Laboratory Evaluation for Utilization of Phosphogypsum through Carbide Slag Highly-Effective Activating Anhydrous Phosphogypsum
2021-06-14CHENShunXIONGGuoqingSUYingHEXingyangXIEYunxuanWANGYunfengGONGMinhua
CHEN Shun, XIONG Guoqing, SU Ying*, HE Xingyang,XIE Yunxuan, WANG Yunfeng, GONG Minhua
(1. School of Civil Engineering, Architecture and Environment, Hubei University of Technology, Wuhan 430068, China; 2. Building Waterproof Engineering and Technology Research Center of Hubei Province, Hubei University of Technology, Wuhan 430070, China; 3. Hubei Jinlu Energy Saving Co., Ltd, Huangshi 435000, China)
Abstract: Carbide slag was used as an activator to improve the activity of anhydrous phosphogypsum.Carbide slag could greatly improve the mechanical strength of anhydrous phosphogypsum than K2SO4. The compressive strength of 11 wt% carbide slag and 1 wt% K2SO4 activated anhydrous phosphogypsum increased greatly to 8.6 MPa at 3 d, and 11.9 MPa at 7 d, and 16.0 MPa at 28 d, respectively. The rate of hydration heat was accelerated and the total hydration heat was increased, and more calcium sulfate dihydrate was formed and cross-linked with other parts which improved the compressive strength of anhydrous phosphogypsum under the effects of different activators. It was indicated that carbide slag was a highly effective and cost-efficient activator. The result provides a highly effective and low-cost method which results in a novel and high valueadded method for the utilization of phosphogypsum in the future.
Key words: anhydrous phosphogypsum; activation; carbide slag; K2SO4; compressive strength
1 Introduction
Phosphogypsum (PG) is a main solid waste generated from wet-process phosphoric acid production[1-4]. It is estimated that the global stockpile of phosphogypsum is about 6 billion tonnes. The global annual production of PG is about 300 million tonnes,while the annual production of China is about 80 million tonnes. The utilization rates of phosphogypsum in the Americas and European countries are generally less than 10%, while the utilization rates in China are overall less than 40%[5,6]. Usable PG consists mainly of calcium sulfate dihydrate about 90% and slightly amounts of silicon, F-, P2O5, and organic substances[7].Only about 15% of PG in the world is reused, such as soil stabilization amendments[8,9], set retarder in cement manufactures and building materials[10,11], while the remaining 85% is released into a PG dam without any further treatment[12,13]. It restricts the widespread utilization of PG because of the complex and unstable impurities in PG[14,15]. Furthermore, parts of impurities in PG leach out under the water environment which causes soil pollution and groundwater contamination[16].Therefore, the advanced method and technology approach for efficient utilization of PG will benefit in the sustainable development of the phosphorus chemical industry and even the ecological environment.
Many researchers have done lots of explorations and works on the utilization of PG. Among those reports, PG has been mostly used as hemihydrate phosphogypsum and anhydrous phosphogypsum(APG) products through calcinating at different temperatures[17]. APG is obtained from the dehydration of the original PG, which contains calcium sulfate(CaSO4) as the main chemical component. Owing to the calcinated process, most impurities in the APG result in stable states. APG preforms slow hydration rate and low strength when it is used as a cementing material directly. Most activators to enhance the hydration rate as well as the mechanical strength,were used such as K2SO4, Ca(OH)2, and MgSO4·7H2O complex, and anhydrous sodium sulphate and Portland cement[18-20]. But APG obtained from long-term hightemperature treatment processes loses its activities because of potential changes in its internal structure.Most commercial chemical reagents as activators are more expensive than APG materials which restrict the widespread usage of APG products. Therefore, it is necessary to find economical and effective activated methods to realize the utilization of PG and APG.
Carbide slag (CS) is a kind of solid wastes with calcium hydroxide as the main component, which is commonly obtained from the preparation of acetylene through calcium carbide hydrolysis. In China, the annual output of CS reached about 34 million tons,and the dry basis discharge already exceeded 20 million tons, and more than 100 million tons of CS had accumulated in the past[21]. Residual CS has occupied space and venue, and results in the surrounding environment contamination through long-term accumulation. Based on CS activating cementitious materials, CS may be used as potential efficient activator for APG.
In this work, CS along with K2SO4was experimentally tried for activating APG obtained from PG at 500 ℃. The mechanical strength, macroscopic performance, and microstructure of activated APG were measured and discussed. The purpose of this work is aimed at developing high effective and cost-efficient phosphogypsum materials, which provides a novel and high-value additional approach for the utilization of PG and APG.
2 Experimental
2.1 Materials
The APG was obtained from PG calcined at 500 ℃ for 1 hour. PG was provided by a phosphate fertilizer plant affiliated by Hubei Yihua Fertilizer Inc., China. The particle size distribution and X-ray diffraction (XRD) pattern of APG and CS were shown in Fig.1. The equivalent chemical composition of APG was shown in Table 1. CS used in this work was obtained from the same place as Hubei Yihua Fertilizer Inc., China. Chemical reagent K2SO4and Ca(OH)2were also used in this work.

Table 1 The equivalent chemical composition of APG and CS/wt%
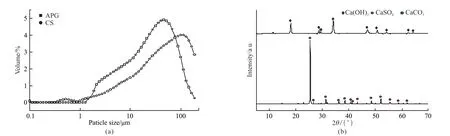
Fig.1 The particle size distribution (a) and XRD patterns (b) of APG and CS
2.2 Mix proportions and preparation process
Different mix proportions for experiments were listed as Table 2. From the reported literature, the optinum content of K2SO4in the samples was 3.3 wt%[22]. Consideration of the difference between APG samples, the dosages of K2SO4were 1 wt%, 3 wt% and 5 wt% in this work. In order to explore the optinum activating effect of CS on APG, different dosages of CS were explored from 1 wt% to 20 wt%. After preliminary exploration, the dosages of CS added in this experiment were 7 wt%, 9 wt%, and 11 wt%. The ratio of water to APG (W/A) was 0.55. Specimens with 40 mm×40 mm×40 mm (GB/T17669.4-1999) were molded at 20 ± 2 ℃, (55 ± 5)% RH for 24 hours, and then cured for 3, 7, and 28 days after being demolded.To characterize the microscopic properties of the specimens, samples were crushed into small pieces, and then immersed into ethanol to interrupt their hydration process. After being dried in a vacuum oven at 40℃ for 48 h, they were used for the measurements of SEM and other tests. Furthermore, these small pieces were also ground to pass through a 45 μm sieve for the measurements of hydration products.

Table 2 Different mix proportions of APG/wt%
2.3 Characterization
The setting time of APG samples was measured in accordance with Chinese standard GB/T 17669.4-1999 through a Vicat apparatus machine.
The mechanical strengths of APG samples were measured by an automatic test machine (TYE-300,China) in accordance with Chinese standard GB/T 28627-2012, and the speed of the load was kept at 2.4 kN·s-1. For each group, three specimens were measured.
The electrical conductivities of different activated APG samples were determined at 20 ℃ with ratio of water to anhydrous phosphogypsum at 10:1 by an electrical conductance meter (DDSJ-308F).
The hydration heat of the APG samples were measured at 25 ℃ by the isothermal calorimetry (TAM AIR, C80). The experimental data were recorded automatically by the instrument.
Phase composition of hydrate APG samples were analyzed with an X-ray diffractometer (XRD, D/Max-RB). During the test process, the scanning rate was kept at 5°/min with the step-length at 0.02° with a scanning range from 5° to 70°.
The mass loss and heat behavior of specimens were measured by a Synchronous thermal analyzer(STA449T, Germany) under different temperatures.Under an argon atmosphere, the test was carried out with the heating rate of 5 ℃·min-1with the heat range from 40 to 500 ℃.
The microstructure of APG samples were investigated through the scanning electron microscope(QUANTA FEG450, FEI). Under high vacuum mode,the tests were implemented with a working distance of 10 mm and the accelerating voltage from 10 to 15 kV.

Fig.2 The electrical conductivities of APG samples suspensions with different activators
3 Results and discussion
3.1 Electrical conductivity
As shown in Fig.2, the electrical conductivities of activated APG were studied, which explained the different influences of activators on hydration processes of APG slurry. From Fig.2(a), when the content of K2SO4was 5 wt%, the conductivity reached 749.2 μS·cm-1as the largest and stabilized at the first time. From Fig.2(b), the conductivity increased faster reaching to 880 μS·cm-1. From Fig.2(c), the total content of CS and K2SO4composite was 12 wt%. Using 7 wt% CS and 5 wt% K2SO4as composite activator,the equilibrium conductivity performed the largest as 1 494 μS·cm-1. The different conductivities of the initial suspensions were due to the dissolution of both APGs and activators[23]. It illustrated the effects of different activators during the hydration process of the APG.
3.2 Setting time
The setting times of activated APG were shown in Fig.3, and the initial setting time (IS) and final setting time (FS) were used to describe the curing behavior of cementitious materials. From Fig.3(a), with the increased dosage of K2SO4, the setting times of APG samples were gradually shortened overall. Compared with the blank group (A0), IS and FS of APG sample greatly reduced by 97.71% and 83.5%, respectively,when the added content of K2SO4was 5 wt%. From Fig.3(b), IS of the CS activated APG samples with content from 7 wt% to 11 wt% reduced from 82.53%to 87.02%, and the final setting time reduced from 83.18% to 84.27%. Compared with Ca(OH)2as 9 wt%added, the same content of CS performed better effect on the setting time of APG sample. From Fig.3(c),it performed different setting times of composite activated APG samples.When 7 wt% CS and 5 wt%K2SO4were added into APG samples, IS reached the shortest as about 7 minutes.
The influence factors of IS and FS mainly included the particle sizes of the materials and the dissolution and sedimentation rate of ions from the particles[24-26]. From Fig.2, it was found that the addition of activators increased the dissolution of ions into the solution based on the electrical conductivities of APG samples. The increased connection points caused by ions between the hydrated particles performed significant effects on the formation of the solidification structures. With the addition of activators, the amount of dissolved ions increased and the dissolution rate became faster, thus IS and FS became shorter. It resulted that the synergy of the granulation effect and reactivity effect determined the overall trend of the setting times of activated APG samples.
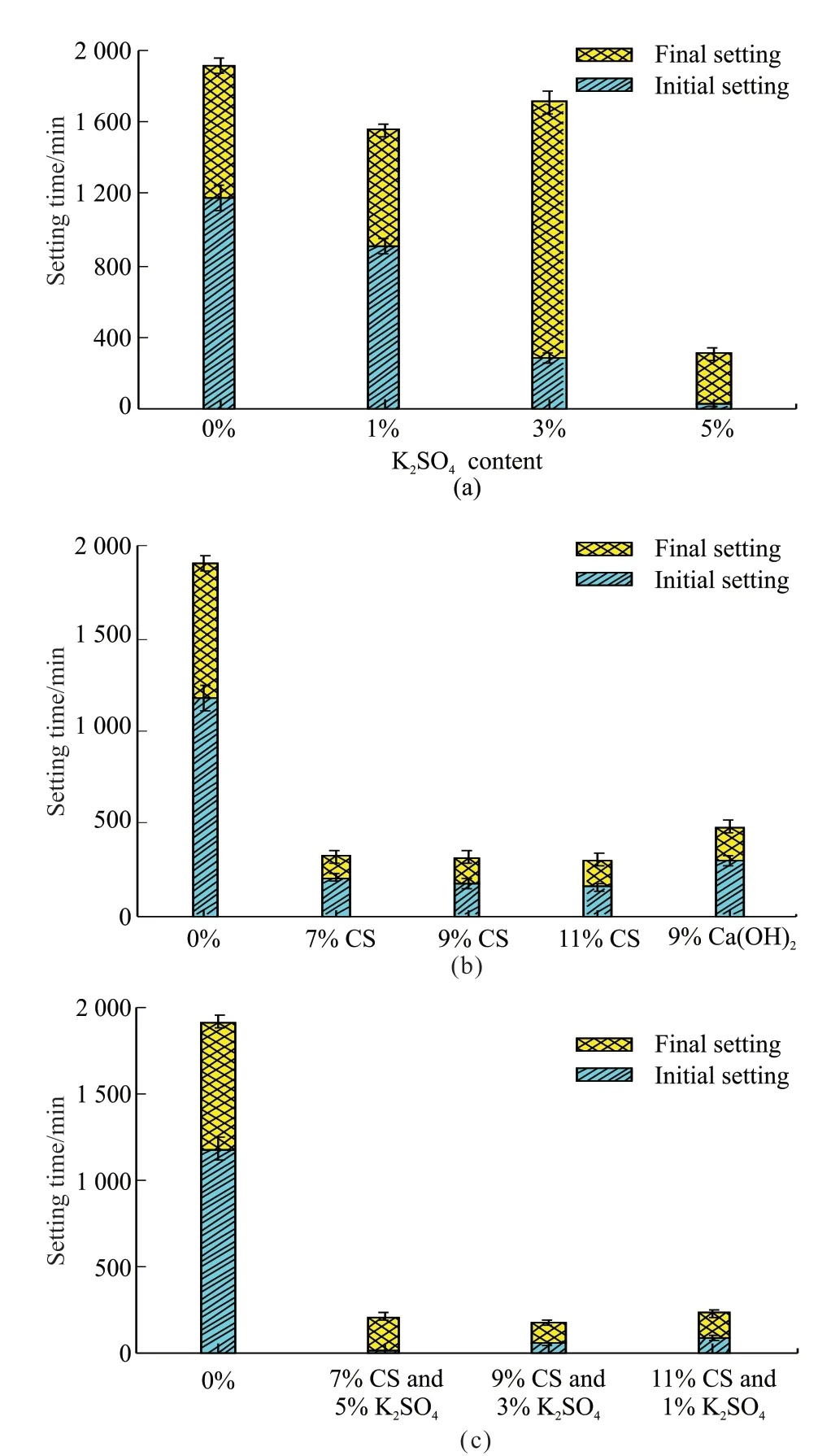
Fig.3 Setting time of different activated APG samples
3.3 Compressive strength
The compressive strength of samples at different curing time was shown in Fig.4. From Fig.4(a), with different contents of K2SO4, the trend of compressive strength of samples performed similarly as the blank but larger in value. From Fig.4(b), CS and Ca(OH)2increased the compressive strength of samples significantly. From Fig.4(c), the compressive strength of APG samples with composite activator performed a smaller increase than that of CS individually.When 11 wt% CS and 1 wt% K2SO4were added, the early compressive strength of activated APG sample developed faster than other contents and later the compressive strength also increased. The compressive strength of activated APG sample at this content reached 8.6 MPa at 3 d, and 11.9 MPa at 7 d, and 16.0 MPa at 28 d, respectively. It means that the activation effects performed fast at the early stage and slowly at the later stage.

Fig.4 Compressive strengths of different groups of activated APG
3.4 Hydration heat
The evolution rate and the total value of hydration heat were shown in Fig.5. From Fig.5(a) and 5(b), the hydration heat of APG blank sample raised rapidly and reached the maximum within 30 minutes while APG was in touch with water. And then, the heat dissipation rate dropped and stabilized at a certain level after 10 hours. The evolution of hydration heat was caused by the dissolution of anhydrous calcium sulfate in water[27].
The early hydration rate of activated APG by 3 wt% K2SO4was higher and performed a peak at 10 mins. After 10 mins, the hydration rate developed very slow at around 10 h. The dissolution of CaSO4and K2SO4provided more SO42-ions in the surrounding environment which potentially accelerated the further dissolution and heat releasing rate of APG. While 7 wt% CS was added, the continuous exothermic process and exothermic rate remained stable within 24 h. From hydration rate curve until 160 h, it showed two continuous small exothermic peaks. The thermal evolution rate remained stable within 40 h and then gradually decreased until to a constant. The maximum releasing heat rate was slightly lower than the peak value of the APG blank sample.

Fig.5 The evolution rate (a) and the total value (b) of hydration heat of APG samples
Samples of APG activated by 7 wt% CS and 5 wt% K2SO4showed an earlier heat peak at 8 min and another at 5 h. In most cases, the hydration heat evolution rate of different activated APG samples stabilized after 160 h. It was consistent with the rapid development of the compressive strength of APG within 7 days. Fig.5(b) showed the cumulative hydration heat of APG samples with different activators in 160 h. When 3 wt% K2SO4was added, the total hydration heat of activated APG sample was higher than blank sample. The total hydration heat of APG activated by 7 wt% CS was higher than that of 3 wt%K2SO4. While the total hydration heat of APG activated by 7 wt% CS and 5 wt% K2SO4was even higher than that of 7 wt% CS.
From the hydration heat data, the ability of CS was stronger than K2SO4, and the composite activator was stronger than both, especially in the early stage. It was related to the exothermic dissolution rate of CaSO4in water and the content of Ca2+and SO42-ions in the solution.
3.5 XRD analysis
As mentioned above, the setting time and the compressive strength were affected by additional activators. The electrical conductivities and hydration heat were used to evaluate the effects of activators on the APG[28]. However, during the hydration process, the hydration products might be the internal reasons for the effects. To investigate the effects of activators on the hydration products of APG samples, the XRD patterns of different activated APG samples at 28 d was shown in Fig.6(a). It indicated that the anhydrous calcium sulfate phase (CaSO4) reduced and the calcium sulfate dihydrate phase (CaSO4·2H2O) increased, compared with blank sample and activated sample by 9 wt% CS.The hydration degree had been getting better. The phase compositions of APG samples activated by 9 wt% CS or by 9 wt% CS and 3 wt% K2SO4were similar. The phase analysis based on XRD patterns explained the reasons for the compressive strength of samples.

Fig.6 XRD patterns: (a) diffent activated APG; (b) different times
To investigate the transformation of hydration products in activated APG, XRD patterns of samples by 9 wt% CS and 3 wt% K2SO4at 3, 7, and 28 d were shown in Fig.6(b). It revealed that the transformation from the anhydrous phase (CaSO4) to the dihydrate phase (CaSO4·2H2O) tended to be stable. The results showed that activated APG hydrated quickly at early age and reacted slowly at later age. Based on phase analysis, the hydration products formed by activators were characterized qualitatively. Furthermore, It also indicated that CS and the composite activator performed a stronger ability to activate APG than K2SO4only.
3.6 TG-DTG analysis

Fig.7 TG-DTG curves of different specimens
The TG-DTG curves of activated APG were shown as Fig.7. From Fig.7(a) and 7(b), the decomposition of Ca(OH)2might take place at the temperature between 380 and 620 ℃ under normal circumstances, and CaSO4·2H2O was between 63 and 250 ℃[29,30]. It indicated that the first peaks were the dehydration of CaSO4·2H2O and the second peaks were the decomposition of Ca(OH)2. From the intensity of peaks between 100 and 200 ℃, it showed that hydrates products of APG activated by CS were more than K2SO4at 28 d. It confirmed that APG activated by CS induced the more formation of CaSO4·2H2O than K2SO4, which implied higher efficiency of CS than K2SO4.
The TG-DTG analysis of APG activated by 9 wt% CS and 2 wt% K2SO4were shown in Fig.8(c) and 8(d). From Fig.8(c), the mass losses of APG samples in the temperature range were 14.54% at 3 d, 15.08% at 7 d, and 15.47% at 28 d individually. It indicated that the contents of hydrates products increased with hydration time. Thus, the hydration rates reached 66.16% at 3 d, 68.62% at 7 d, and 70.40% at 28 d. The TG-DTG analysis and XRD patterns of APG samples explained the developments of their compressive strength and the generations of CaSO4·2H2O in the samples. It indicated that the activated efficiency of CS was higher than K2SO4at early stage, and the composite activator was higher than both, which were related to the generations of CaSO4·2H2O.
3.7 Microstructure analysis

Fig.8 SEM images of different activated APG: (a) blank sample at 28 d; (b) 9 wt% CS and 3 wt% K2SO4 at 28 d; (c) 9 wt% CS and 3 wt% K2SO4 at 3 d; (d) 9 wt% CS and 3 wt% K2SO4 at 7 d
From Fig.8(a), it showed the morphology and distribution of hydration products of blank APG sample at 28 d. Generally, the morphologies of crystal were related to the periods of dissolution and precipitation[31].While the time of dissolution and precipitation were longer, the crystals formed thicker. However, the thicker crystals were difficult to overlap closely and formed more pores, and the interconnection between the crystals became weak[32]. The microstructure of blank APG sample performed relatively loose and contained relatively bigger pores among calcium sulfate dihydrate. The calcium sulfate dihydrate crystals were mainly rough plate-like with weak inter-joints between crystals. The poor mechanical properties of APG samples were due to the weakness of the contact points of calcium sulfate dihydrate. SEM images of APG samples by 9 wt% CS and 3wt% K2SO4at 28 d were shown in Fig.8(b). When the nucleation rate of APG sample was higher, the crystal size was smaller and the porosity was lower[33]. With the content of activators increasing, more calcium sulfate dihydrate with long rod shape in the hydration products formed.
SEM images of APG activated by 9 wt% CS and 3 wt% K2SO4at 3, 7, and 28 d were shown as Fig.8(b), (c), and (d). The APG samples performed fine needle-like crystals in the hydration products. During the hydration process, the number of fine needlelike crystals decreased and the density increased and formed a dense whole part. When the hydration process reached at 28 d, the bonds between the crystals were further strengthened and formed denser structure.
4 Conclusions
The CS withD50about 29.2 μm had high efficiency for activating APG. 11 wt% CS improved the compressive strength of APG up to 6.9 MPa at 3 d, 9.9 MPa at 7 d and 14.8 MPa at 28 d, which was seldom reported in the literatures.
CS and K2SO4can activate APG, which benefits from the effects of CS and K2SO4on the dissolutionnucleation-growth process of APG.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of Nano Silver Modification on the Dielectric Properties of Ag@TiO2/PVDF Composites
- Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres
- Effects of Cracks on the Mass Transfer of Polymer Electrolyte Membrane Fuel Cell with High Performance Membrane Electrode Assembly
- Refinement of TiB2 Powders with High-speed Planetary Mill and Its Effect on TiB2 Sinterability
- Fabrication of Ordered Meso-macroporous HPW/TiO2 Catalyst for Efficient Heterogeneous Oxidative Desulfurization
- The Preparation of Porous Activated Slag Granules/TiO2 Photocatalyst and Its De-NOx Performance
