentrations from zero to 16.8 g/L (Fig.5a). The maximum intracellular total saccharide content reached 49.2% DW in the 16.8-g/L NaHCO3group.However, taking the biomass concentration into account, the maximum intracellular total saccharide productivity was detected in the 8.4-g/L NaHCO3group, which was up to 79 mg/(L·d).
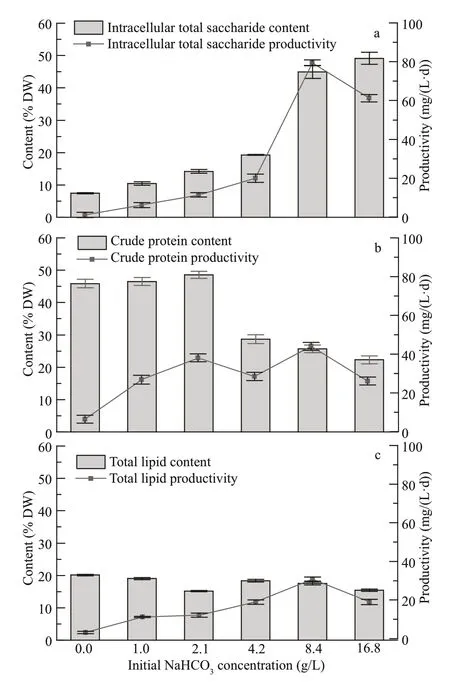
Fig.5 Biochemical composition of Cyanobacterium sp.SCSIO-45682 under diff erent initial NaHCO 3 concentrations
3.4 Influences of diff erent initial NaHCO 3 concentrations on crude protein and total lipid production of Cyanobacterium sp. SCSIO-45682
The crude protein content ofCyanobacteriumsp.SCSIO-45682 markedly declined under initial NaHCO3concentrations from 2.1 g/L to 16.8 g/L(Fig.5b). The crude protein content of the 16.8-g/L NaHCO3group decreased to 22.3% DW, which was only 45.9% of that of the 2.1-g/L NaHCO3group(achieved the highest content compared with other groups) (P<0.05). Whereas, due to the high biomass yield, the 8.4-g/L NaHCO3group exhibited the highest crude protein productivity of 44 mg/(L·d).
The total lipid content remained relatively stable ranging between 15.2% DW and 20.1% DW under diff erent initial NaHCO3concentrations (Fig.5c).The 8.4-g/L NaHCO3group showed the maximum total lipids productivity of 31 mg/(L·d) among all cultures.
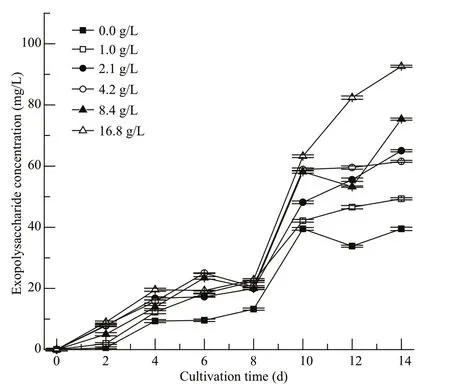
Fig.6 Variation of exopolysaccharide concentration of Cyanobacterium sp. SCSIO-45682 under diff erent initial NaHCO 3 concentrations
3.5 Accumulation of EPS of Cyanobacterium sp.SCSIO-45682 under diff erent initial NaHCO 3 concentrations
As shown in Fig.6, the EPS concentration greatly ascended with increment ofinitial NaHCO3concentrations. No significant diff erences in EPS concentrations among diff erent treatments were observed from Day 0 to Day 4 between the treatments.The 4.2 and 8.4-g/L NaHCO3groups showed higher EPS concentrations than others’ from Day 4 and Day 8. Notably, the EPS accumulation in all groups increased rapidly from Day 8 to Day 10. At the end of cultivation, the 16.8-g/L NaHCO3group showed the highest EPS concentration of 93 mg/L, 22.9% higher than that of the 8.4-g/L NaHCO3group (P<0.05).
3.6 Pigment and phycocyanin production of Cyanobacterium sp. SCSIO-45682 under diff erent initial NaHCO 3 concentrations

Fig.7 Pigment composition by HPLC of Cyanobacterium sp. SCSIO-45682 under diff erent initial NaHCO 3 concentrations
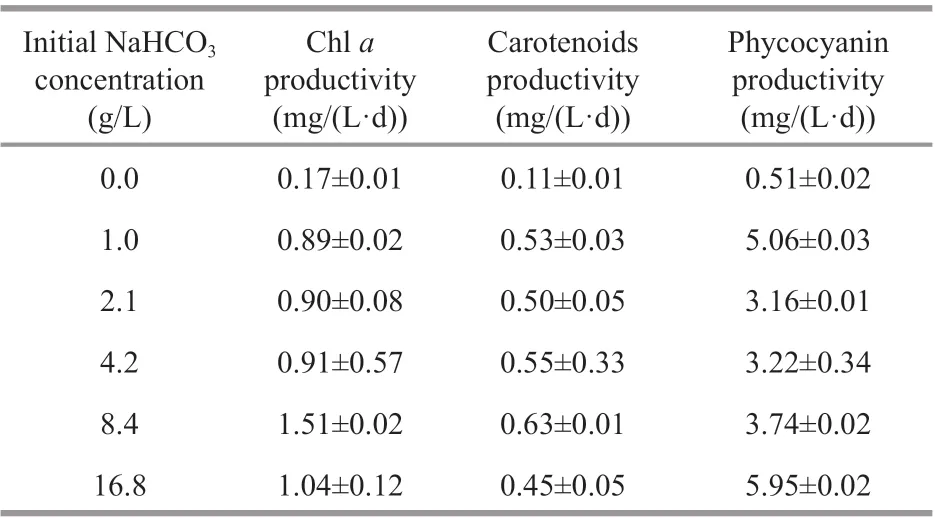
Table 2 Pigments and phycocyanin productivities of Cyanobacterium sp. SCSIO-45682 under diff erent initial NaHCO 3 concentrations
As shown in Table 2, the chl-aproductivity variation was consistent with that of the biomass productivity under diff erent initial NaHCO3concentrations. The highest chl-aproductivity and total carotenoids productivity were observed in the 8.4-g/L NaHCO3group, up to 1.51 mg/(L·d) and 0.63 mg/(L·d), respectively. The pigments profile by HPLC (Fig.7) suggested thatCyanobacteriumsp.SCSIO-45682 possessed five major pigments which were identified as myxoxanthophyll, zeaxanthin,chla, chlaisomer, and β-carotene, respectively.According to peak area of the zeaxanthin under diff erent initial NaHCO3concentrations, there was a declining trend of zeaxanthin with the increase of NaHCO3concentrations. The highest phycocyanin productivity was found in the 16.8-g/L NaHCO3group, reaching 6.0 mg/(L·d) (Table 2).
4 DISCUSSION
In terms ofindustrialization, the screening of algal strains that can adapt to extreme environments, grow rapidly, and accumulate high-value metabolites shall be the first step towards downstream biotechnological production. In this work, one novel cyanobacterial strain was isolated and identified asCyanobacteriumsp. SCSIO-45682. The growth adaptability and biochemical composition of this strain under diff erent initial NaHCO3concentrations were investigated.
In this work, NaHCO3rather than CO2was utilized as dissolved inorganic carbon source, considering the comparatively lower solubility and the high cost of carbon capture, compression, and transportation of CO2; the use of high alkalinity can also improve the transfer of CO2into the culture medium (Chi et al.,2014). According to previous studies,C.aponinumPCC 10605 could effi ciently use both NaHCO3and CO2as carbon source (Gris et al., 2017), andC.aponinumOUC1 may preferentially take up bicarbonate because of a sophisticated CO2concentrating mechanism(Badger and Price, 2003; Meng et al., 2018).
The results showed thatCyanobacteriumsp.SCSIO-45682 had good adaptation to high concentrations of NaHCO3(up to 16.8 g/L, the same NaHCO3concentration in the Zarrouk formula)(Zarrouk, 1966) (Fig.4a & b), which may provide a theoretical possibility for the monoculture ofCyanobacteriumsp. SCSIO-45682 in outdoor open ponds. Vonshak et al. (1983) reported that a monoculture ofSpirulinacould be sustained both in the laboratory and outdoors under at least 16.8-g/L NaHCO3. Furthermore, high concentrations of NaHCO3improved the growth ofCyanobacteriumsp.SCSIO-45682 (Fig.4b), which is highly significant because the accumulation of biomass is the primary index to evaluate the potential of capable algal strains.It was noteworthy that biomass yield ofCyanobacteriumsp. SCSIO-45682 under high NaHCO3concentrations was much higher than that of previously reported cyanobacterial strains, perhaps due to the good adaptation to low irradiance ofCyanobacteriumsp. SCSIO-45682 observed in our laboratory (data not shown), and similar adaptation was observed inAphanizomenon(Bradburn et al.,2012).Cyanobacteriumsp. SCSIO-45682 reached a final biomass concentration of 2.5 g/L at 0.1-mol/L(8.4 g/L) NaHCO3. Gris et al. (2017) reported thatC.aponinumPCC 10605 reached a final biomass of 0.513 g/L using NaHCO3as carbon source under 150 μmol photons/(m2·s), the same light intensity as that ofCyanobacteriumsp. SCSIO-45682. Chi et al.(2013) reported that an extremely alkalihalophilic cyanobacteriumEuhalothecesp. reached a final biomass concentration of 4.79 g/L in medium with 1-mol/L (84.0 g/L) NaHCO3. Zhu et al. (2018)reported thatSpirulinareached a biomass concentration of 1.55 g/L at 0.3-mol/L (25.2 g/L)NaHCO3cultivated in Erlenmeyer flasks, and obtained a biomass concentration of 2.24 g/L using a 1.0-m2floating horizontal photobioreactor with NaHCO3as carbon source. In addition, the medium ofCyanobacteriumsp. SCSIO-45682 reached the highest pH value of 10.5 at 4.2-g/L NaHCO3rather than at higher NaHCO3concentrations (Fig.4c),because suffi cient bicarbonate/carbonate can control high pH by acting as a strong buff er (Chi et al., 2013),which may alleviate the negative eff ects of extremely high pH on the growth ofCyanobacteriumsp.SCSIO-45682, and similar results were also found in the cultivation ofSpirulina,Synechocystissp. andCyanothecesp. in high NaHCO3concentrations (Chi et al., 2014; Zhu et al., 2018).
The findings also indicated that high concentrations of NaHCO3promoted the accumulation of polysaccharide ofCyanobacteriumsp. SCSIO-45682.Cyanobacteria can absorb carbon and convert it into macromolecules such as polysaccharide, protein, and lipid, and the regulation of cultivation parameters is a feasible method to manipulate carbon allocation and to produce desirable metabolites (González-Fernández and Ballesteros, 2012). In the present work, the intracellular total saccharide content increased with the increment of NaHCO3concentration, and accounted for 49.2% DW in the 16.8-g/L NaHCO3group (Fig.5a), reaching a considerably high carbohydrate content in comparison to cyanobacterial strains ofSpirulinamaxima (13%–16% DW),Synechoccussp. (15% DW), andAnabaenacylindrical(25%–30% DW) (Becker, 2004).Furthermore, the results showed that the crude protein content in the 16.8-g/L NaHCO3group concomitantly declined by 51.4% (P<0.05) compared with the group with no NaHCO3added (Fig.5b), which might explain the corresponding raise in the intracellular total saccharide content ofCyanobacteriumsp.SCSIO-45682. This was in accordance with the findings inPorphyridiumpurpureum, whose carbohydrate content significantly increased with the decline of protein content with the consumption of NaNO3(Li et al., 2019). For total lipid content,Cyanobacteriumsp. SCSIO-45682 grown under diff erent initial NaHCO3concentrations remained relatively stable (15.2%–20.1% DW) (Fig.5c).Karatay and Dönmez (2011) reported that crude lipids extracted fromC.aponinum(45.0% DW) could act as a novel source for biodiesel production.
Additionally, high concentrations of NaHCO3improved the EPS secretion ofCyanobacteriumsp.SCSIO-45682. The protective response of cyanobacteria with an extracellular polymeric gel layer of EPS may inhibit the diff usion ofions through the cell surface (Kumar et al., 2007), which may explain the enhancement of EPS yield and viscosity of medium with the increase of NaHCO3concentration.It was noteworthy that EPS-Ca secreted byC.aponinumcan up-regulate the interleukin-10(Gudmundsdottir et al., 2015) as well as inhibit the expression of spleen tyrosine kinase and the encoding gene for the Dectin-1 receptor, which showed remarkable immunomodulatory activities in vitro(Gudmundsdottir et al., 2019). In the present study,the EPS yield ofCyanobacteriumsp. SCSIO-45682 ascended during cultivation. By the final day of culture, the 16.8-g/L group obtained the maximum EPS yield of 93 mg/L (Fig.6). The EPS productivity of the 16.8-g/L NaHCO3group was 6.6 mg/(L·d)(Fig.6), higher than that ofSynechocystissp. PCC 6803,Anabaenasp. C5, andNostocsp. 2S9B (Su et al., 2007). Salinity and nutrient composition of the medium forCyanobacteriumsp. SCSIO-45682 may play a significant role in the synthesis of EPS, as increasing salinity can aff ect the EPS secretion of cyanobacterial strains ofAnabaenasp.,Aphanocapsahalophyta, andCyanothece(Sudo et al., 1995;Nicolaus et al., 1999; Su et al., 2007).
Another thing should be mentioned is that high concentrations of NaHCO3induced the production of chlaand phycocyanin ofCyanobacteriumsp.SCSIO-45682. Photosynthetic pigments are major members oflight-harvesting compounds responsible for capturing light energy in cyanobacteria (Liu and Blankenship, 2019). Specifically, chlorophylls,carotenoids, and phycobiliproteins play a crucial part in the structure and photo-protection of the photosynthetic apparatus of cyanobacteria. These key pigments might help capture and assimilate light energy and promote the photosynthesis activity,which contributes to the boost of biomass production(Viola et al., 2019). This may elucidate the result that the chl-aproductivity agreed with the variation of biomass productivity (Tables 1 & 2). However, there was a declining trend of zeaxanthin with the increase ofinitial NaHCO3concentrations. This is probably due to the photoprotection of zeaxanthin. The stress conditions (e.g. high light intensity and carbon limitation) could obviously enhance photoinhibition in cyanobacteria (Ibelings and Maberly, 1998). The pigment zeaxanthin could protect PSII by decreasing the level of singlet oxygen (Kusama et al., 2015). In the present study, the accumulation of zeaxanthin inCyanobacteriumsp. SCSIO-45682 was found in treatments grown in low NaHCO3concentrations.The growth was inhibited by limited inorganic carbon,and average light absorption by every cell could increase due to the lower cell density, thus zeaxanthin accumulated to protect against photoinhibition.Similar results were observed inSynechococcussp.andCyanobacteriumaponinumPCC 10605(Masamoto and Furukawa, 1997; Gris et al., 2017).Therefore,Cyanobacteriumsp. SCSIO-45682 can be considered as a promising cyanobacterial strain cultivated under high concentrations of NaHCO3to obtain polysaccharide. A further attempt at a pilotscale outdoor culture with 8.4-g/L NaHCO3was thus proposed in this work, aiming to prevent biological contamination and harvest large quantities of biomass with high-value molecules.
5 CONCLUSION
Alkalinity plays a significant role in the growth and biochemical composition ofCyanobacteriumsp.SCSIO-45682 by preventing contamination and acting as an inorganic carbon source. The present work showed thatCyanobacteriumsp. SCSIO-45682 had good adaptation to the NaHCO3concentration of 16.8 g/L, which was comparable toSpirulina.Furthermore, high concentrations of NaHCO3enhanced the biomass, intracellular total saccharide,EPS, chla, and phycocyanin yields ofCyanobacteriumsp. SCSIO-45682. Hence, it may be practicable to cultivateCyanobacteriumsp. SCSIO-45682 in alkaline systems to obtain polysaccharide and to further explore its potential of commercial applications.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of the current study are available from the corresponding author on reasonable request.
7 CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
