Development of Cementitious Materials Utilizing Alkali-activated Yellow River Silt
2021-06-14WANGBaominWANGWanliLIANGXiaoxiaLIUHuiHANJunnanZHAOLuYANGXingxingYANJifei
WANG Baomin, WANG Wanli, LIANG Xiaoxia*, LIU Hui, HAN Junnan,ZHAO Lu, YANG Xingxing, YAN Jifei
(1.School of Civil Engineering, Dalian University of Technology, Dalian 116024, China; 2.Yellow River Institute of Hydraulic Research,Zhengzhou 450003, China)
Abstract: The possibility of preparing cementitious materials by the alkali-activated method using Yellow River sediment (The second largest river in China) as raw material and the modification effect on different slag addition were investigated. Sodium silicate and calcium hydroxide were used as the activator, and the specimens were prepared by the press molding method. The hydration process, hydration products, pore characteristics, and mechanical properties were investigated using SEM/EDS, FTIR, TG/DTG, XRD, MIP, and uniaxial compressive strength experiments, respectively. The results showed that the compressive strength of the modified yellow river silt-based cementitious material was significantly increased when the water glass dosage was 12 wt% (Ms=1.8)) and the slag dosage was 40%, and its 90-day maximum compressive strength could reach 53 MPa.
Key words: yellow river silt; alkali-activation; blast-furnace slag; compression molding
1 Introduction
With the technological progress, Yellow River sediment has been accepted and valued by more and more scholars as an available resource. The main components of Yellow River sediment are alkali-soluble minerals quartz and sodium feldspar, which have certain volcanic ash activity and can provide the active silica-alumina components required for the synthesis of geopolymers by alkali activation reaction[1,2]. Until now, minimal research was conducted on the feasibility of converting the Yellow River silt into construction materials, especially focusing on improving the properties of the Yellow River silt as a cementitious material. Alkali-activation is added to the industrial manufacture as chemistry additives to cement[3,4],and it is also a chemical process that changed vitreous structure into well-compacted cementitious composites[5,6]. Alkali-activated cementitious materials have many advantages, including environmental friendliness, fast strength growth, good durability,and high-erosion resistance[7-10]. Liet al[11]used the Yellow River silt as the main material and fly ash as a mineral additive to produce building material. The highest strength of the modified Yellow River was 20.3 MPa, and the corresponding softening coefficient was 0.86 at 90 days. Wanget al[12]used the Yellow River silt as the main material and 10 wt% blast-furnace slag as a cementitious material to produce artificial flood-prevention stone. The result showed that the highest was 12.3 MPa (curing for 28 days) with 5 wt%Ca(OH)2and 0.5 wt% NaOH.
However, the modified Yellow River silt strength could not meet the current requirements of high strength. Thus it is necessary to improve the modified Yellow River silt strength and durability. This study aims to produce an environmental-friendly and high strength (the compressive strength is 30-50 MPa at 90 days) cementing material. This paper provides a detailed analysis to analyze alkali-activated species’effect, alkali dosage, blast-furnace slag, and curing age on compressive strength. Micro tests investigated the mechanism of strength formation. The result showed that water glass (Ms=1.8) and Ca(OH)2have an activation effect on the Yellow River silt. The blastfurnace slag can significantly improve the modified material strength, and the main hydration product is C-S-H gel.
2 Experimental
2.1 Materials
In this work, the Yellow River silt (YRS) was obtained from Mengzhou, Jiaozuo City, Henan Province (in China). Blast-furnace slag (BFS) was used as an addition produced from Shandong Luxin Building Materials Co. LTD. The blast-furnace slag used in this work is S105 according to GB/T 18046-2008. The particle size distributions of the Yellow River silt were tested by GSL-101BI laser particle size distribution measuring instrument, shown in Fig.1.The X-ray diffraction (XRD) and X-ray fluorescence(XRF) were carried to determine different minerals and chemical compositions of the Yellow River silt and blast-furnace slag using a German Bruker SRS 3400 instrument. The result of XRF and XRD of Yellow River sediment are shown in Fig.2 and Table1,respectively. The main minerals of Yellow River silt are quartz and albite, which are alkali-soluble minerals,and the main chemical compositions of Yellow River silt are SiO2and Al2O3. Given the fineness, main minerals, and chemical composition of the Yellow River silt, it can be presumed that it possesses a specific pozzolanic activity. So, the Yellow River silt’s potential pozzolanic activity was activated by the alkali-activated method to prepare cementitious materials. The alkali activators include the following two types: water glass(the modulus ratio SiO2/Na2O,Ms=1.8), Ca(OH)2(analytically pure, power, content>99%). The test water was potable.
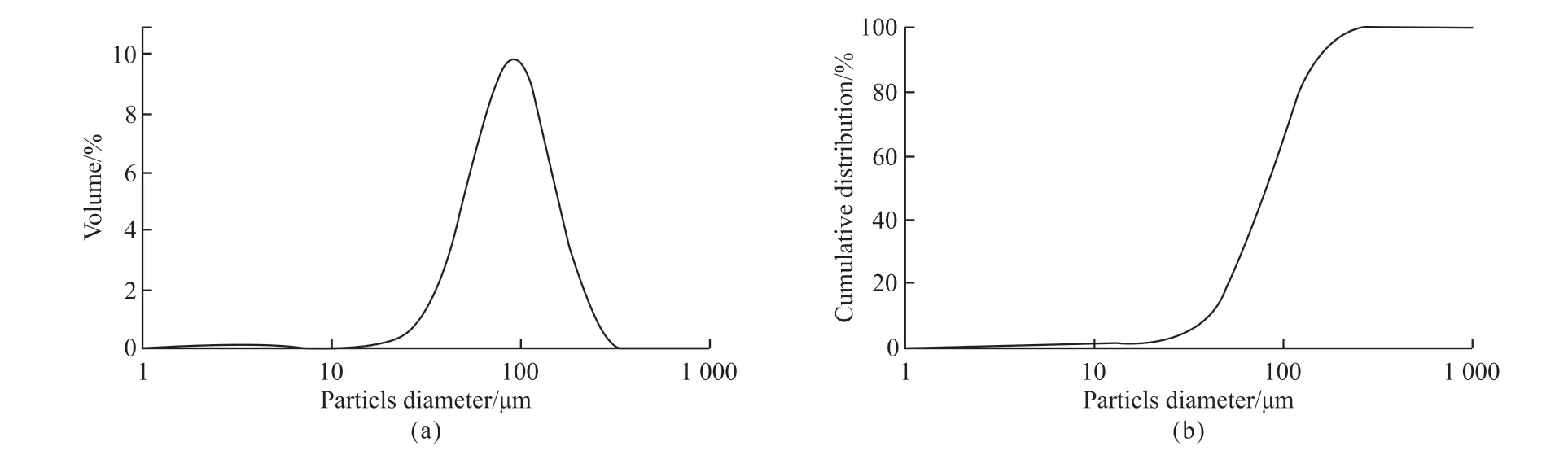
Fig.1 Particle size distribution curves of Yellow River silt: (a) Particle size distribution curve; (b) Particle size cumulative distribution curve
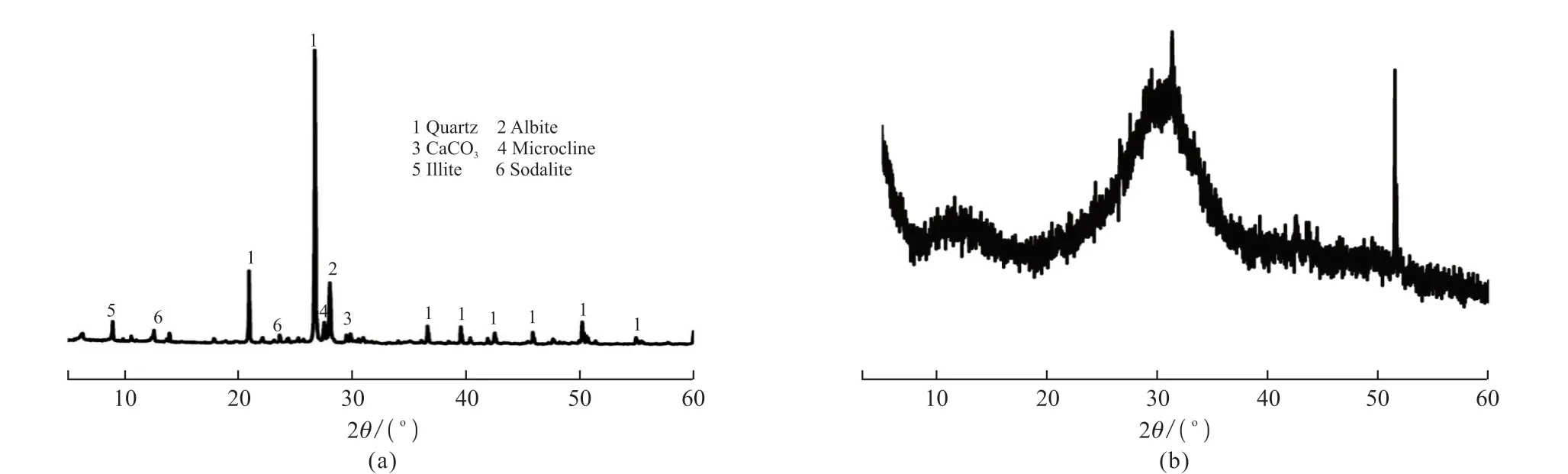
Fig.2 XRD patterns of (a) Yellow River silt ; (b) Blast-furnace slag

Table 1 Chemical composition of Yellow River silt and blast-furnace slag by XRF/wt%
2.2 Sample processing
The test samples were prepared using the press forming method. The forming mold is a steel cylindrical mold, and the samples were prepared by mixing Yellow River silt with blast-furnace slag at different ratios before adding activators and water to the paste. After vibrating and mixing, the fresh mixtures were poured into the steel cylindrical mold,and a machine pressed the samples under the pressure of 35 kN for 5 min. Then, the pieces were removed from the steel cylindrical mold.
The size of the modified Yellow River sediment sample isΦ50 mm×50 mm. Finally, the samples were placed in the basement with a temperature of 20±3℃ and relative humidity of 80 ± 5°. According to previous research, the modified Yellow River sediment performance is mainly affected by four variables: the samples’ density, the water content of the samples,alkali-activator, and mineral additions. The materials mixture used to prepare the Yellow River silt is shown in Table 2.
After curing for 3, 7, 28, and 90 days, the sample’s compressive strength was tested by an electronic universal testing machine with 300 kN capacity and a constant displacement rate of 0.5 mm/min. After the sample crushing, the debris from the crushed samples was dried in the vacuum dryer. Parts of the dried samples were ground in an agate mortar for microscopic tests. The hydration mechanism,hydration process, and hydration products of modified Yellow River silt were investigated using XRD, FTIR,TG/DTG. The XRD patterns were measured by a D8 ADVANCE automatic diffractometer (Bruker AXS Co., Germany) with Cu radiation and 2θin the range from 5-55° at a scanning rate of 0.5 °/min. The FTIR spectra were taken in an EQUINOX55 spectrometer(Bruker Co., Germany) in the frequency range of 400-2 000 cm-1. TG/DTG was measured using a Mettler Toledo TGA/DSC 1 synchronous thermal analyzer in the range from 0-100 ℃ in an atmosphere of nitrogen with a heating rate of 10 ℃/min. The modified Yellow River silt’s microstructure was investigated with a QUANTA 450 environmental scanning electron microscope (FEI Co., Hillsboro, OR, USA). Pore structure and porosity of modified Yellow River silt was analyzed by an AUTOPORE IV 9500 porosimeter Micromertics Instrument Corp., Norcross, GA, USA)with a maximum pressure of 33 000 psi.
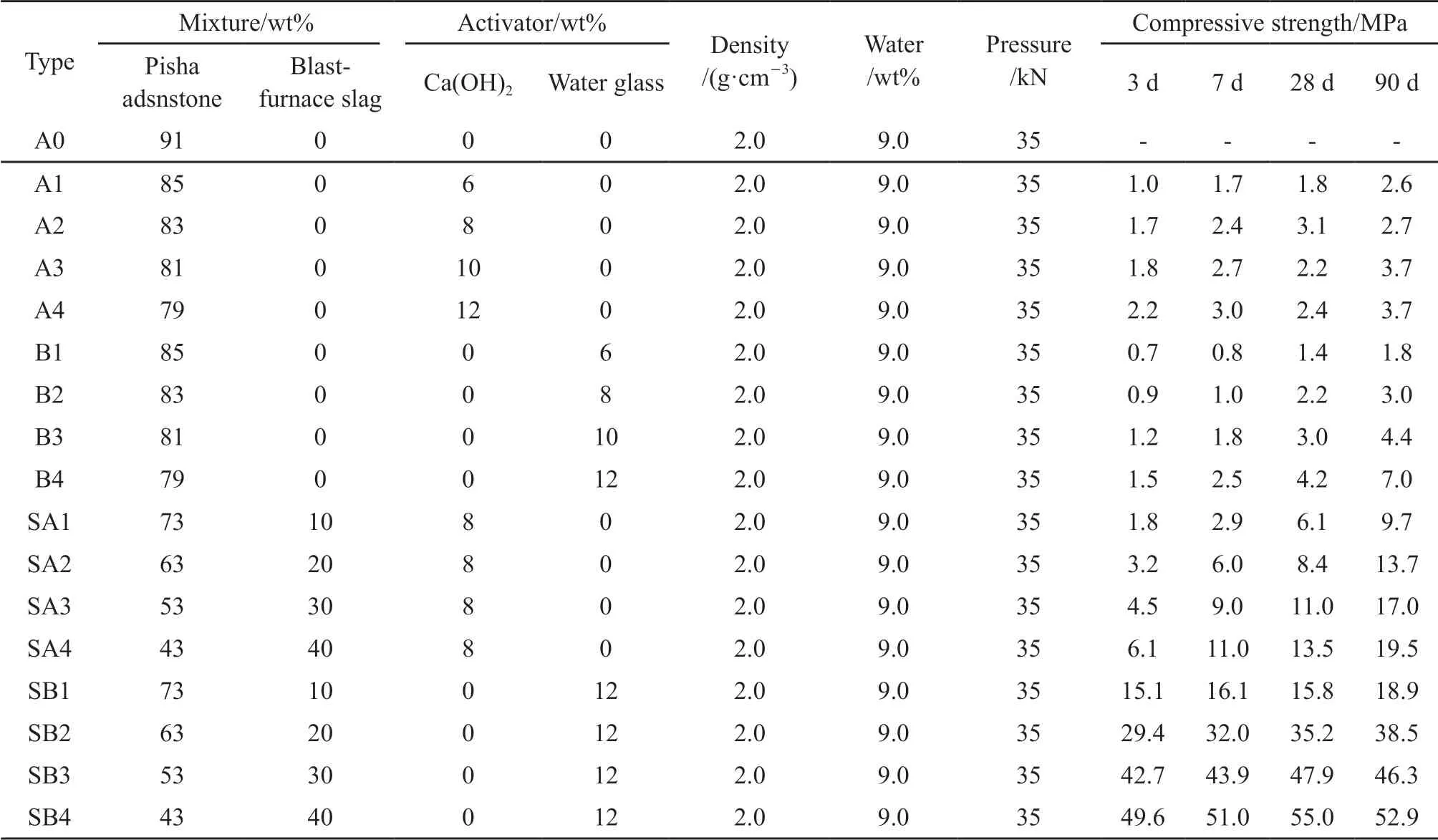
Table 2 Mix proportion and test results for modified Yellow River silt
3 Results and discussion
3.1 Compressive strength testing
3.1.1 Yellow River sediment modified by different alkali-activator
The alkaline activators were determined to be Ca(OH) and water glass by pre-experiments. In this paper, the tests are not described in detail. The compressive strength of Yellow River Sediment material at different curing ages with varying Ca(OH)2and water glass additions is shown in Fig.3. The strength of the Yellow River Sediment improves slightly with the increase of Ca(OH)2. With different Ca(OH)2additions, the modified Yellow River silt shows different trends with the growth of curing ages. When the addition of Ca(OH)2is 6 wt%-8 wt%,the strength increases with the development of curing periods, and when the addition of Ca(OH)2exceeds 10 wt%, the strength of modified materials decreases significantly after curing for 28 days. The carbonization of the excessive Ca(OH)2has negatively affected the development of compressive strength. So,the optimal dosage of Ca(OH)2shall be 8 wt%.
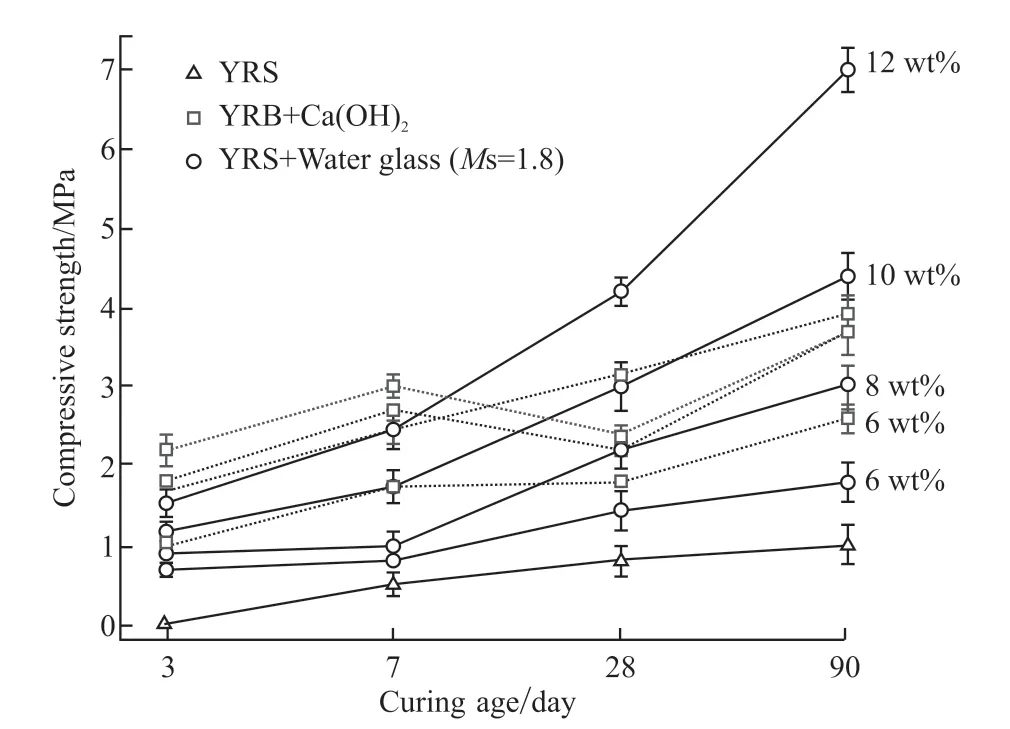
Fig.3 The compressive strength of Yellow River silt at different curing ages with an extra dosage of Ca(OH)2 and water glass (Ms=1.8)
It can also be seen from Fig.3, the compressive strength of the Yellow River Sediment material can be significantly improved with the addition of water glass, and it increased with the increase of water glass(Ms=1.8) addition and the growth of curing age. When the addition of water glass (Ms=1.8) is 12 wt%, the Yellow River silt material’s compressive strength was 7 MPa at 90 days. Given some economic factors, the optimal dosage of water glass (Ms=1.8) shall be 12 wt%. `
Comparison of the modification effect of the two activators on the Yellow River sediment is as follows.In the early curing ages, the compressive strength of the Yellow River Sediment modified by Ca(OH)2shows a significant improvement than that of the Yellow River silt modified by water glass(Ms=1.8). However, the result is opposite in the late age (the dosage of water glass (Ms=1.8)>8 wt%). Compared with water glass(Ms=1.8), Ca(OH)2could quickly stimulate the activity of Yellow River silt and produce the gel that provides strength. However, water glass (Ms=1.8) has a better activation effect and greater activation degree on Yellow River silt at a late age.
After the mixing of yellow river sediment with alkaline activator, some of the silica and alumina components in Yellow River sediment with high activity will be dissolved and depolymerized under the action of hydroxyl and alkali metal ions to produce silica and alumina monomer; These monomers then interact with each other to produce dimer, trimer,etc,and finally after condensation and precipitation reaction to produce gels. When using calcium hydroxide as the activator, the early strength increases faster because of the introduction of calcium ions, which generates a large amount of C-S-H gel in the early hydration stage.The solubility of calcium hydroxide in the aqueous solution is low, and its solubility is influenced by temperature. When the amount of calcium hydroxide is significant, too much-uninvolved calcium hydroxide and the C-S-H gel generated by the hydration reaction undergo a carbonation reaction to generate calcium carbonate crystals. The generation of a moderate amount of calcium carbonate crystals is beneficial to the development of compressive strength[13], but the formation of excessive calcium carbonate crystals causes strength deterioration. When using water glass as the activator, a large number of C-S-H gels don’t form in the early stage of hydration, so its early strength development is slower compared with the calcium hydroxide activated system. Many geopolymer gels are developed with the curing age extension, and the specimen’s compressive strength is significantly increased.
3.1.2 Compressive strength of Yellow River silt at different blast-furnace slag addition with different alkali-activator
For the low compressive strength of alkalimodified Yellow River sediment material, taking into account the mechanical properties and economic factors, the material properties were improved by blending with slag. The effects of different slag addition on the material properties were investigated.
The effect of different slag additions on the compressive strength of the Yellow River sediment with 8% Ca(OH)2at different curing ages is shown in Fig.4(a). The 3-day strength of A2 is only 1.7 MPa, and the 90-day strength increases by 1 MPa compared with the 3-day strength. Compared with the A2, the 3-day and 90-day compressive strengths of SA2 increase by 10.7 and 13.2 MPa, respectively, and the compressive strength of the material increases with the growth of slag addition, and when the slag addition is 40%, the 90-day compressive strength reached 23.4 MPa, which was 19.7 MPa higher than that of the A2.
The influence on the compressive strength of Yellow River sediment with 12 wt% water glass at different curing ages of various slag additions is shown in Fig.4(b)). The compressive strength of SB2 increased 5.5 times compared with B4, and the compressive strength of SB4 at 90 days increased from 7 to 53 MPa. Additionally, the compressive strength of SB2 tends to grow with the extension of the curing age,while the compressive strength of SB4 increases after 28 days of curing but decreases slightly at 90 days. The reasons are mentioned above.
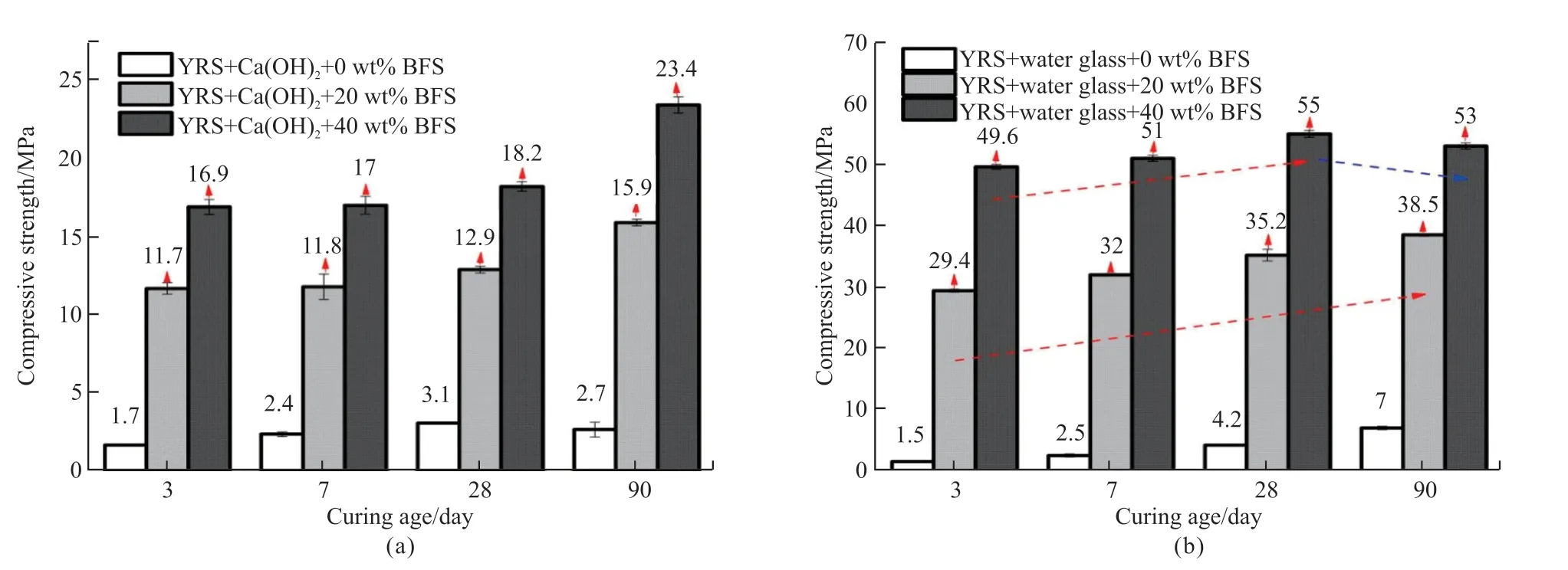
Fig.4 The effect of curing age and the dosage of blast-furnace slag on the compressive strength of Yellow River silt with 8 wt% water glass and 12 wt% water glass (Ms=1.8): (a) 8 wt% Ca(OH); (b) 12 wt% water glass
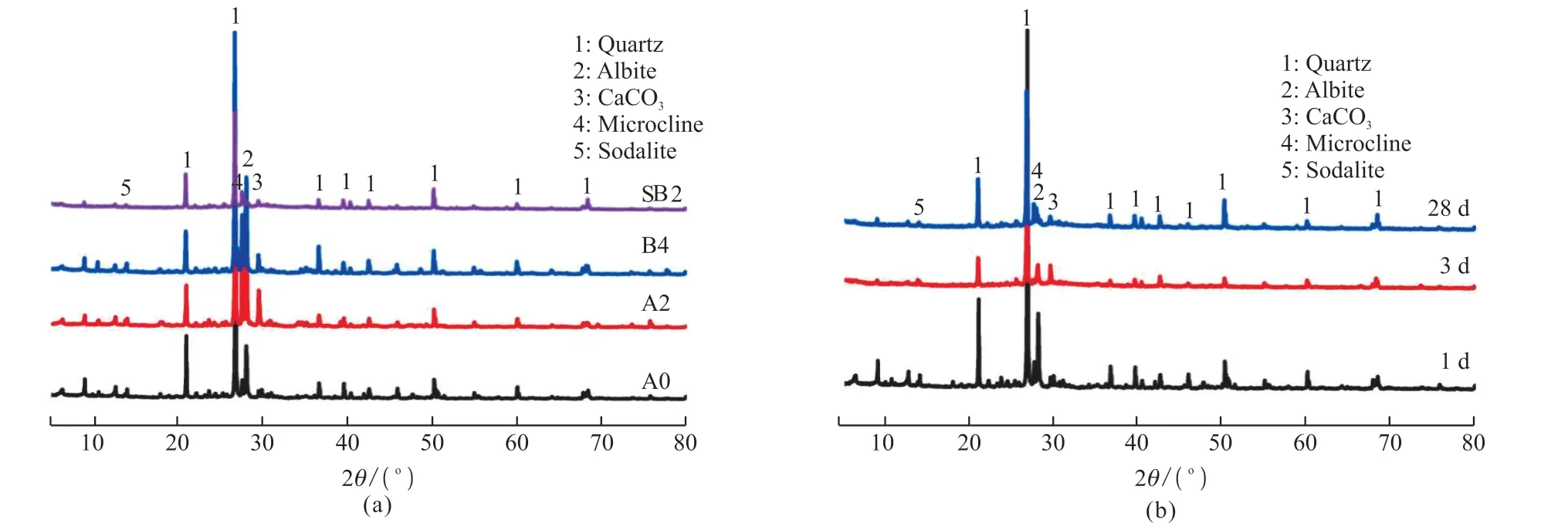
Fig.5 The XRD pattern of modified Yellow River Sediment: (a) A0, A2, B4, and SB2 at 28 days; (b) SB2 at different ages
In conclusion, the modified yellow river sediment composite material’s compressive strengths increase significantly by different activators, mainly when using water glass as the activator. The slag composition is a charge overbalanced aluminosilicate frame-work with many active silica-alumina components, and its reactivity is much higher than that of yellow river sediment. The ion exchange between the slag and the activator solution allows H-, Ca2+, Na+into the slag particles. The Al-O-Si bond is hydrolytically broken under metal particles’ action, accompanied by the destruction of the glassy network structure inside the slag, releasing many active silica-alumina components and generating a large amount of C-S-H gel, which makes the yellow river silt-slag and make the early strength of the composites increase rapidly. Besides,the content of calcium in the raw material and its status play an essential role in determining the reaction pathway and physical properties of the final hydration product, and the degree of depolymerization (DP)in the alkali activation reaction primarily control the reactivity of the material. A formula is described as follow:
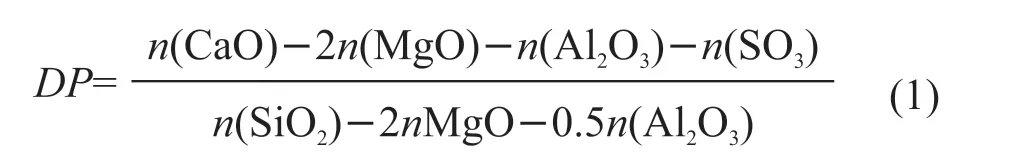
Slag as a Ca-rich modifier will essentially reduce the degree of polymerization of raw materials[14].The introduction of many calcium ions increases the disorder of the active silica-alumina components in the Yellow River sediment, which reduces the degree of polymerization (Eq.(1)[15]), accelerates the active silicaalumina components’ dissolution, and promotes the alkali activation reaction.
Considering of compress strength requirements,economic and ecological factors, the optimal dosage is 12 wt% water glass, 20 wt%-40 wt% blast-furnace slag and Yellow River silt.
3.2 XRD analysis of hydration process
After curing for 28 days, XRD analysis is performed on untreated Yellow River silt (A0),Ca(OH)2-activated Yellow River silt (A2), water glassactivated Yellow River silt (B4), and water glassactivated Yellow River silt- slag composite materials(SB2), respectively (Fig.5(a)). As we can see from Fig.5(a), sodalite and microcline are chemically stable and do not participate in reactivity reduction reactions,so they are present in all composite systems. The alkali-activated blast-furnace slag generates C-S-H to provide the samples compressive strength. C-S-H is an amorphous gel with poor crystallinity, so no obvious peak appears in the XRD pattern. Compared with the XRD diffractogram of A0, the diffraction peaks of CaCO3in the A2 XRD diffractogram were significantly enhanced, indicating that more CaCO3was generated in the Yellow River sediment modified by calcium hydroxide, which is formed partly by the reaction of untreated Ca(OH)2with CO2in the air, and partly by carbonization of C-S-H and Ca(OH)2generated by hydration.
As shown in Fig.5(b), the XRD peaks of SB2 curing for 3 days and 28 days are significantly different. After curing for 3 days compared with 1 day,the quartz diffraction peak substantially decreases,which indicates that the water glass (Ms=1.8) promotes the quartz in the Pisha/blast-furnace slag composite to participate in the hydration reaction. In other words,the hydration reaction of the Yellow River sediment is completed within 3 days. Given the result of compressive strength, the modified Yellow River silt’s strength does not increase significantly at a late age.
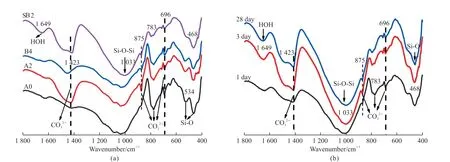
Fig.6 The FTIR spectra of modified Yellow River silt: (a) A0, A2, B4, and SB2 at 28 days; (b) SB2 at different ages
3.3 FTIR analysis of hydration process
The spectra of A0, A2, B4, and SB2 curing for 28 days and the spectra of SB2 at different curing ages are shown in Fig.6.
As shown in Fig.6(a), there is no significant difference in the four samples’ absorption peaks’number and position. The absorption peak at 468 and 534 cm-1are Si-O bending vibrations. The absorption peak at 1 033 cm-1is Si-O asymmetric stretching vibration. The absorption peak at 1 635 cm-1is O-H bending vibration[16-18]. The modified Yellow River sediment’s absorption peaks at 696, 875 and 1 423 cm-1are C-O-C stretching vibrations, indicating CO32-in the modified material.
According to the results of XRD, it can be determined that these CO32-belong to CaCO3. The relative strength of the absorption peak reflects the content of the group. The higher the peak, the more the content. The displacement of the peak generally represents the change of the degree of polymerization of the group. The displacement from low wavenumber to high wavenumber indicates that the degree of polymerization of the group increases. The peak at 1 423 and 875 cm-1of the Ca(OH)2-activated Yellow River sediment is stronger than others, which means the content of CaCO3increases. However, the peak at 1 033, 534, and 468 cm-1are not significantly changed,so the activation degree of Ca(OH)2is limited. The change of CO32-peak (1 420-1 440 cm-1) in B2 compared with A0 indicates that water glass (Ms=1.8)can activate the sediment and promote the hydration reaction to produce hydration products. FTIR of SB2 shows that the peak of CO32-is deepened, and the peaks of Si-O-Si (1 033 cm-1) and Si-O (468 cm-1)become more vigorous and narrower because water glass (Ms=1.8) promotes the reaction of blast-furnace slag and Yellow River silt to form C-S-H gel and reduces the degree of polymerization of C-S-H gel[17].
The blast-furnace slag in the composite system provides more active Si and Al to participate in the hydration reaction, generates more hydration products,and improves the compressive strength. As shown in Fig.6(b), there is no significant difference in the number, strength, and position of the absorption peaks in the FTIR spectra of SB2 after curing for 3 and 28 days, and there are two significant changes in the FTIR spectra of SB2 after curing for 3 and 1 day. On the one hand, the peak of the CO32-(1 423 cm-1) has become stronger, and the peak width has become narrower.Because the modified Yellow River silt hydration is carried out in the early curing age, and the hydration products C-S-H and Ca(OH)2have been generated and carbonized. On the other hand, the Si-O-Si group at 1 033 cm-1and the Si-O group at 468 cm-1enhance,indicating silica gel material is generated in the modified Yellow River silt[19-22]. The modified Yellow River silt’s main hydration product is the C-S-H gel to provide compressive strength.
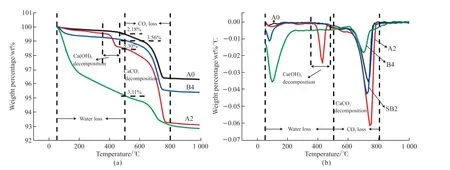
Fig.7 Thermogravimetric analyses (TG and DTG) of A0, A2, B4, and SB2 at 28days: (a)TG; (b) DTG
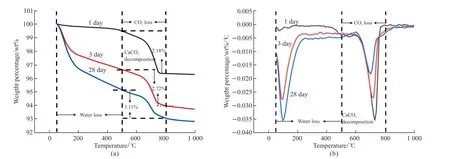
Fig.8 Thermogravimetric analyses (TG and DTG) of SB2 at different age: (a) TG; (b) DTG
3.4 TG/DTG analysis of hydration process
The TG and DTG curves of A0, A2, B4, and SB2 after curing for 28 days are shown in Fig.7,reflecting the changes of mineral phases before and after the Yellow River silt by different alkali-activators(Ca(OH)2and water glass(Ms=1.8)) and the effect of blast-furnace slag on the reaction process of modified Yellow River silt. The weight loss process can be divided into three stages: 1. The temperature range from 50 to 100 ℃ is the stage of losing free water; 2.The temperature range from 100 to 500 ℃ is the stage of losing the bound water and interlayer water (C-S-H gel,etc); 3. The temperature range from 500 to 800 ℃is the stage of CaCO3decomposition[2].
TG analysis results (Fig.7(a)) show a significant weight loss peak in Yellow River silt modified by Ca(OH)2. According to the results of DTG (Fig.7(b)), a decomposition peak (about 420 ℃) can be found. This peak is the decomposition of residual Ca(OH)2—the weight loss rate increase from 2.18 wt% to 5.30 wt% at the temperature ranging from 500 to 800 ℃. Therefore,sample A2 contains more CaCO3. CaCO3is partly derived from the carbonization of C-S-H and Ca(OH)2.TG analysis results (Fig.7(a)) of untreated Yellow River silt (A0) and water glass-activated Yellow River silt (B4) show the weight loss rate increases from 0.45 wt% to 0.53 wt% at the temperature from 100 to 500℃ and the weight loss rate increases from 2.18 wt% to 3.56 wt% at the temperature from 500 to 800 ℃, which means 12 wt% water glass has a specific activation effect on Yellow River silt. After adding 20 wt% blastfurnace slag, the weight loss rate of the composite modified material at the temperature of 500 to 800 ℃is 3.11 wt%. The result of DTG (Fig.8(b)) shows that the decomposition peak of C-S-H gel and CaCO3were significantly enhanced because the carbonization of the hydration product of the blast-furnace slag (C-S-H gel)produces a large amount of CaCO3. Simultaneously,the blast-furnace slag provides active Si and Al to promote the activated reaction and has a large amount of hydrated gel. According to the analysis result, the addition of blast-furnace slag can significantly improve the strength and durability of the modified Yellow River silt, which further confirms that the existence of blast-furnace slag activates the potential activity of the Yellow River silt. Fig.8 shows the TG/DTG curves of SB2 at different curing ages (1, 3, and 28 days). The curve can also be divided into three stages:
1. The temperature range from 50 to 100 ℃ is the stage of losing free water; 2. The temperature range from 100 to 500 ℃ is the stage of losing the bound water and interlayer water (C-S-H gel,etc.). In this stage, the weight loss rate at 1, 3, and 28 days are 0.45 wt%, 2.24 wt%, and 3.52 wt%, respectively. The weight loss rate keeps increasing, indicating that hydration products increase with the growth of age. However, the weight loss rate of 3 days is significantly higher than that of 28 days, so the modified material has a large degree of hydration in the early curing age; 3. The temperature range from 500 to 800 ℃ is the stage of CaCO3decomposition[23]. In this temperature range, the modified material’s weight loss rate increases gradually with the increase of the curing age. It goes from 2.18 wt% to 2.72 wt% and then to 3.11 wt%. At 1, 3, and 28 days, the content of CaCO3in SB2 is 100/44×2.18 wt%=4.95 wt%, 100/44×2.72 wt%=6.18 wt% and 100/44×3.11 wt%=7.07 wt%, respectively. At 3 days,the carbonization rate of C-S-H gel in the modified Yellow River silt increases (2.72 wt%-2.18 wt%)/2.18 wt%=24.8%, compared with that of 1 day. At 28 days increase (3.11 wt%-2.72 wt%)/2.72 wt%=14.3%,compared with that of 3 days. With the increase of curing age, the carbonization rate of C-S-H barely increases significantly or even decreases. Therefore,blast-furnace slag can effectively control carbonization in the late curing age. Based on the changes of the modified Yellow River silt’s compressive strength, it can be known that carbonization reactions do not harm compressive strength.
3.5 SEM/EDS analysis of hydration product
Fig.9 shows the scanning electron microscopy images of A0 and SB2. The point established in Fig.9(c,d) is detected by energy dispersive spectrometer(EDS). The EDS result is shown in Table 3. According to the microstructure and morphology of the modified material, the reaction product is mainly C-S-H gel.
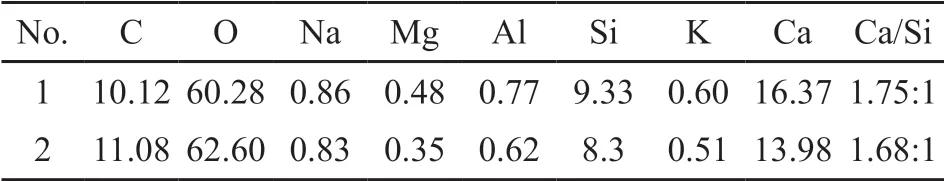
Table 3 Average values of the primary elemental molar ratios observed in EDS
The SEM of untreated Yellow River silt (A0)is shown in Fig.9(a). The untreated Yellow River silt has poor internal cementation and many cracks. The untreated Yellow River silt particle is mainly held together by mechanical bite friction and small adhesive material. Fig.9(b) is the SEM of the modified Yellow River silt (SB2) at 3 days. When the blast-furnace slag is added to the modified material, the matrix morphology changes fundamentally. It can be seen that the structure of the modified sample is relatively compact and uniform, and the crack decreases so that the loose sediment has formed a whole. It can be seen from Fig.9(c,d) that there is a homogenous and dense gel on the surface of the modified Yellow River silt. To confirm that the gel is C-S-H, EDS is used to detect the chemical composition of it. The result shows that the gel’s main elements were Ca, Si and O, and the Ca/Si ratio is about 1.7/1, which is similar to Ca/Si in C-S-H(Ca1.5SiO4·nH2O).

Fig.9 Scanning electron microscope images of modified Yellow River silt
Moreover, a large amount of literatures[24,25]indicates that the blast-furnace slag’s hydration product is mainly C-S-H, so the gel is determined to be C-S-H gel. The blast-furnace slag can provide more active Si and Al and promote the hydration reaction of Yellow River silt. Besides, the C-S-H gel has a large specific surface area, which can fill the internal pores to enhance the modified Yellow River silt’s compressive strength.
3.6 The result of MIP
The mercury injection test is carried out by AutoPorelV9500 floor mercury instillator. The result shows that the pore size distribution and porosity of the modified Yellow River silt (SB2) are significantly improved, and the total amount of mercury is reduced compared with untreated Yellow River silt (A0). As shown in Fig.10, the median pore diameter decreases from 8.6 to 7.9 nm, the average pore diameter decreases from 386.8 to 103.3 nm, and the porosity decreases from 39% to 17%.
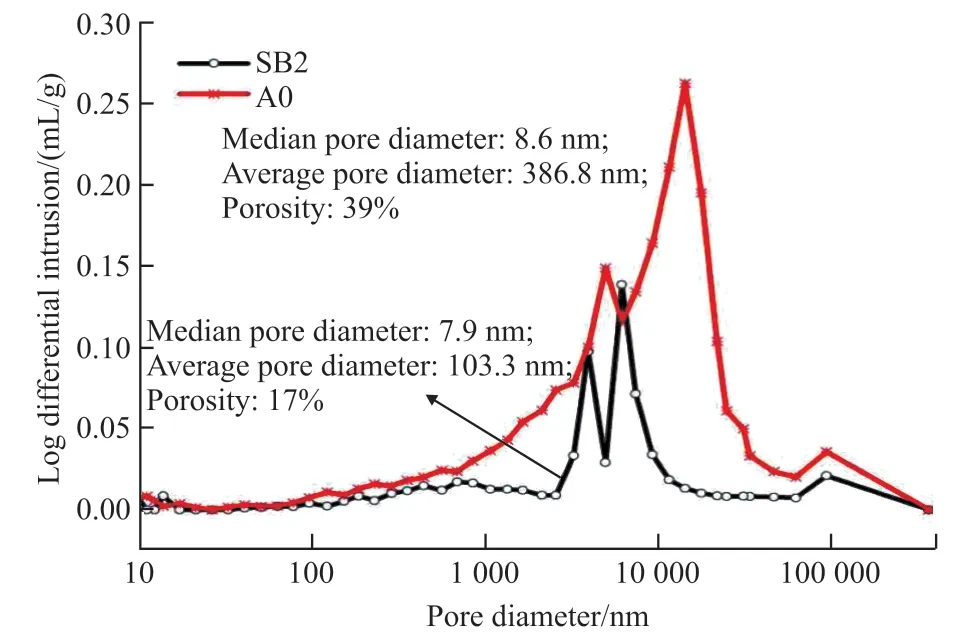
Fig.10 The pore structure of A0 and SB2 at 28 day
Therefore, the pore diameter of the modified Yellow River silt/blast-furnace slag decrease.Combined with the changes of compressive strength,it can be seen that the blast-furnace slag can promote the formation of C-S-H gel and the densification of the matrix, thus leading to stronger mechanical properties.
4 Conclusions
a) The Yellow River silt is modified by Ca(OH)2and water glass (Ms=1.8). In the early curing age, the compressive strength of Ca(OH)2-Yellow River silt is higher than that of water glass (Ms=1.8)-Yellow River silt. The result is the opposite in the late curing age. In the Yellow River silt, the optimal dosage of Ca(OH)2is 8 wt%, and the optimal dosage of water glass (Ms=1.8)is 12 wt%.
b) The blast-furnace slag can significantly improve the compressive strength of the modified Yellow River silt. The compressive strength of Ca(OH)2-activated Yellow River silt increases with the addition of blastfurnace slag. The maximum strength of 90 days is 23.4 MPa, which does not reach the target strength. The compressive strength of water glass (Ms=1.8)-Yellow River silt increases significantly with the addition of blast-furnace slag, and the 90 days strength reached up to 53 MPa, which meets the target strength. The blastfurnace slag significantly promotes the polymerization reaction of C-S-H gel by providing active Si and Al.
c) Modified Yellow River silt’s hydration product is C-S-H gel. The C-S-H gel can compact the matrix and improve the modified Yellow River silt matrix’s mechanical properties.
The high-performance cementing material made by Yellow River silt should meet the requirements of durability and compressive strength. Therefore, it is necessary to conduct further research on freeze-thaw resistance, wear resistance, weathering resistance, and corrosion resistance of the modified Yellow River silt.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Effect of Nano Silver Modification on the Dielectric Properties of Ag@TiO2/PVDF Composites
- Preparation and Photocatalytic Performance of Double-Shelled Hollow W18O49@C3N4@Ti3C2 Microspheres
- Effects of Cracks on the Mass Transfer of Polymer Electrolyte Membrane Fuel Cell with High Performance Membrane Electrode Assembly
- Refinement of TiB2 Powders with High-speed Planetary Mill and Its Effect on TiB2 Sinterability
- Fabrication of Ordered Meso-macroporous HPW/TiO2 Catalyst for Efficient Heterogeneous Oxidative Desulfurization
- The Preparation of Porous Activated Slag Granules/TiO2 Photocatalyst and Its De-NOx Performance
