Taxonomic reassessment of Polysiphonia hainanensis(Rhodomelaceae, Rhodophyta) based on molecular and morphological analyses*
2021-06-15HuaqiangTANWenhuaLIUPingLI
Huaqiang TAN , Wenhua LIU , Ping LI ,
1 Guangdong Provincial Key Laboratory of Marine Biotechnology, Institute of Marine Sciences, Shantou University, Shantou 515063, China
2 Southern Marine Science and Engineering Guangdong Laboratory(Guangzhou), Guangzhou 511458, China
Abstract Polysiphonia hainanensis was previously described from Hainan, China, but DNA sequence had never been provided and its phylogenetic position in Polysiphonieae has not been clear so far. The classification of Polysiphonieae has changed recently based on the molecular analyses and detail morphological anatomy,consequently, the generic assignment of some species must be reassessed. In this study, we analyzed phylogenetic relationships of P. hainanensis and the related taxa in Polysiphonieae and Streblocladieae based on rbc L sequences, with specimens collected from the type locality and the area around. Two genetic types were detected, and formed a clade with Bryocladia thyrsigera with strong bootstrap support,in which the divergence was less than 0.81%. Furthermore, based on morphological analyses, we found characteristics of Chinese specimens, number of pericentral cells, percurrent axis, determinate branchlets,branching pattern, spermatangial axes development, and arrangement of tetrasporangia, etc., were consistent with those of Bryocladia. Combined with morphological and molecular analyses, P. hainanensis was taken a synonymous of B. thyrsigera, and gametophytes of this species were found from China for the first time.
Keyword: Bryocladia; phylogeny; Polysiphonieae; rbc L
1 INTRODUCTION
PolysiphoniaGreville is the largest genus of red algae, in which 705 species names are present and about 193 are currently recognized species (Guiry,2020). These species are common and widely distributed on virtually all coasts of the world(Womersley, 1979). The members in this genus have a wide range of morphological variability that has led to much debate as to how species should be defined and classified, and the generic circumscription has been in an almost constant state of flux since its original description (Mamoozadeh and Freshwater,2012; Díaz-Tapia et al., 2017a; Huisman et al., 2017).
Recently, based on integrative molecular and morphological studies, the tribal and generic classification of Polysiphonieae was reviewed, and Polysiphonieae had been segmented into Polysiphonieae and Streblocladieae (Díaz-Tapia et al., 2017a). As the tribal classification had been changed, the generic circumscription ofPolysiphoniaalso had been improved, for example, thePolysiphoniaspecies with multinucleate trichoblast cells have been combined intoVertebrataand thePolysiphoniaspecies with plastids lying exclusively on radial walls of pericentral cells have been combined intoMelanothamnus(Díaz-Tapia et al., 2017b).
In China, 3 genera and 34 species of Polysiphonieae and Streblocladieae were described based on morphological characteristics (Xiang, 2004; Xia,2011; Xia and Wang, 2012); nevertheless, molecular analyses have never been performed, and their phylogenetic relationships remain unknown.Considering the recent taxonomic rearrangements in the Polysiphonieae (Díaz-Tapia et al., 2017a, b), the tribal and generic assignments require revision.
PolysiphoniahainanensisXia et Wang were previously reported from China based on morphological characteristics (Xia and Wang, 2012),but has not been listed in the AlgaeBase (Guiry, 2020).It is unusual in having rhizoids in open connection with pericentral cells and 10(-12) pericentral cells.The rhizoids character is typical of the tribe Polysiphonieae and is unknown in Streblocladieae,and most species with more than four pericentral cells are in the Streblocladieae (Díaz-Tapia et al., 2017a, b,2018). Therefore, it is uncertain on the relationship ofP.hainanensisand its related taxa.
During our sampling surveys in Guangdong and Hainan provinces, we found numerous specimens with characters that matched the main features ofP.hainanensis. This study aims to conduct a taxonomic reassessment onP.hainanensisand clearify the phylogenetic position among the related taxa based on molecular and morphological analyses. Furthermore,the morphological observation of sporophyte, male and female gametophytes was conducted.
2 MATERIAL AND METHOD
Specimens collected from Nan’ao Island, Shantou City (23°24.32′N, 117°3.42′E), Honghai Bay,Shanwei City (22°39.32′N, 115°34.21′E), Yinggehai,Ledong City (18°28.43′N, 108°46.50′E), Boao,Qionghai City (19°9.27′N, 110°35.11′E) (Fig.1).They were fixed in 5% formalin in seawater, and voucher specimens were deposited in the herbarium at the Laboratory of Marine Algae, Shantou University,China. Sections for microscopic examination were cut into slices and stained with 1% aceto carmine.Mounted slides were made with fresh or stained fragments. Photomicrographs were taken using a digital Olympus DP20 camera (Olympus, Tokyo,Japan) attached on the microscope Olympus BX53.
DNA was extracted from silica-gel samples using a DNeasy Plant Kit (TIANGEN Biotech, Beijing,China). Partial sequences of therbcL gene were amplified using one pair primers designed by ourselves:TanF72, 5′-AAAAATGGGATACTGGGATC-3′ and TanR1144, 5′-CCACAATGGATACCACCTGA-3′.Polymerase chain reaction (PCR) cycle was amplified:94 °C for 6 min, 30 cycles at 94 °C for 45 s, 52 °C for 1 min, 72 °C for 1 min, and a final extension at 72 °C for 10 min, with a Mycylcer thermal cycler (Bio-Rad,USA). Each 20 μL PCR solution consisted of 10-μL 2×Taq PCR Master Mix, 1-μL template DNA, 1 μL of each primer (10 mmol/L), and 7-μL ddH2O. The sequencing was performed by the BGI Biotech Co.Ltd. (Shenzhen, China). All sequences generated in the present study have been accessioned in GenBank(MN709123–MN709126).
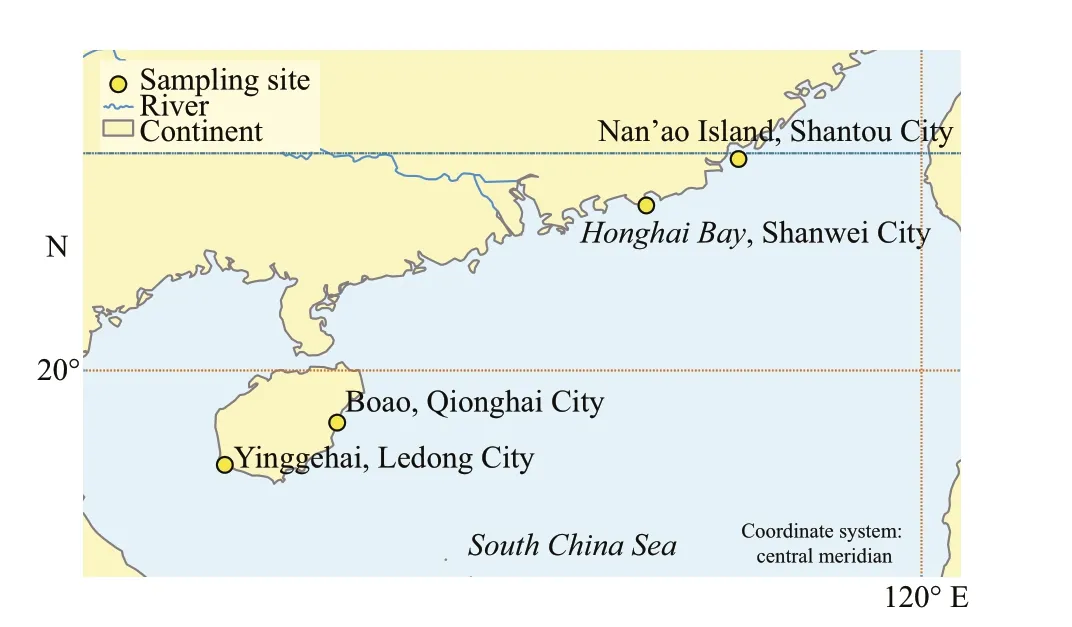
Fig.1 Collection map
FourrbcL sequences generated in the present study and others 45 sequences obtained from GenBank were aligned with ClustalX v2.1 (Larkin et al., 2007)and corrected manually using MEGA v5 (Tamura et al., 2011). Model parameters were estimated in Modeltest v3.7 (Posada and Crandall, 1998).GTR+I+G model was selected for sequences analyses used in the construction of both Bayesian Inference(BI) and maximum likelihood (ML) trees. Bayesian inference was performed MrBayes v3.2 (Ronquist et al., 2012). The MCMC runs were carried out for 3 million generations each with one cold chain and three heated chains, with sampling and printing every 100 generations. Summary trees were generated using a burnin value of 7500. The ML tree was constructed using PhyML 3.0 software (Guindon and Gascuel,2003) with 3 000 bootstrap replicates. Pairwise distance estimation used the Kimura two-parameter model. Two species ofPterosiphoniawere selected as the out-group based on our phylogenomic analyses of the major lineages of the Rhodomelaceae (Díaz-Tapia et al., 2018).
3 RESULT
3.1 Molecular analysis
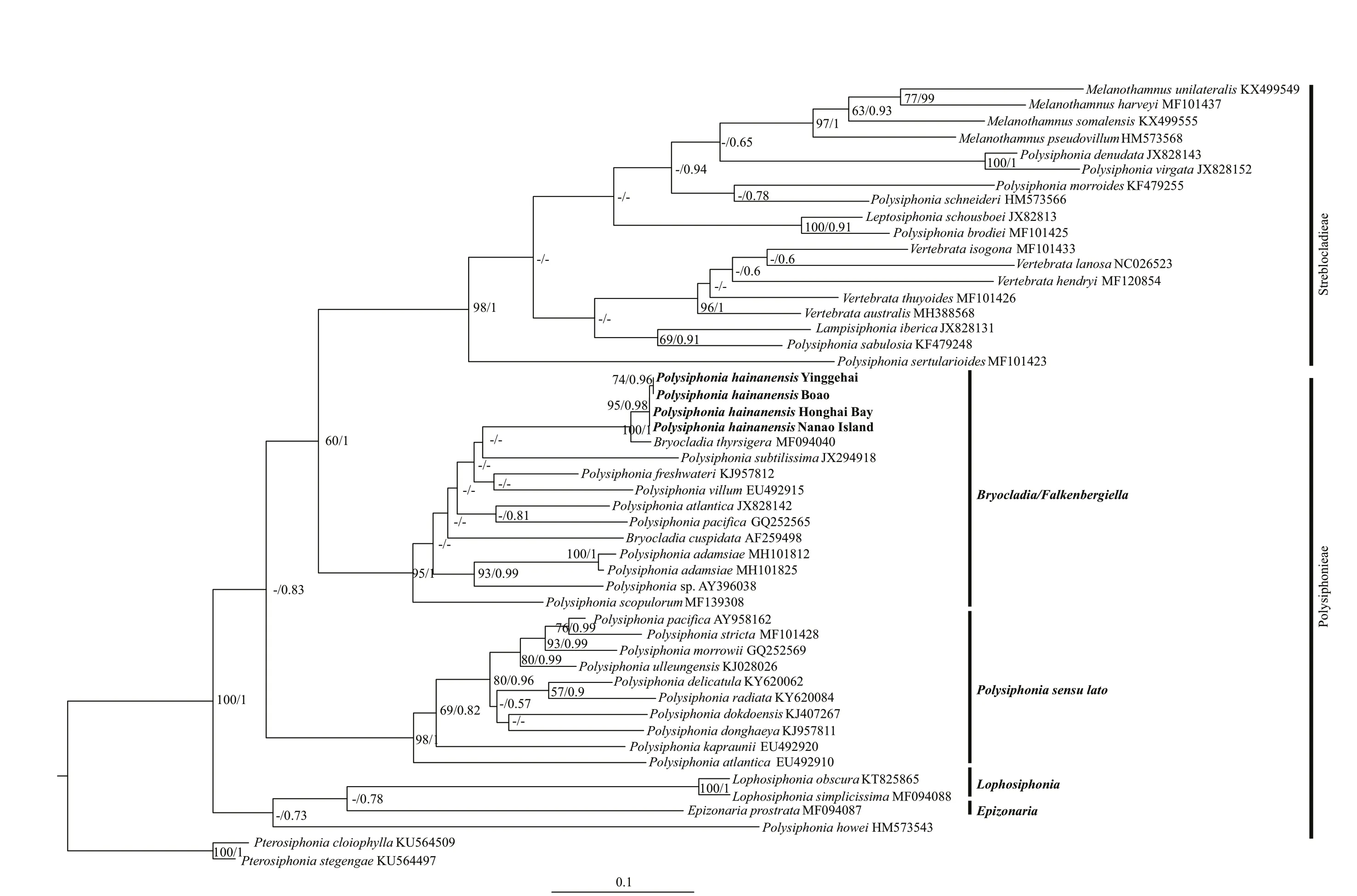
Fig.2 Phylogenetic tree based on rbc L sequences inferred from maximum-likelihood (ML) analysis using the GTR+I+G evolution model
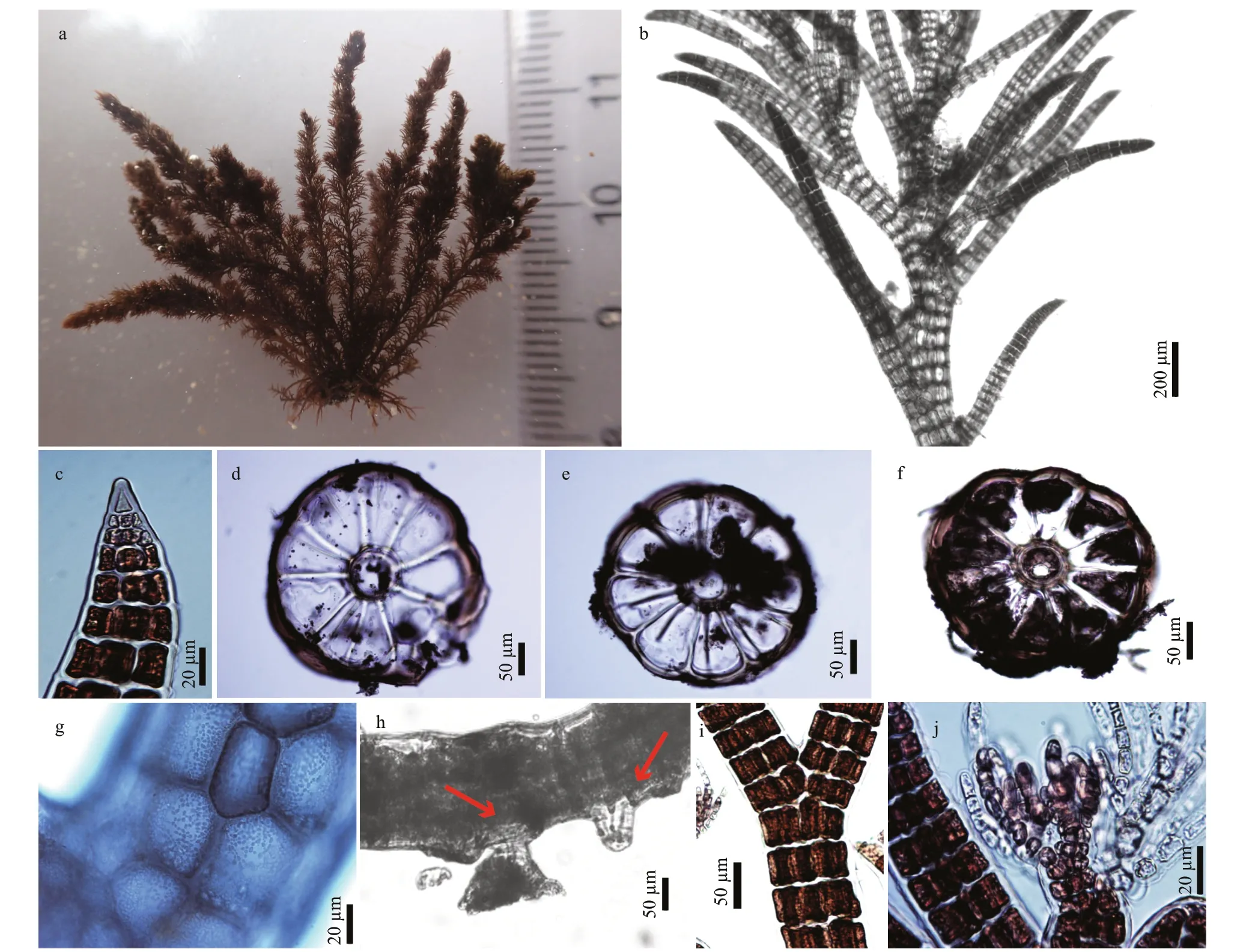
Fig.3 Vegetative structures of Bryocladia thyrsigera
FourrbcL sequences were generated, and one of them was from the specimen collected in the type locality. Our sequences were compared to those related ones from GenBank and the phylogenetic trees recoveredP.hainanensisas a member of the Polysiphonieae (Fig.2). Within the Polysiphonieae,three clades were resolved: 1)Bryocladia/Falkenbergiella; 2)Polysiphoniasensustricto; and 3)LophosiphoniaandEpizonaria.P.hainanensiswas nested inBryocladia/Falkenbergiellawith high bootstrap value. Sequences from the four specimens ofP.hainanensiswere essentially identical (0.07%divergence) and formed a stable clade withBryocladiathyrsigera(J. Agardh) F. Schmitz from Brazil(MF094040) with 100% bootstrap support. The genetic divergence betweenP.hainanensisandB.thyrsigerawas 0.74%–0.81%. On the other hand,Polysiphoniawas not monophyletic, andP.hainanensiswas distant from the generic type speciesPolysiphoniastricta(MF101428) with about 8% genetic divergence.
3.2 Species description
Bryocladiathyrsigera(J. Agardh) F. Schmitz in Falkenberg, 1901, p169.
PolysiphoniathyrsigeraJ. Agardh, 1847, p. 17.
PolysiphoniahainanensisXia et Wang, 2012,p. 963–965.
Type locality: La Guayra, South America.
Thalli were brownish red or brownish black,saxicolous, 2–7 cm high, and grew in tufts with an entangled prostrate system and erect axes (Fig.3a).Erect axes had a distinct main axes arising from prostrate axes (Fig.3a). Main axes were percurrent and borne lateral indeterminate branches in 1/4 spirally arrangement every 1–7 segments, and the axes oflateral indeterminate branches mostly borne determinate branches in 1/4 spirally arrangement every 0–4 segments (Fig.3b). Main axes and indeterminate branches grew via a dome-shaped apical cell that divided slightly obliquely, whereas determinate branches grew via a spinous apical cell that divided almost transversely (Fig.3c).

Fig.4 Reproductive structures of Bryocladia thyrsigera
Axes were ecorticate, consisting of a central axial cell and 8–10(-12) pericentral cells (Fig.3d–f).Plastids were discoid, lying all along the cell walls of pericentral cells (Fig.3g). Prostrate axes were 100–180 μm in diameter, and segments were wider than long, 0.2–0.3 L/D (Fig.3h). Unicellular rhizoids which were open connection with pericentral cells,were 15–40 μm in diameter, with or without a discoid haptera at the terminal part (Fig.3h). Erect axes grew from an apical cell 3–51 μm in diameter with segments 0.2–0.5 L/D, increasing in diameter to 280–350(-450) μm in mid and basal parts, with segments 0.2–0.3 L/D (Fig.3i). Trichoblasts were formed by uninucleate cells, 1–3 times dichotomously divided,and only found in mature female plants (Fig.3j).
Procarps were formed on a fertile trichoblast(Fig.4a). Cystocarps were globose, and 250–300 μm in diameter (Fig.4b). Carpospores are clavate, 60–100 μm long (Fig.4c). Spermatangial branches were cylindrical, colorless, 150–180 μm long, 30–50 μm in diameter, without a sterile tip, developed from the entire trichoblast primordium in clusters on short axis with 1/4 spirally arrangement (Fig.4d–f).Tetrasporangia were arranged in straight series and composed of one per fertile segment of determinate branches, and their stichidia were in clusters on short axis (Fig.4g & h). The mature ones are divided tetrahedrally, 40–50 μm in diameter, and surround by two presporangial cover cell (Fig.4h & i).
Habitat: Thalli grow in the intertidal zone attached on rocks.
Domestic distribution: Guangdong, Hainan.
Foreign distribution: America, Africa, and Western Atlantic (Guiry, 2020).
Specimens examined: STU20130405017 (I–XXXVI) were collected from Hainan on November 2013 by Tan Huaqiang; STU20140103015 (I–XII)were collected from Guangdong on April 2014 by Tan Huaqiang; STU20150402012 (I–VI) were collected from Guangdong on February 2015 by Tan Huaqiang.
4 DISCUSSION
TherbcL sequences from our specimens collected from four sides were essentially identical (0.07%divergence), and formed a clade withBryocladiathyrsigerafrom Brazil with strong bootstrap support(100%), among which the divergence was 0.74%–0.81%. The previous studies ofrbcL sequence data inPolysiphoniasensulatodetected comparatively low intraspecific, with values ranging 0–1.3% (McIvor et al., 2001; Kim et al., 2004; Kim and Yang, 2005).Therefore, it suggests that the Chinese and Brazilian specimens should be the same species. Because theBryocladia/Falkenbergiellaclade was distant from the type speciesP.strictaof the genusPolisiphoniasensulato, Huisman et al. (2017) had suggested that a separate genus might be appropriate for theBryocladia/Falkenbergiella. Since molecular information of the type species of this genus,B.cervicornis(Kützing) F. Schmitz, is unavailable,Bryocladiashould still be valid.
The genusBryocladiahave the following key morphological characteristics (Schmitz and Falkenberg, 1897; De Toni, 1903; Dawes and Mathieson, 2008; Suárez et al., 2015): 1) thallus comprised of prostrate branches, and erect branches,having a percurrent axis; 2) all branches ecorticate,densely clothed with short, determinate, spine-like,exogenous polysiphonous branchlets, spirally arranged, and with up to 12 pericentral cells; 3)tetrasporangia single in a segment, in a straight series, stichidia in clusters on short axis; and 4)spermatangial capitula replace trichoblasts, conoidal,with or without sterile tip, in clusters on short axis.By comparison, we found that morphological characteristics of Chinese specimens marched the genusBryocladia(Table 1). Furthermore,morphological comparison (Table 2) suggestedB.thyrsigera,P.hainanensisand newly collected specimens from China are conspecific.
The number of pericentral cells was commonly used to distinguish species ofPolysiphoniasensulato(Stuercke and Freshwater, 2008). Xia and Wang(2012) mentioned their new species was closely related toPolysiphoniahoweiandVertebratahendryivar.luxuriansbased mainly on the morphological character of more than ten pericentral cells. However,in our phylogenic tree, we foundB.thyrsigerawas placed in theBryocladia/Falkenbergiellaclade,P.howeiwas near to theEpizonariaclade, andV.hendryiwas placed in the family of Streblocladieae.Futher more, in this study, the other eight species(Bryocladiacuspidate,Lampisiphoniaiberica,Leptosiphoniaschousboei,Polysiphoniaadamsiae,and threeVertebrataspecies) ofPolysiphoniasensulato, all having more than four pericentral cells were also not closely related and scattered in Polysiphonieae and Streblocladieae. It suggests the number of pericentral cells may originate based on parallel evolution, and is not applied well to determine therelationship of species amongPolysiphoniasensulato.
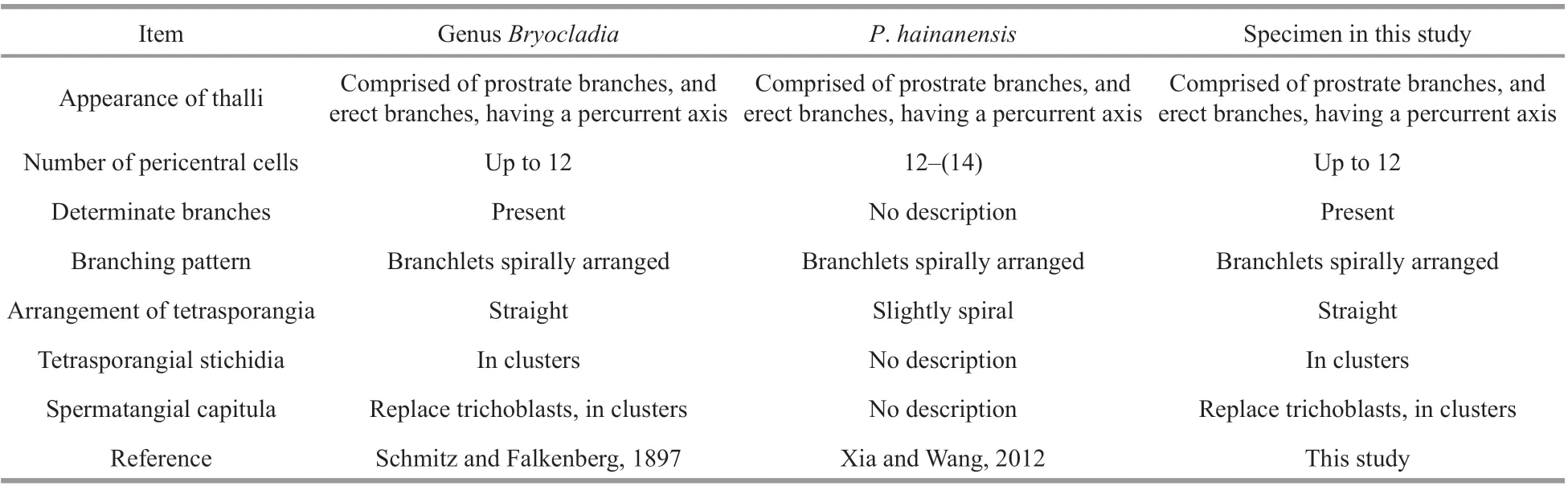
Table 1 Morphological comparison of Polysiphonia hainanensis and Genus Bryocladia
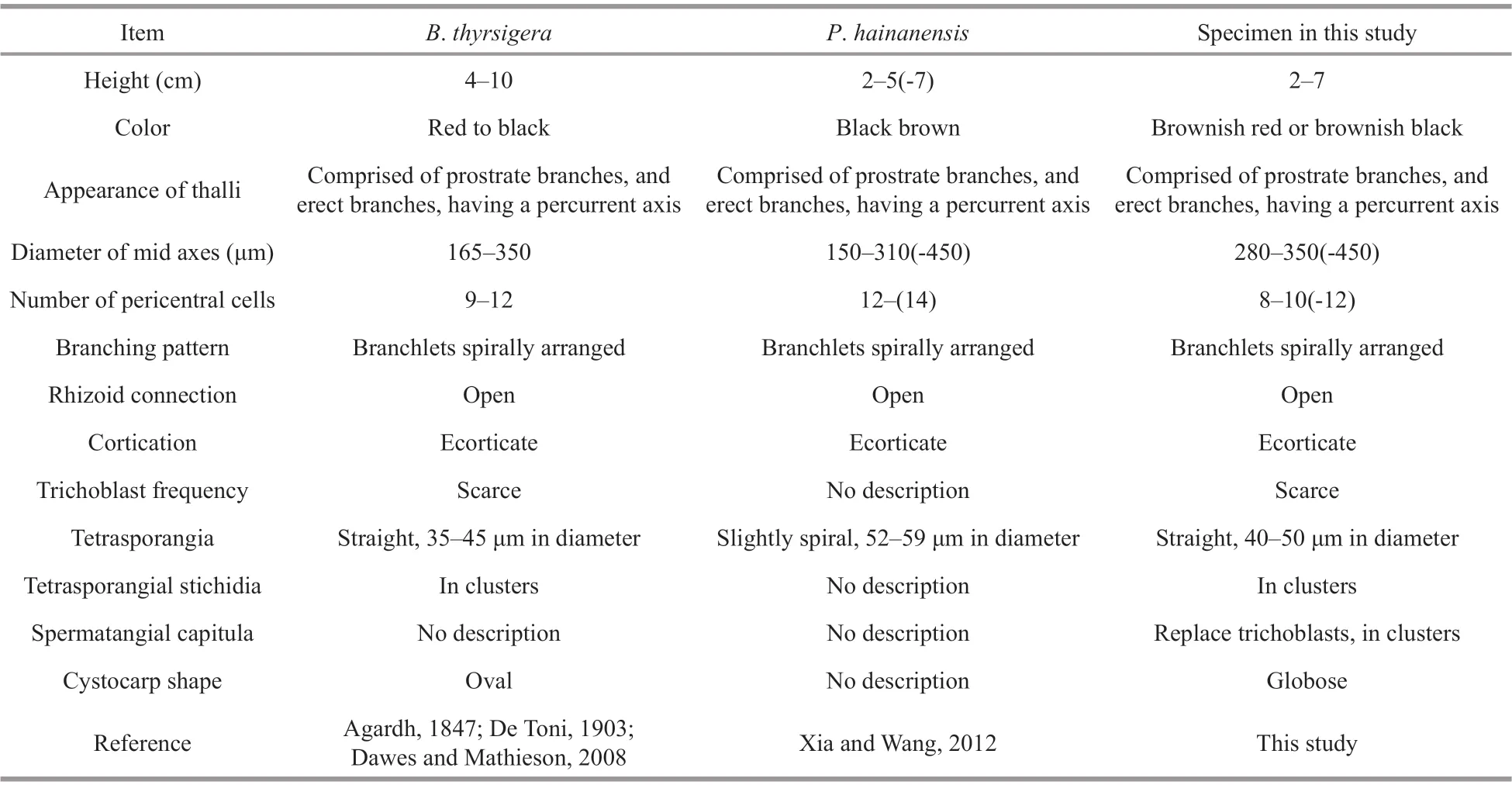
Table 2 Morphological comparison of Polysiphonia hainanensis and Bryocladia thyrsigera
5 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Sequence data that support the findings of this study have been deposited in GenBank under the accession numbers MN709123–MN709126.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
