Cembranoids of Soft Corals:Recent Updates and Their Biological Activities
2021-06-10MarsyaYonnaNurrachmaDeamonSakaragaAhmadYogiNugrahaSitiIrmaRahmawatiAsepBayuLindaSukmariniAkhirtaAtikanaAnggiaPrasetyoputriFauziaIzzatiMegaFerdinaWarsitoMasteriaYunovilsaPutra
Marsya Yonna Nurrachma ·Deamon Sakaraga ·Ahmad Yogi Nugraha ·Siti Irma Rahmawati ·Asep Bayu ·Linda Sukmarini ·Akhirta Atikana ·Anggia Prasetyoputri ·Fauzia Izzati ·Mega Ferdina Warsito ·Masteria Yunovilsa Putra
Abstract Soft corals are well-known as excellent sources of marine-derived natural products.Among them,members of the genera Sarcophyton,Sinularia,and Lobophytum are especially attractive targets for marine natural product research.In this review,we reported the marine-derived natural products called cembranoids isolated from soft corals,including the genera Sarcophyton,Sinularia,and Lobophytum.Here,we reviewed 72 reports published between 2016 and 2020,comprising 360 compounds,of which 260 are new compounds and 100 are previously known compounds with newly recognized activities.The novelty of the organic molecules and their relevant biological activities,delivered by the year of publication,are presented.Among the genera presented in this report,Sarcophyton spp.produce the most cembranoid diterpenes; thus,they are considered as the most important soft corals for marine natural product research.Cembranoids display diverse biological activities,including anti-cancer,anti-bacterial,and anti-infl ammatory.As cembranoids have been credited with a broad range of biological activities,they present a huge potential for the development of various drugs with potential health and ecological benefi ts.
Keywords Cembranoids·Diterpene·Soft corals·Sarcophyton ·Sinularia ·Lobophytum ·Anti-bacterial·Anti-cancer·Anti-infl ammatory
1 Introduction
The ocean represents the largest habitat on earth,covering over 70% of the earth surface and harboring a large number of marine organisms whose living environments are quite different from those of their land-based counterparts [1– 3].The extreme ocean conditions,e.g.high pressure,high salinity,hypoxia,and low light levels [4],lead marine organisms to synthesize the highly diverse and unique biological and chemical entities.As a result,the ocean is an important source of natural products with remarkable bioactivities for (novel) drug discovery.Among marine organisms,sessile animals such as soft corals have been shown to have strong chemical defense systems,which are refl ected in the almost infi nite structural diversity and complexity of their secondary metabolites.Hence,these organisms have long attracted the interest of natural product chemists for drug discovery research and development [5].
Soft corals (phylum,Cnidaria; class,Anthozoa; subclass,Octocorallia; order,Alcyonaceae; family,Alcyoniidae) have been studied as sources of marine-derived natural products since the nineteenth century [6].They are generally found in Indo Pacifi c reefs,whereas Gorgonian octocorals dominate the biomass in coral reef environments of the north-western Atlantic Ocean and the Caribbean Sea [7].The subclass Octocorallia including soft corals,gorgonians,and sea pens,are the most commonly studied corals for drug discovery [8].The main natural product isolated from soft corals is cembranoids,which act as chemical defense compounds against fi sh predators.Generally,these metabolites are obtained from the generaSarcophyton,Sinularia,Lobophytum,Eunicea,andClavularia[7,9– 11].Among all,the fi rst three genera attract the most interest in the study of cembranoids [6].
Cembranoids are derived from the cyclization of geranylgeranyl pyrophosphate [12],as shown from the double bonds of the cembrane skeleton having the E geometry observed in geranylgeraniol,diterpene alcohol.Theyare a class ofisoprenoid and consist of a fourteen-membered carbocyclic ring with an isopropyl residue at position 1,and three methyl groups at positions 4,8,and 12 [9,13,14].Cembrane diterpenoids have diverse structural variations with many functional groups (lactone,epoxide,furan,ester,aldehyde,hydroxyl,carboxyl moieties) and cyclizations that allow them to be grouped into several families [15,16].According to the review of Yang et al.[15],the cembranetype diterpenoids may be classifi ed as shown in Fig.1,which are:
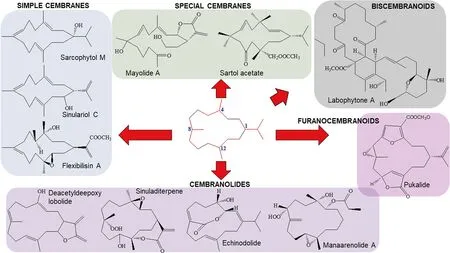
Fig.1 Chemical structures of chembranoid molecules.The isoprene unit of the basic carbon skeleton of cembranoids is bonded head-to-tail (red colors)
(1) Simple cembranes include the isopropyl cembranes,isopropenyl cembranes,and isopropyl/isopropenyl acid cembranes subtypes.
(2) Cembranolides possess a 14-membered carbocyclic nucleus generally fused to a 5-,6-,7-,or 8-membered lactone ring.Cembranolides include the subtypes 5-membered lactone,6-membered lactone,7-membered lactone,8-membered lactone.
(3) Furanocembranoids possess a 14-membered carbocyclic nucleus as well as a furan heterocycle.They also have a butenolide moiety involving C-10–C-12 and C-20.
(4) Biscembranoids possess a 14-6-14 membered tricyclic backbone of tetraterpenoids.
(5) Special cembranes include the subtypes secocembranes,13-membered carbocyclic cembranoids,cembrane glycosides,cembrane africanane,and other cembranes.
This review highlights secondary metabolites isolated from the generaSarcophyton,Sinularia,Lobophytumand their biological activities reported in the literature between 2016 to mid-2020.The literatures were collected from different online databases,including Pubmed and Google Scholar,presenting the research progress on secondary metabolites isolated from soft corals within the last fi ve years.This review summarizes the potential application of biomolecules (360 compounds) isolated from these three genera,covering the chemistry as well as the biological activity of their secondary metabolites,with special reference to cembranoids.
2 Cembranoids
2.1 Cembranoids Reported from Genus Sarcophyton
A total of 169 cembranoid compounds were isolated fromSarcophytoncollected from various geographical areas (Table 1).Out of those,128 were new compounds and 41 were previously known compounds with newly discovered activities.Eleven of the new compounds were newly discovered and have not been thoroughly tested for their biological activities.
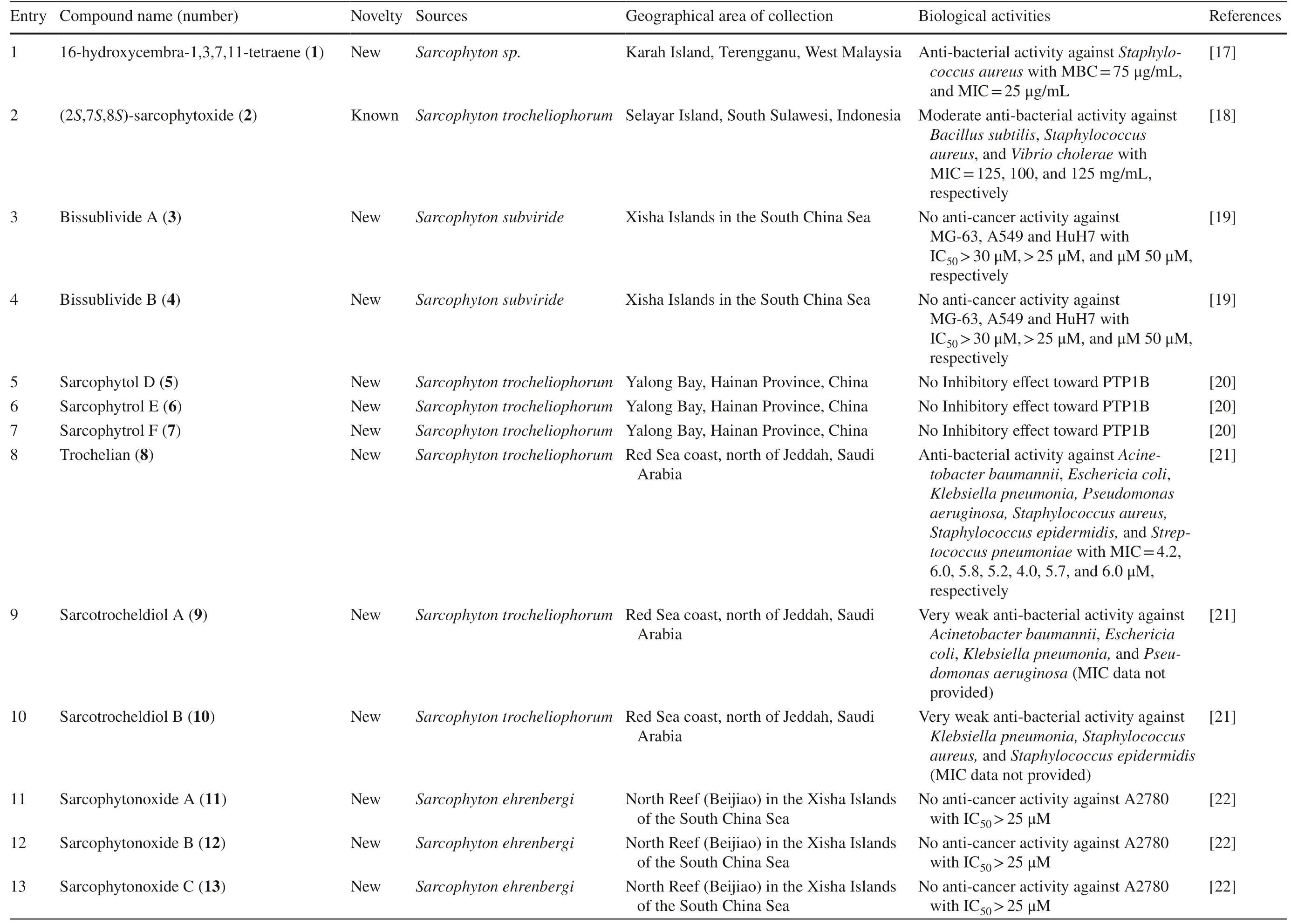
Table 1 The biological activities of cembranoid isolates from genera Sarcophyton
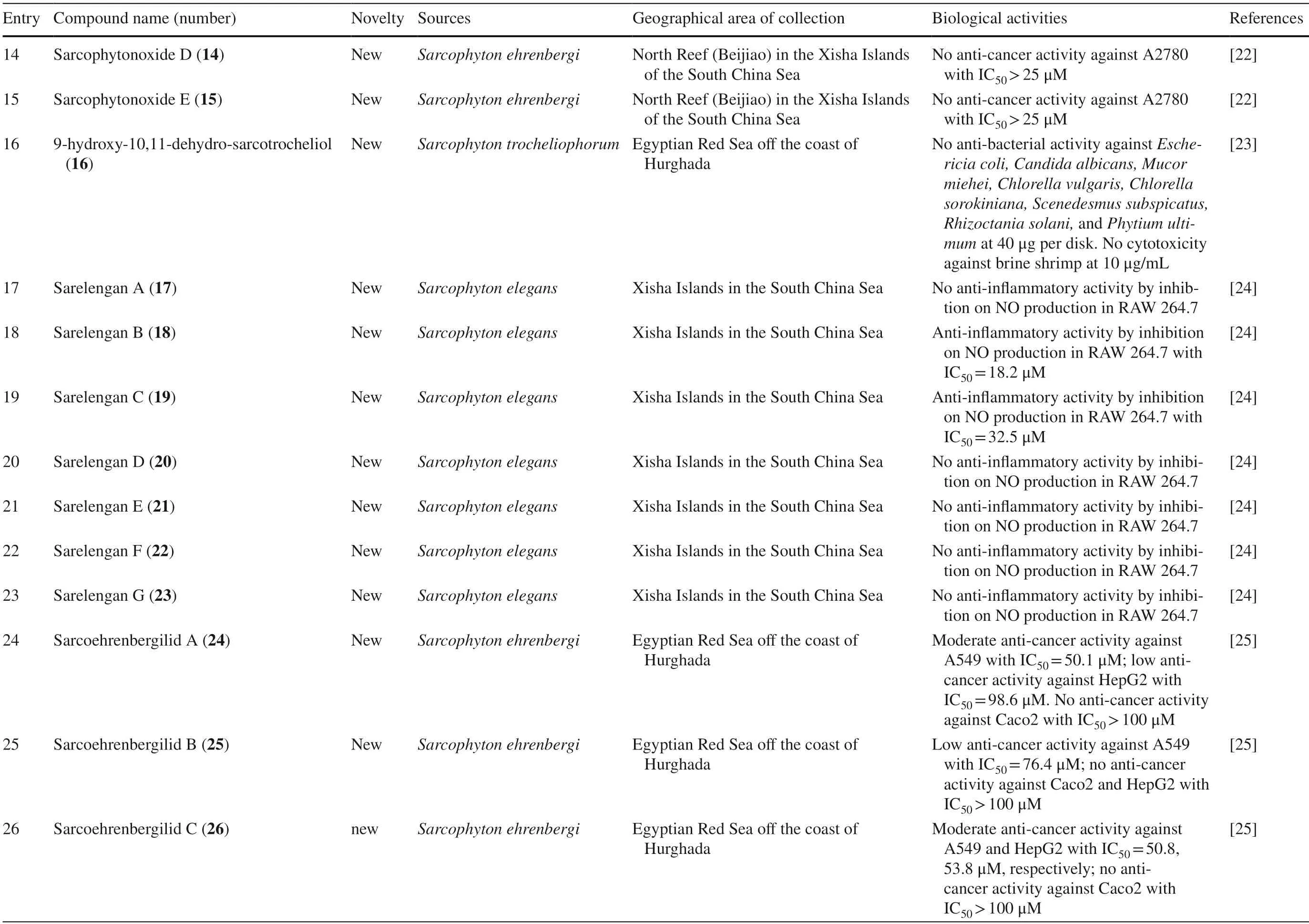
Table 1 (continued)
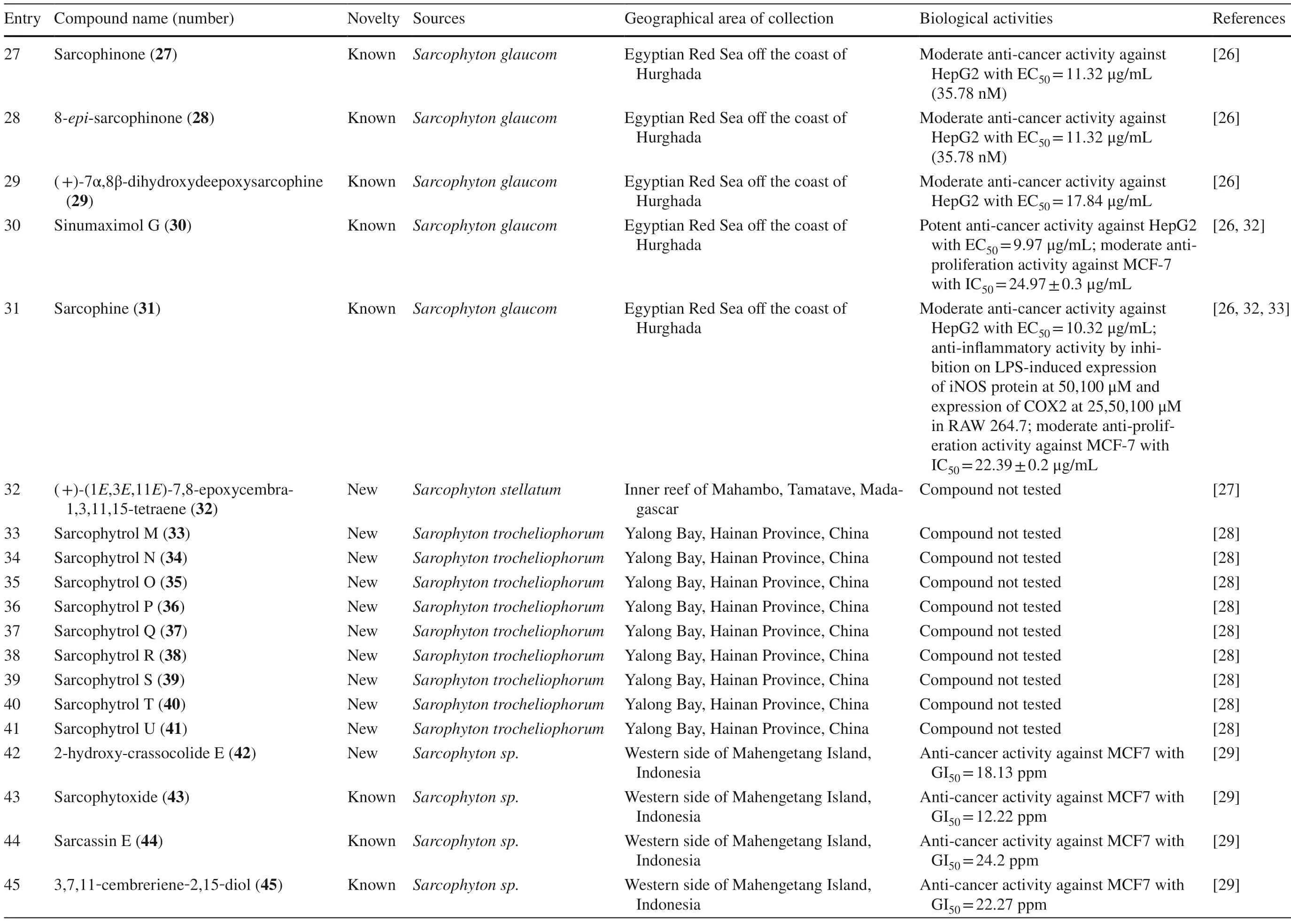
Table 1 (continued)
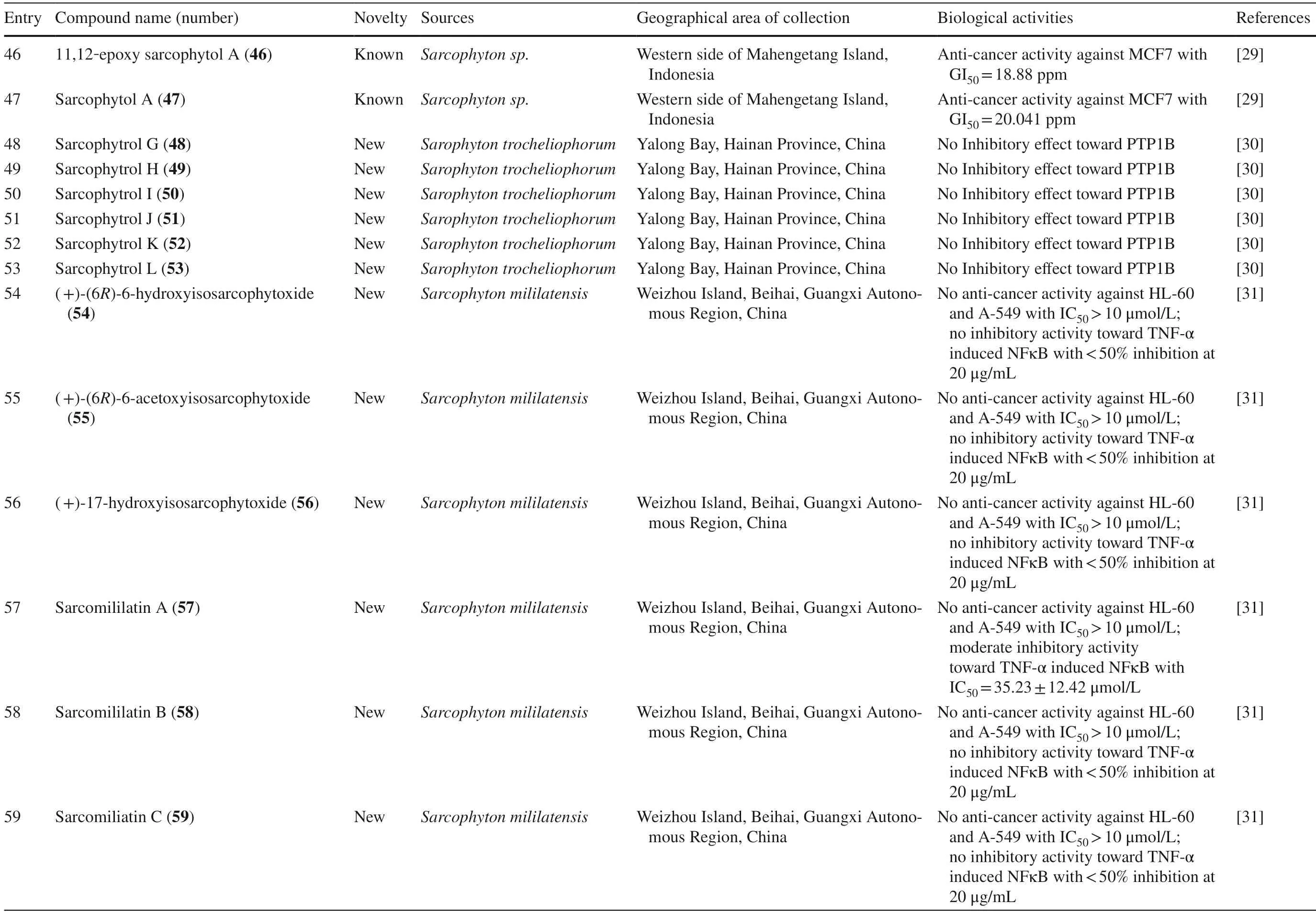
Table 1 (continued)
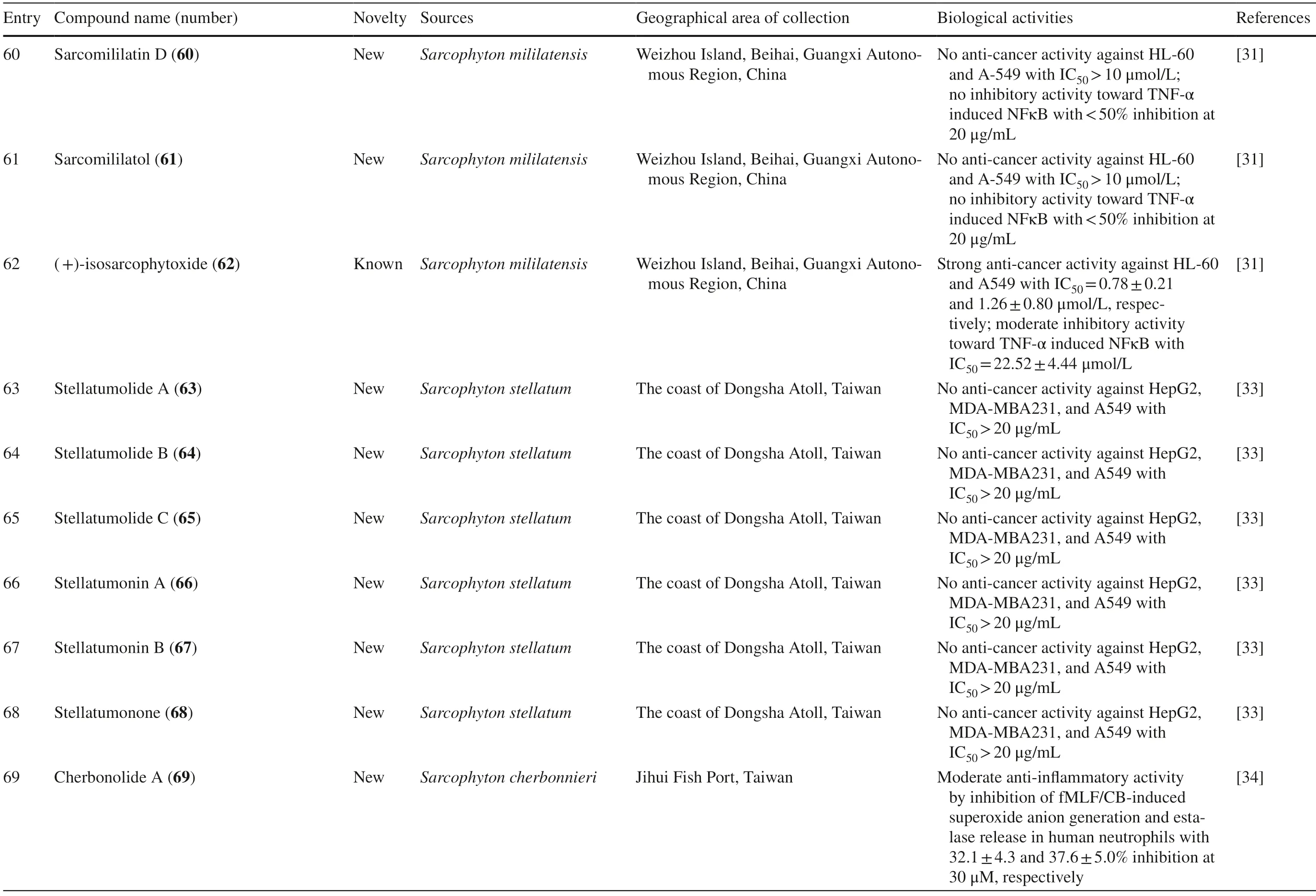
Table 1 (continued)

Table 1 (continued)
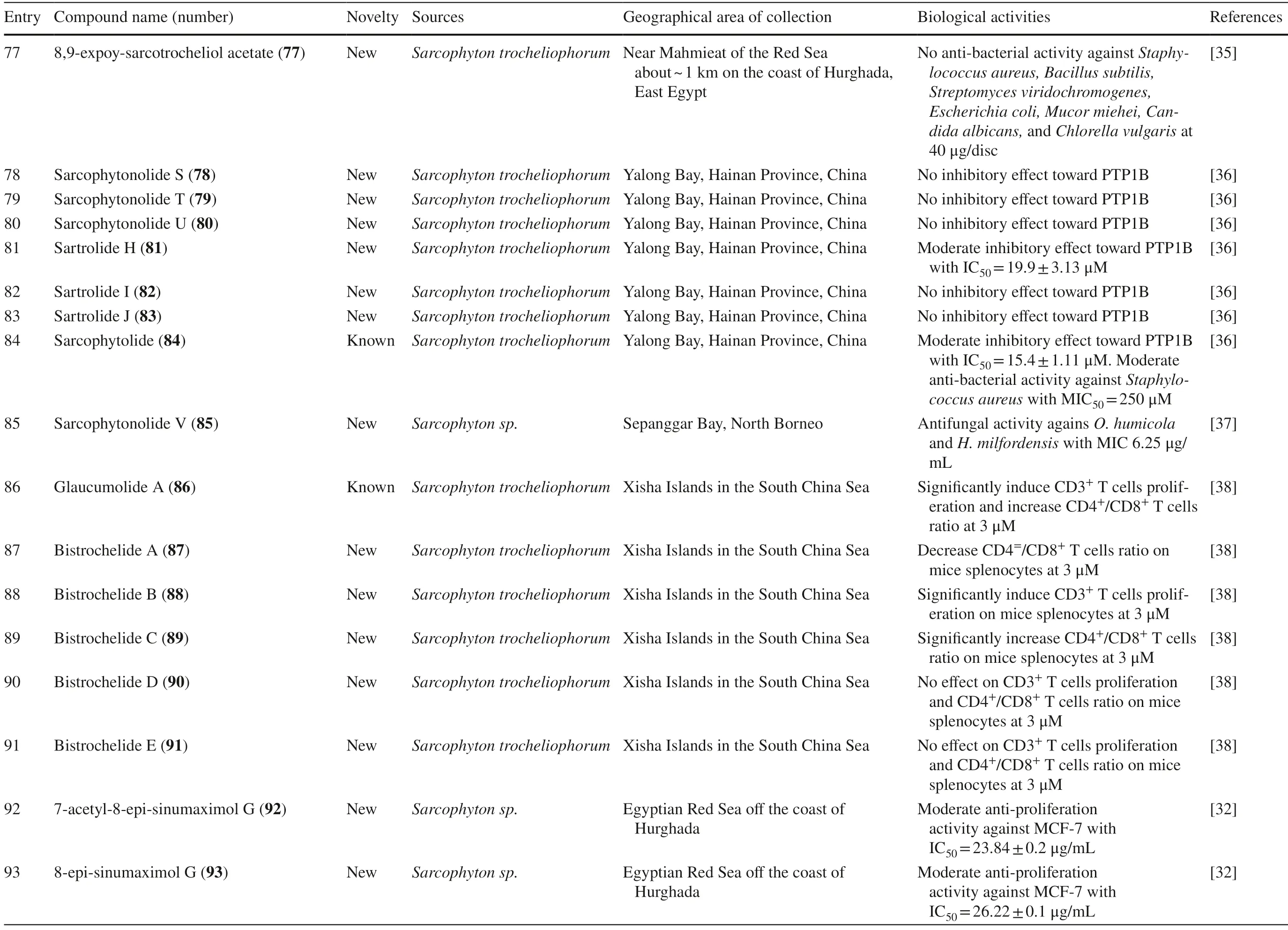
Table 1 (continued)
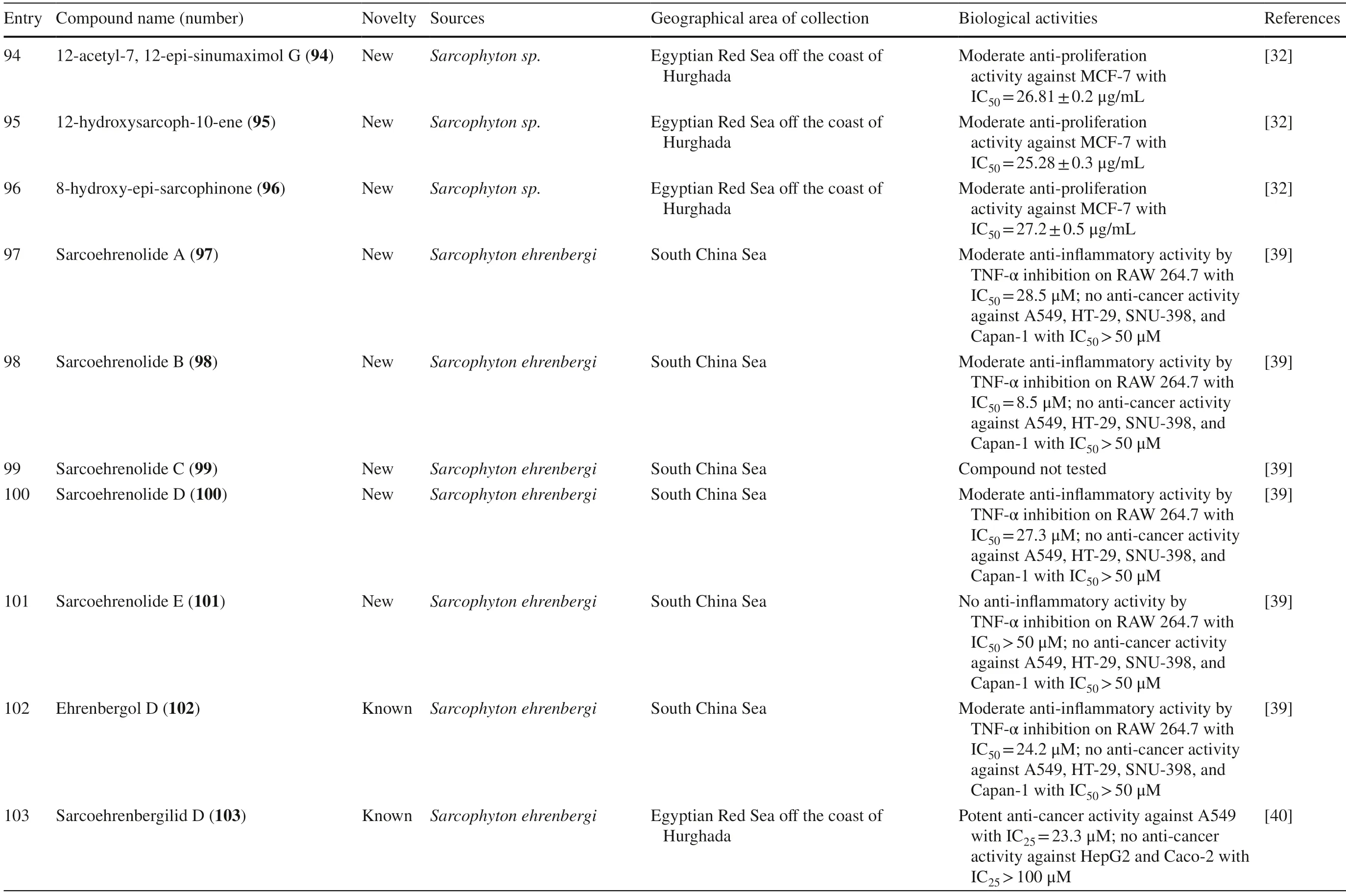
Table 1 (continued)
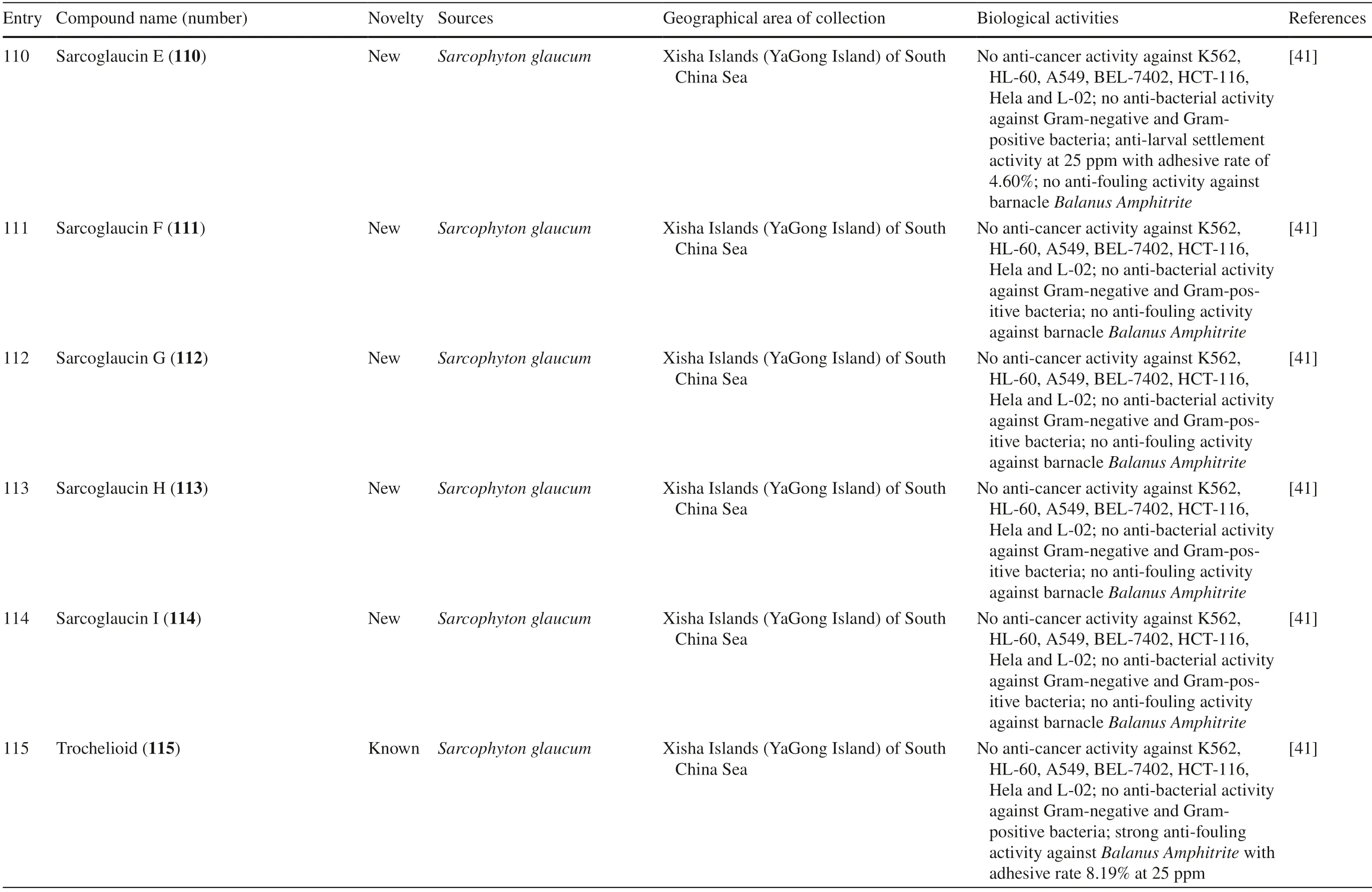
Table 1 (continued)
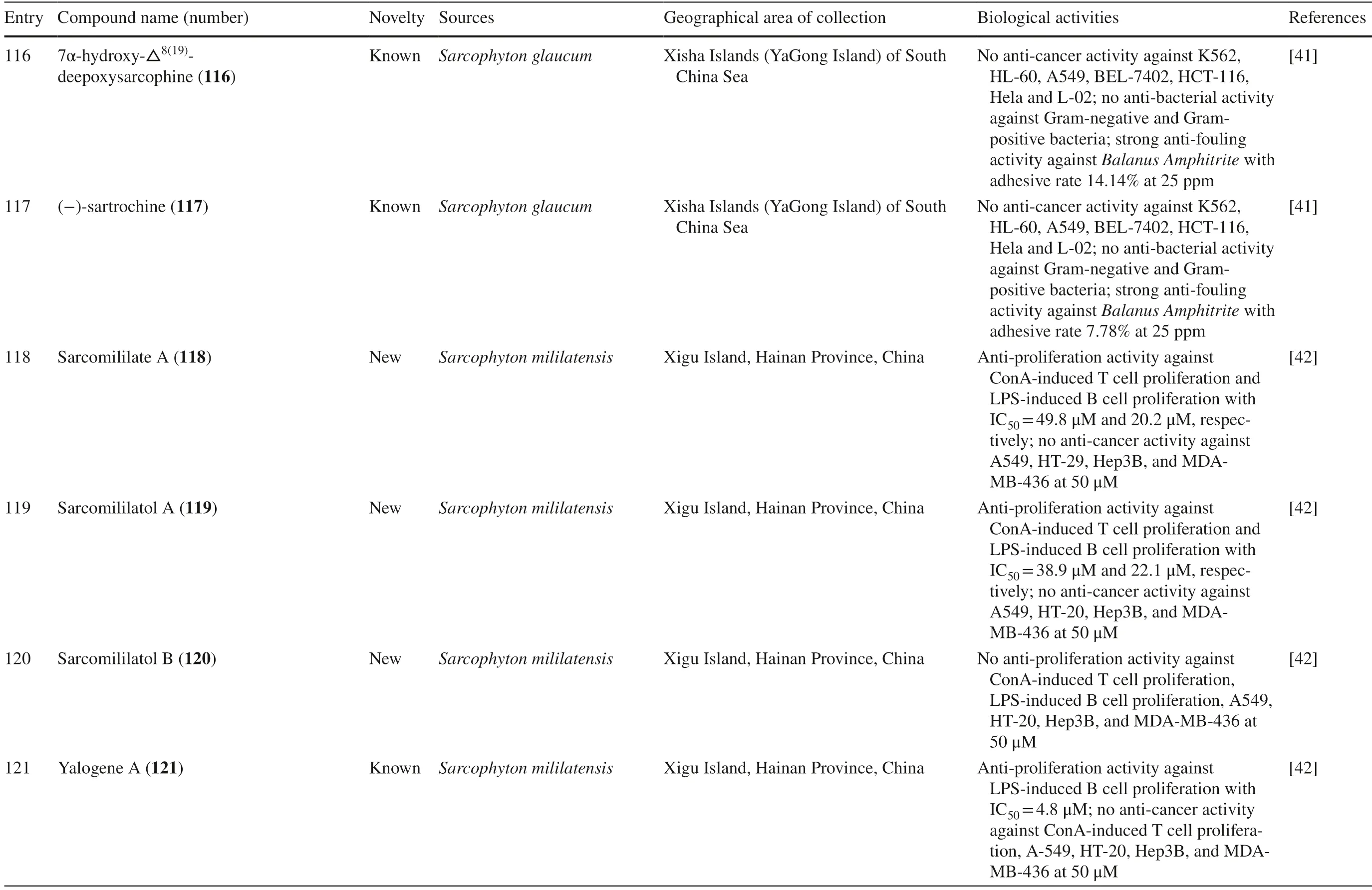
Table 1 (continued)
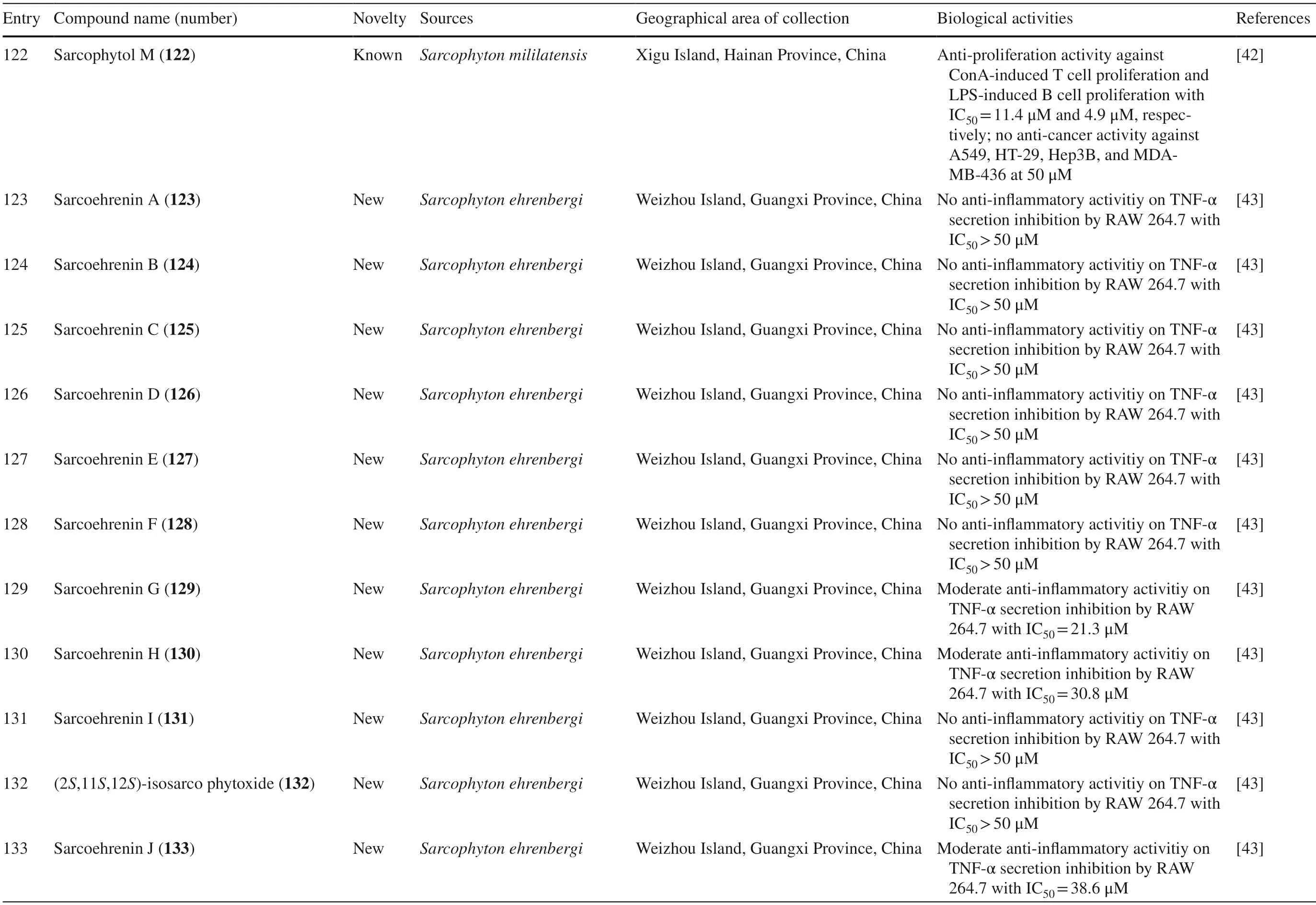
Table 1 (continued)
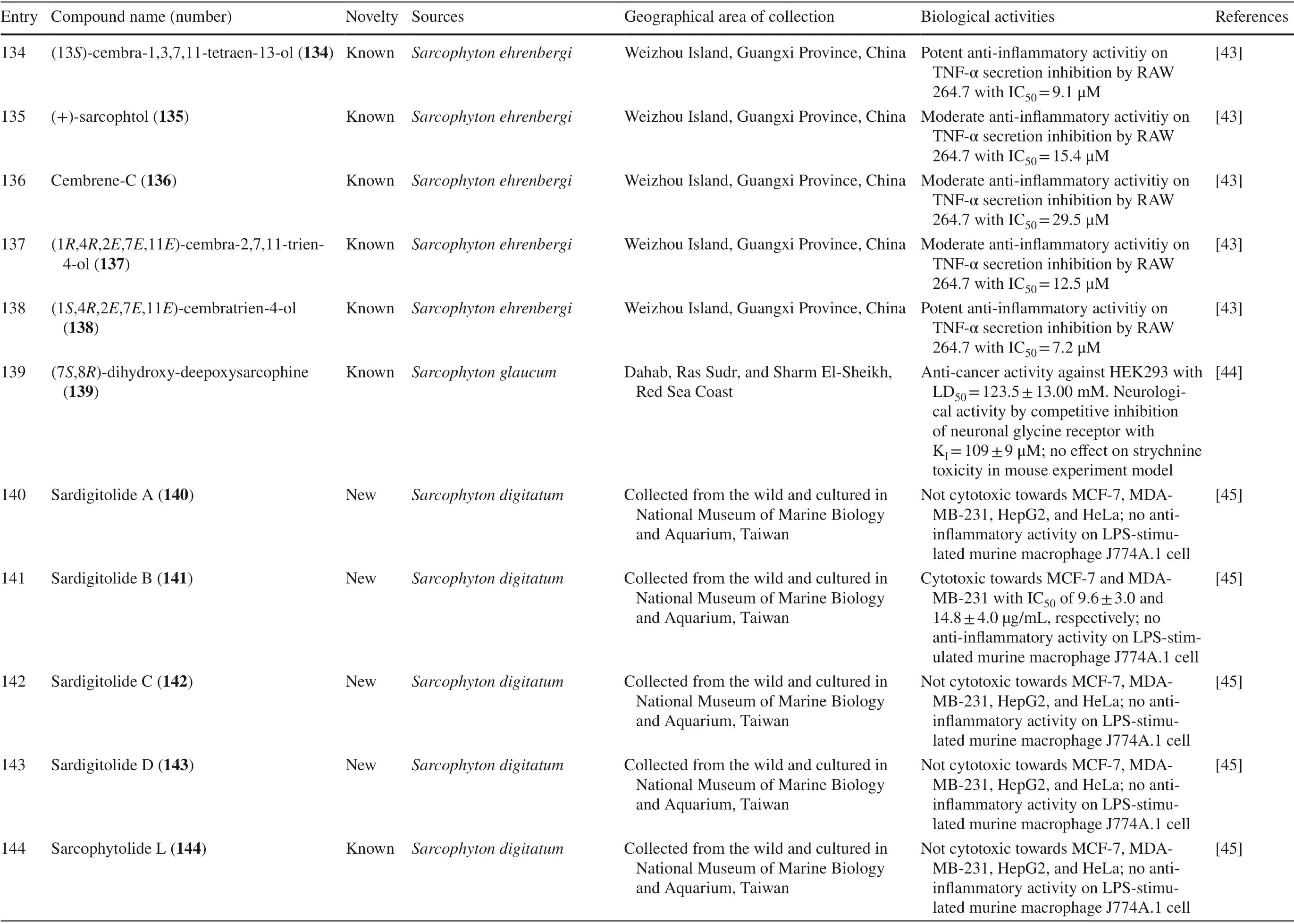
Table 1 (continued)
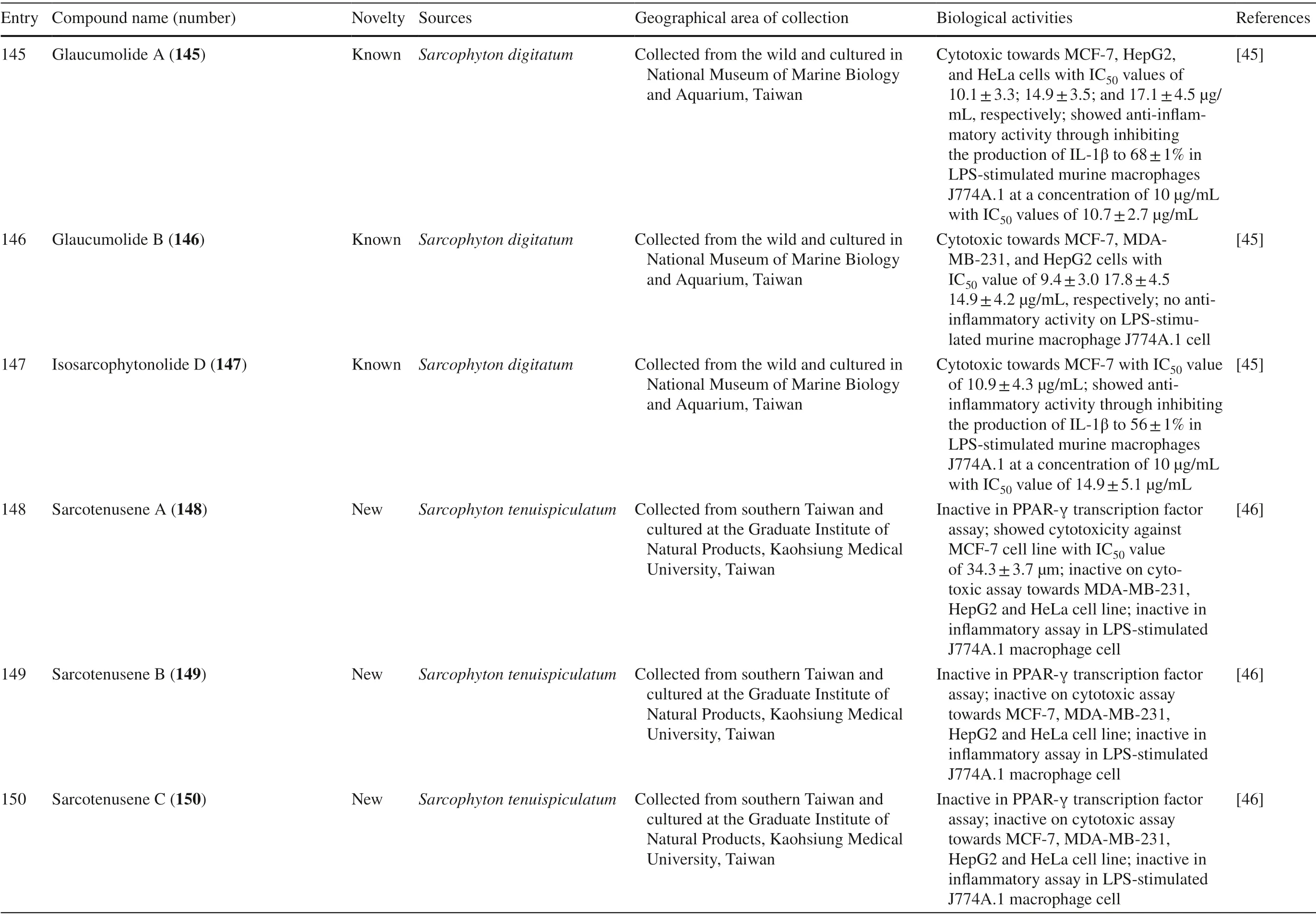
Table 1 (continued)
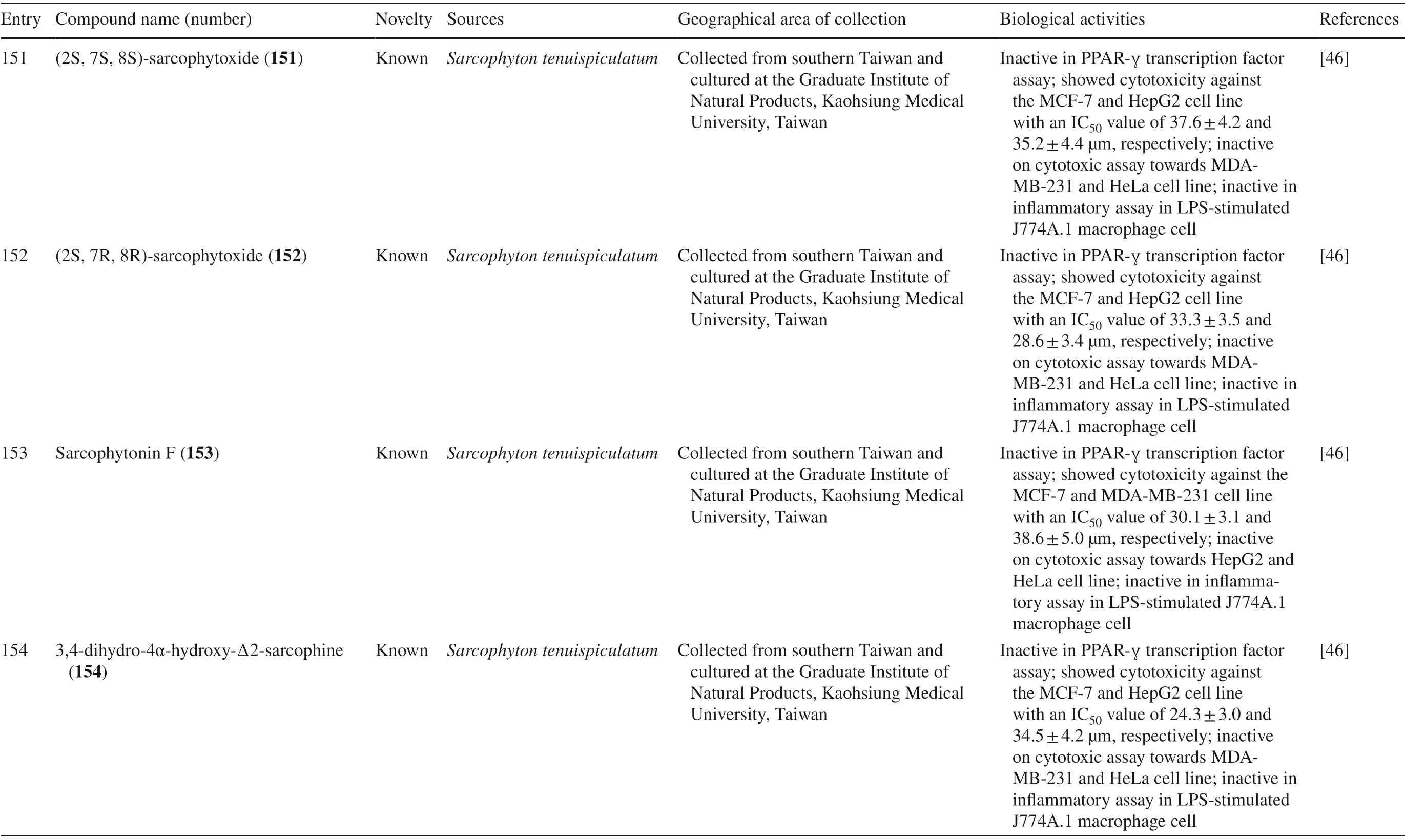
Table 1 (continued)

Table 1 (continued)

Table 1 (continued)
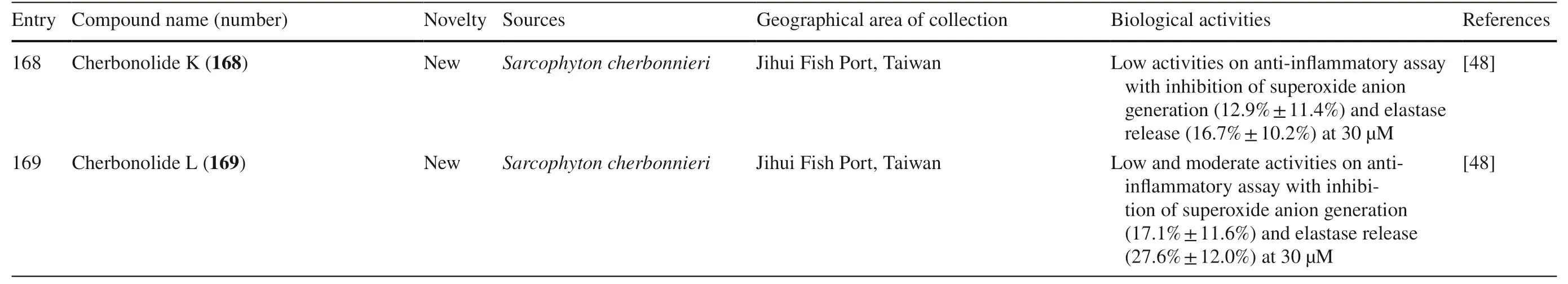
Table 1 (continued)
Cembrane diterpenes have been isolated in a number of different locations.Fresh soft coralSarcophytonsp.from Karah Island,Terengganu,West Malaysia yielded a new cembrane diterpene,16-hydroxycembra-1,3,7,11-tetraene 1 (Fig.2) [17].The compound is a colorless oil,[α]D 25:− 9.3 (c 0.18,CHCl3) with the molecular formula of C20H32O (HR-MS m/z 289.2486 [M+H]+,calcd.289.2526).A known compound cembranoid diterpene compound,sarcophytoxide 2,was isolated as yellow crystalline needles (~ 0.5% yield) from the n-hexane fraction ofSarcophyton trocheliophorumcollected in Selayar Island,South Sulawesi,Indonesia (Fig.3).This compound has a molecular formula of C20H30O2(m/z 325 [M+Na] +,ESI–MS positive ion) and been tested for its new anti-microbial activity (Table 1,entry 2) [18].Two new biscembranoid-like compounds,bissubvilides A-B 3–4 were isolated fromSacrophyton subviridein Xisha Islands,South China Sea.These compounds have been tested for their anti-cancer activity but showed no activity (Table 1,entries 3,4) [19].S.trocheliophorumfrom Yalong Bay,China,yielded three new highly oxidative cembranoids sarcophytols D-F 5–7.Unfortunately,none of them showed activities on protein tyrosine phosphatase 1B (PTP1B) inhibitory eff ect (Table 1,entries 5–7) [20].Another study isolated a new tetracyclic biscembrane hydrocarbon,trocheliane 8 (C40H58),along with two new cembranoid diterpenes,sarcotrocheldiols A-B 9–10 (C20H34O3),from the same species in Red Sea coast,Saudi Arabia [21].These cembranoids were isolated as gummy materials with m/z of 538.4528 (M +,HREIMS) and 322.2500 (M +,HREIMS),respectively.
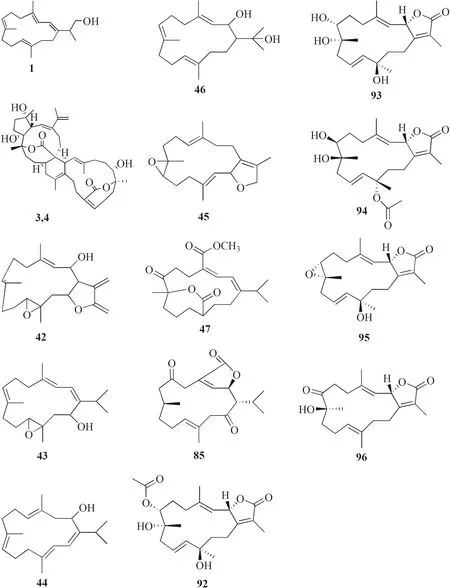
Fig.2 Cembranoids isolated from Sarchophyton sp.(1,42–47,85,92–96) and Sarcophyton subviride (3,4)
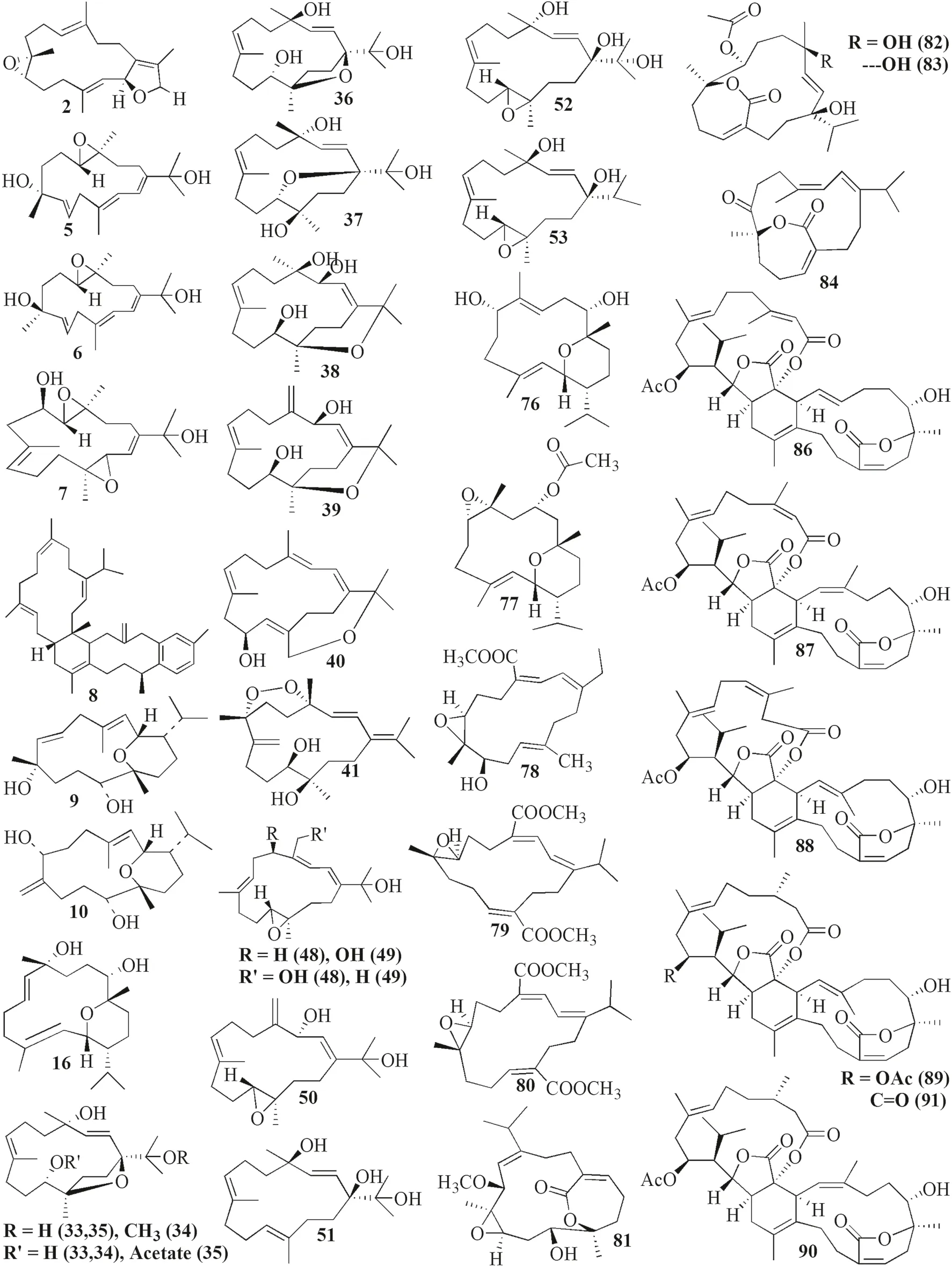
Fig.3 Cembranoids isolated from Sarchophyton trocheliophorum
Five new compounds,sarcophytonoxides A-E 11–15 were isolated fromSarcophyton ehrenbergiin North Reed (Beijiao) in the Xisha Islands,South China Sea (Fig.4).HRESIMS analysis revealed sarcophytonoxides A,C and E are isomers with the molecular formula of C22H32O 4 .Meanwhile,sarcophytonoxides B and D have molecular formula of C22H32O5and C20H30O3,respectively.However,these compounds have been tested for anti-cancer activity against human ovarian cancer cell line A2780,however,they showed no eff ect (Table 1,entries 11–15) [22].A new pyrane-based cembranoid diterpene,9-hydroxy-10,11-dehydro-sarcotrocheliol 16,was isolated fromS.trocheliophorum.However,this compound showed no anti-bacterial activity against multiple microorganisms (Table 1,entry 16) [23].A study isolated two novel biscembranoids,sarelengans A-B 17– 18,along with fi ve new cembranoids,sarelengans C-G 19–23 fromSarcophyton elegansin Xisha Islands,South China Sea with only 18 and 19 exhibited anti-infl ammatory activity (Table 1,entries 17–23) [24].S.ehrenbergifrom the Egyptian Red Sea off the coast of Hurghada yielded three novel cembrene diterpenoids sarcoehrenbergilids A-C 24–26 [25].Sarcoehrenbergilids A was found as a white crystal with a molecular formula of C21H32O5(m/z at [M+Na] + of 387.2142) while Sarcoehrenbergilids B and C were isomers observed as a white powder with a molecular formula of C20H30O5(m/z at [M+Na] + of 373.1986).Another speciesSarcophyton glaucomfrom the same area was reported to yields fi ve new diterpenes,sarcophinone 27,8-epi-sarcophinone 28,(+)-7α,8β-dihydroxydeepoxysarcophine 29,sinumaximol G 30,and sarcophine 31 [26].Several new cembranoids,(+)-(1E,3E,11E)-7,8-epoxycembra-1,3,11,15-tetraene 32 fromSarcophyton stellatumin Inner reef of Mahambo,Tamatave,Madagascar [27],and sarcophytrols M-U 33–41 fromS.trocheliophorumin Yalong Bay,Hainan Province,China [28],was discovered but their activities have not been tested (Table 1,entries 32–41).

Fig.4 Cembranoids isolated from Sarchophyton ehrenbergi
A study reported a new cembranoid,2-hydroxy-crassocolide E 42,and five known cembranoids,sarcophytoxide 43,sarcassin E 44,3,7,11cembreriene2,15diol 45,11,12epoxy sarcophytol A 46,and sarcophytol A 47 fromSarcophytonsp.in the western side of Mahengtang Island,Indonesia,with newly discovered anti-cancer activities against breast cancer MSF-7 (IC50< 30 mg/L) (Table 1,entries 42–47) [29].Six new cembranoids related to 33–41,Sarcophytrols G-L 48–53 was also isolated fromS.trocheliophorumfrom Yalong Bay,Hainan Province,China (Fig.3).These compounds were tested for their inhibitory activity against PTP1B but showed no eff ect (Table 1,entries 48–53) [30].Eight novel cembranetype diterpenoids were also discovered fromSarcophyton mililatensisisolated from Guangxi Autonomous Region,China,namely (+)-(6R)-6-hydroxyisosarcophytoxide 54,(+)-(6R)-6-acetoxyisosarcophytoxide 55,(+)-17-hydroxyisosarcophytoxide 56,sarcomililatins A-D 57–60,and sarcomililatol 61.Most of these compounds did not exhibit anti-cancer and anti-inflammatory activities,except for 57,which showed a moderate anti-infl ammatory activity (Table 1,entries 54–61).Along with these newly discovered compounds,a known compound (+)-isosarcophytoxide 62 was also isolated and reported to have strong anti-cancer and moderate anti-infl ammatory activity (Table 1,entry 62) [31].
Sarcophyton stellatumfrom the coast of Dongsha Atoll,Taiwan,was reported to yield seven new cembrane-based diterpenoids,stellatumolides A-C 63–65,stellatumonins A-B 66–67,and stellatumonone 68 (Fig.5).Unfortunately,none of these compounds was found to have anti-cancer activity as tested (Table 1,entries 63–68) [33].Within the same country,more precisely in Jihui Fish Port,a study reported fi ve new cembranoids,cherbonolides A-E 69–73,a biscembranoid peroxide,bischerbolide peroxide 74,and a known cembranoid,isosarcophine 75,fromSarcophyton cherbonnieri[34].
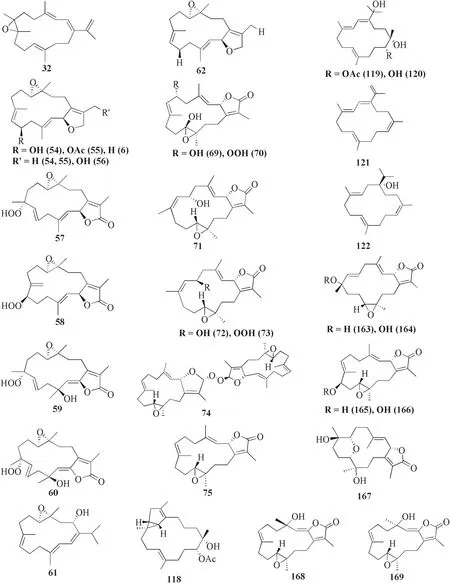
Fig.5 Cembranoids isolated from Sarcophyton stellatum (32),Sarcophyton mililatensis (54–62,118–122),Sarcophyton cherbonnieri (69–75,163–169)
Several studies isolated new compounds as well as known compounds with newly discovered biological activities fromS.trocheliophorumin three different locations.From near Mahmieat of the Red Sea,Hurghada,Eas Egypt,two new pyrane-based cembrane diterpenoids 9-hydroxy-7,8-dehydro-sarcotrocheliol 76,and 8,9-expoy-sarcotrocheliol acetate 77,were tested for their antibacterial activity but were proved as inactive (Table 1,entries 76,77) [35].From Yalong Bay,Hainan Province,China,six new highly oxidative cembranoids were discovered,sarcophytonolides S-U 78–80,and sartrolides 81–83.These new compounds were tested for their anti-diabetic activity along with a known compound sarcophytolide 84,but only 81 and 84 possessed the activity (Table 1,entries 78–84) [36].A known biscembranoid,glaucumolide A 86,together with fi ve new biscembranoids,bistrochelides A-E 87–91,were isolated from this species in Xisha Islands in the South China Sea.Following testing for their immunological activities,86–89 were found to aff ect T-lymphocyte proliferation and differentiation,while 90–91 lacked this activity (Table 1,entries 86–91) [38].
A new cembranolide diterpene with anti-fungal activity,sarcophytonolide V 85,was discovered fromSarcophytonsp.in Sepanggar Bay,North Borneo [37].In the Egyptian Red Sea off the coast of Hurghada,a study onSarcophytonsp.also isolated fi ve new cembrane-type diterpenoids with moderate anti-cancer activity,namely 7-acetyl-8-episinumaximol G 92,8-epi-sinumaximol-G 93,12-acetyl-7,12-epi-sinumaximol G 94,12-hydroxysarcoph-10-ene 95,and 8-hydroxy-epi-sarcophinone 96 (Table 1,entries 92–96) [32].A study onS.ehrenbergifrom South China Sea reported fi ve new cembranoids,sarcoehrenolides A-E 97–101,and a known cembranoid,ehrenbergol D 102.Compound 99 has not been tested for its biological activities,while the others were tested for their anti-cancer properties but were found to be inactive (Table 1,entries 97–101).Most of these compounds have anti-infl ammatory activity,except for 101 [39].Another study on the same species from the Egyptian Red Sea off the coast of Hurghada isolated three known cembrene diterpenoids,sarcoehrenbergilids D-F 103–105,which were reported to have anti-cancer activities (Table 1,entries,103–105) [40].
Sarcophyton glaucumfrom Xisha Islands of the South China Sea was reported to yield nine new cembrane diterpenes,sarcoglaucins A-I 106–114,along with three known analogues,trochelioid 115,7α-hydroxy-8(19)-deepoxysarcophine 116,and (−)-sartrochine 117 (Fig.6).None of them possessed anti-cancer and anti-bacterial activities (Table 1,entries 106–114) [41].A new diterpenoid,sarcomililate A 118,two new cembranoids,sarcomililatols A-B 119–120,and two known related diterpenoids,yalogene A 121 and sarcophytol M 122,were isolated fromSarcophyton mililatensisin Xigu Island,Hainan Province,China.Most of them were active as an anti-cancer agent,except for 120 (Table 1,entries 119–121) [42].

Fig.6 Cembranoids isolated from Sarchophyton elegans (17–23,and Sarchophyton glaucum (27–31,106–117,139)
Another study onS.ehrenbergifrom Weizhou Island,Guangxi Province,China,isolated eleven new cembrane diterpenes,sarcoehrenins A-I 123–131,(2S,11S,12S)-isosarcophytoxide 132,and sarcoehrenin J 133.These compounds were tested for their anti-infl ammatory potentials; however,only 129 and 130 were active.In addition,this study also discovered new anti-infl ammatory activity on fi ve known compounds within the same species,13S)-cembra-1,3,7,11-tetraen-13-ol 134,(+)-sarcophtol 135,cembrene-C 136,(1R,4R,2E,7E,11E)-cembra-2,7,11-trien-4-ol 137,and (1S,4R,2E,7E,11E)-cembratrien-4-ol 138 (Table 1,entries 123–138) [43].Lastly,a known trans-diol derivative of sarcophine,(7S,8R)-dihydroxy-deepoxysarcophine 139 was isolated fromS.glaucumin Dabah,Ras Sudr,and Sharm El-Sheikh,Red Sea Coast,and revealed that 139 exhibited anti-cancer and neurological activities (Table 1,entry 139) [44].
Furthermore,S.digitatumwhich cultured in the National Museum of Marine Biology and Aquarium,Taiwan contained seven biscembranoids and one cembranoid.Four out of seven biscembranoids were unreported compounds namely sardigitolides A-D 140–143 (Fig.7).The other three biscembranoids were reported before and namely sarcophytolide L 144 and glaucumolides A-B 145–146.The only known cembranoid collected from this species namely isosarcophytonolide D 147.The reported cembrane-type diterpenoid from S.digitatumwas reported to display various anti-cancer and anti-inflammatory activities [45].Another study reported nine cembranoids fromSarcophyton tenuispiculatumwhich culture at Kaohsiung Medical University,Taiwan.The three novel cembranoids sarcotenusenes A-C 148–150 were mostly inactive in PPAR-transcription factor assay; cytotoxic assay towards MCF-7,MDA-MB-231,HepG2 and HeLa cell line; and inflammatory assay.Moreover,(2S,7S,8S)-sarcophytoxide 151,(2S,7R,8R)-sarcophytoxide 152,sarcophytonin F 153,3,4-dihydro-4α-hydroxy-∆2-sarcophine 154,A hydroperoxide obtained by autoxidation of dihydrofuranocembranoid 155,and (+)-7α,8βdihydroxydeepoxysarcophine 156 were also displayed various results on the abovementioned assay [46].Additionaly,six cembranoids were isolated from S.roseumcollected in Dahab,Red Sea,Egypt.The new cembranoid sarcoroseolides A-D 157–160 and the known cembranoid 2-epi-sarcophine 161 and 2R,7R,8R-dihydroxydeepoxysarcophine 162 were being assessed for its anti-infl ammatory and anti-cancer activities [47].Lastly,Sarcophyton cherbonniericollected from Jihui Fish Port,Taiwan,contained seven novel cembranoid that possessed various anti-infl ammatory activities through inhibition of superoxide anion generation and elastase release,namely cherbonolides F-L 163–169 [48].
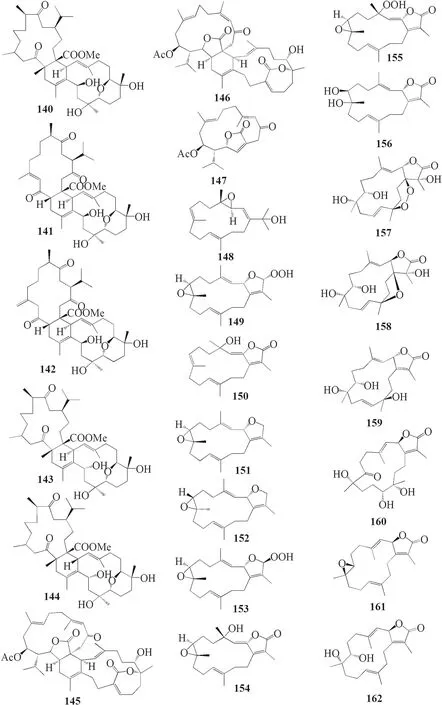
Fig.7 Cembranoids isolated from Sarchophyton digitatum (140–147),Sarchophyton tenuispiculatum (148–156) and Sarchophyton roseum (157–162)
2.2 Cembranoids from Genus Sinularia
The present study reported 42 cembranoid compounds isolated fromSinulariasp.collected from various geographical areas (Fig.8).Twenty-nine of those were new compounds and the other 13 were previously known compounds with newly discovered activities.One of the new compounds was newly discovered and had not been thoroughly tested for their biological activities.

Fig.8 Cembranoids reported from Sinularia erecta (170–172),Sinularia gravis (173),Sinularia nanolobata (174–177),Sinularia compacta (178–180),Sinularia sandensis (181),Sinularia sp.(182–183,192–194,400–203),Sinularia fl exibilis (184–191,195–199) and Sinularia scabra (204–211)
Soft coralSinularia erectafrom the South China Sea yielded three new norcembranoids,sinulerectols A-C 170–172 [49],whereas a new-non tested cembranoid diterpene named isodecaryiol 173 was collected from MadagascarSinularia gravis[50].Three new non-active cembranoids from Taiwan were isolated from S.nanolobatanamely nanolobols A-C 174–176 along with one known biologically active cembranoid sinulariol C 177 [51].Sinularia compactafrom the South China Sea contained three new cembranoid diterpenes namely 5-epi-sinuleptolide 178,michaolide F 179,and 20-acetylsinularolide B 180 [53].S.sandensiswas reported to produce a known compound 7-acetylsinumaximol B 181 [55].Kamada et al.isolatedSinulariasp.from Sabah,Malaysia and discovered a new cembranoid named sinularolide F 182 and a known cembranoid named denticulatolide 183 [56].Taiwanese S.fl exibilisproduced seven compounds,three of which were new compounds with no biological activities named fl exibilisins D-E 184–185 and fl exibilisolide H 186 (Table 2,entries 17–19).The other four compounds were known compounds with various biological activities,namely 11-dehydrosinulariolide 187¸ 11-episinulariolide acetate 188,(S)-14-deoxycrassin 189,and sinulariolide 190 [57].
Table 2 The biological activities of cembranoid isolates from genera Sinularia
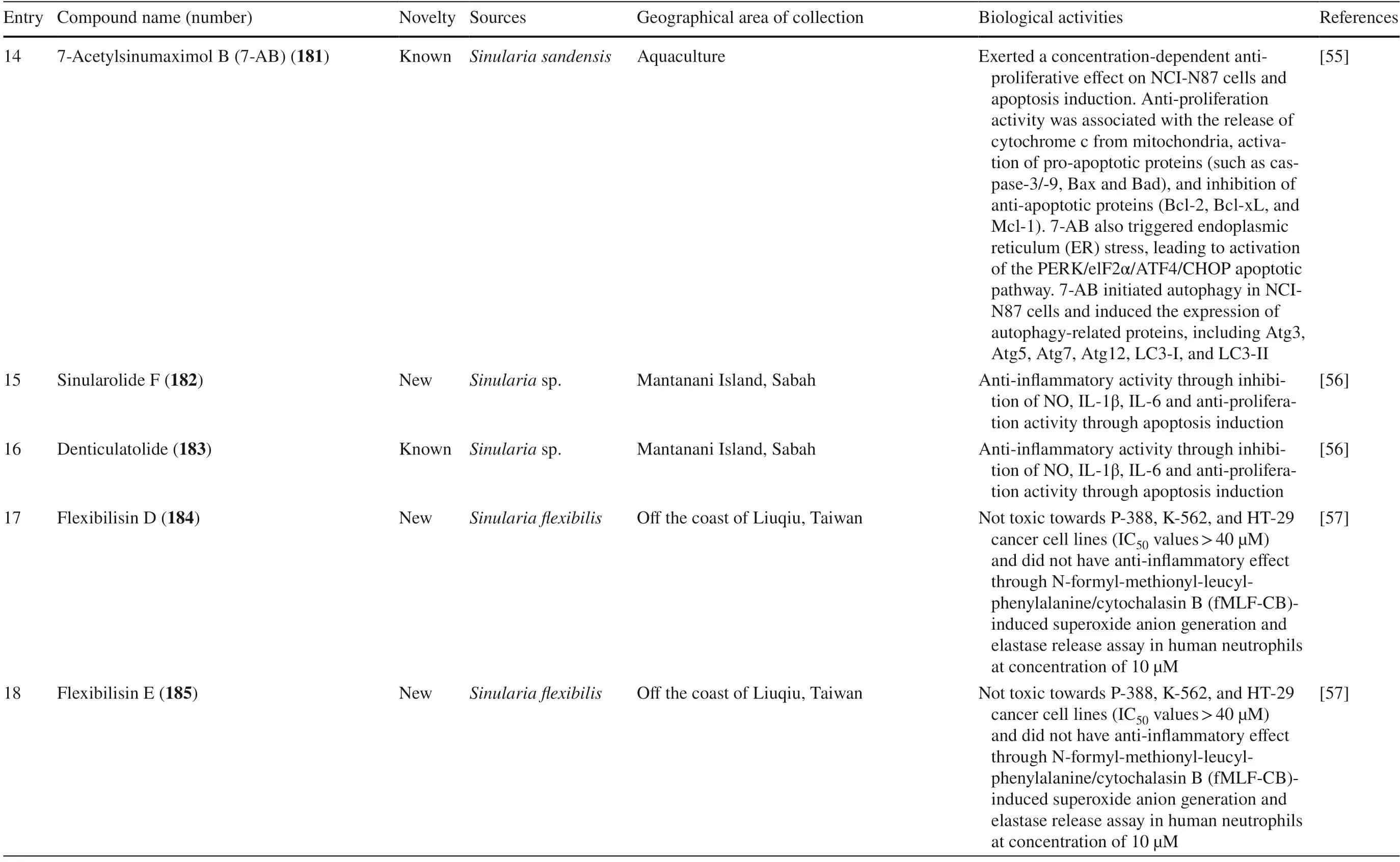
Table 2 (continued)
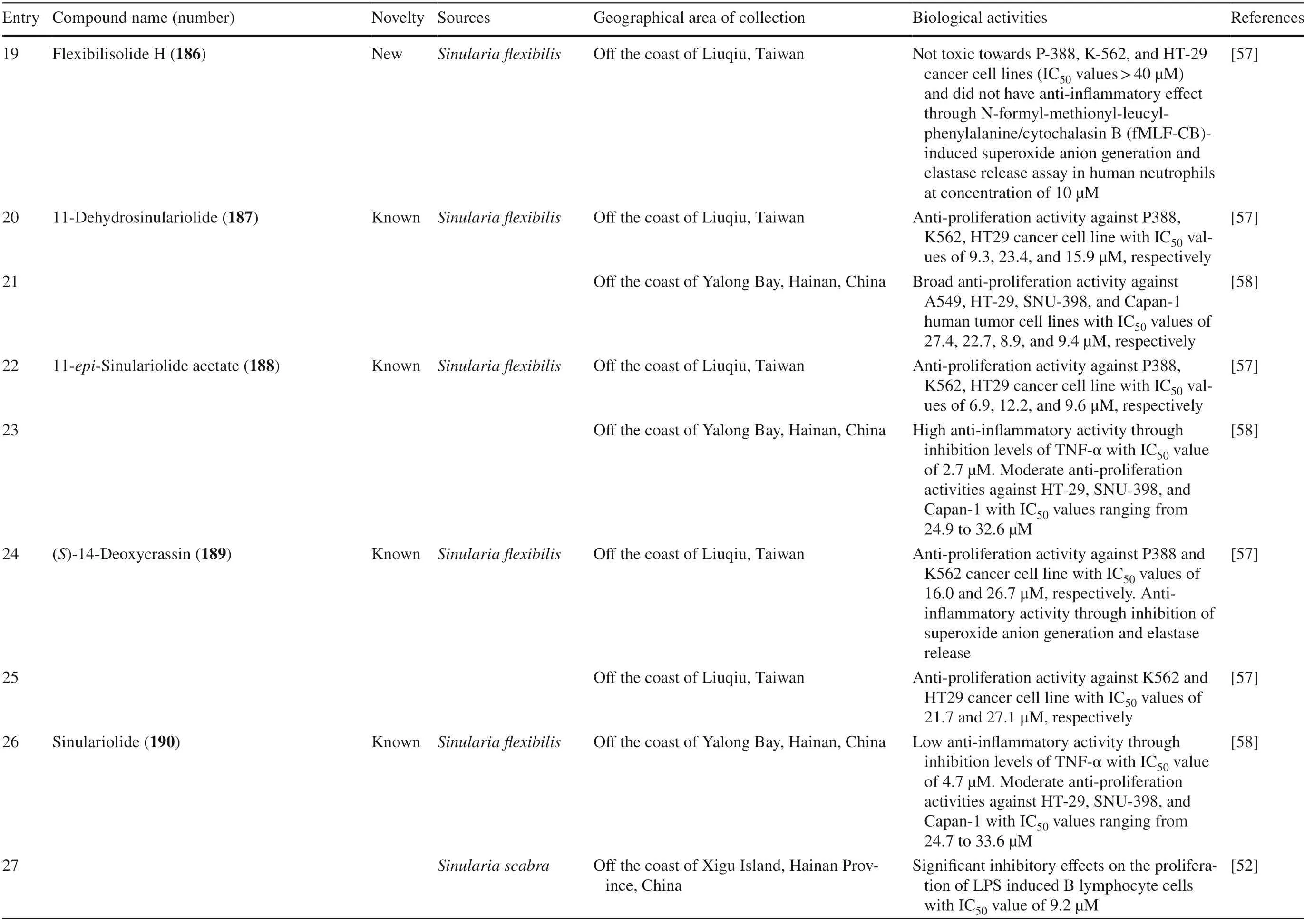
Table 2 (continued)
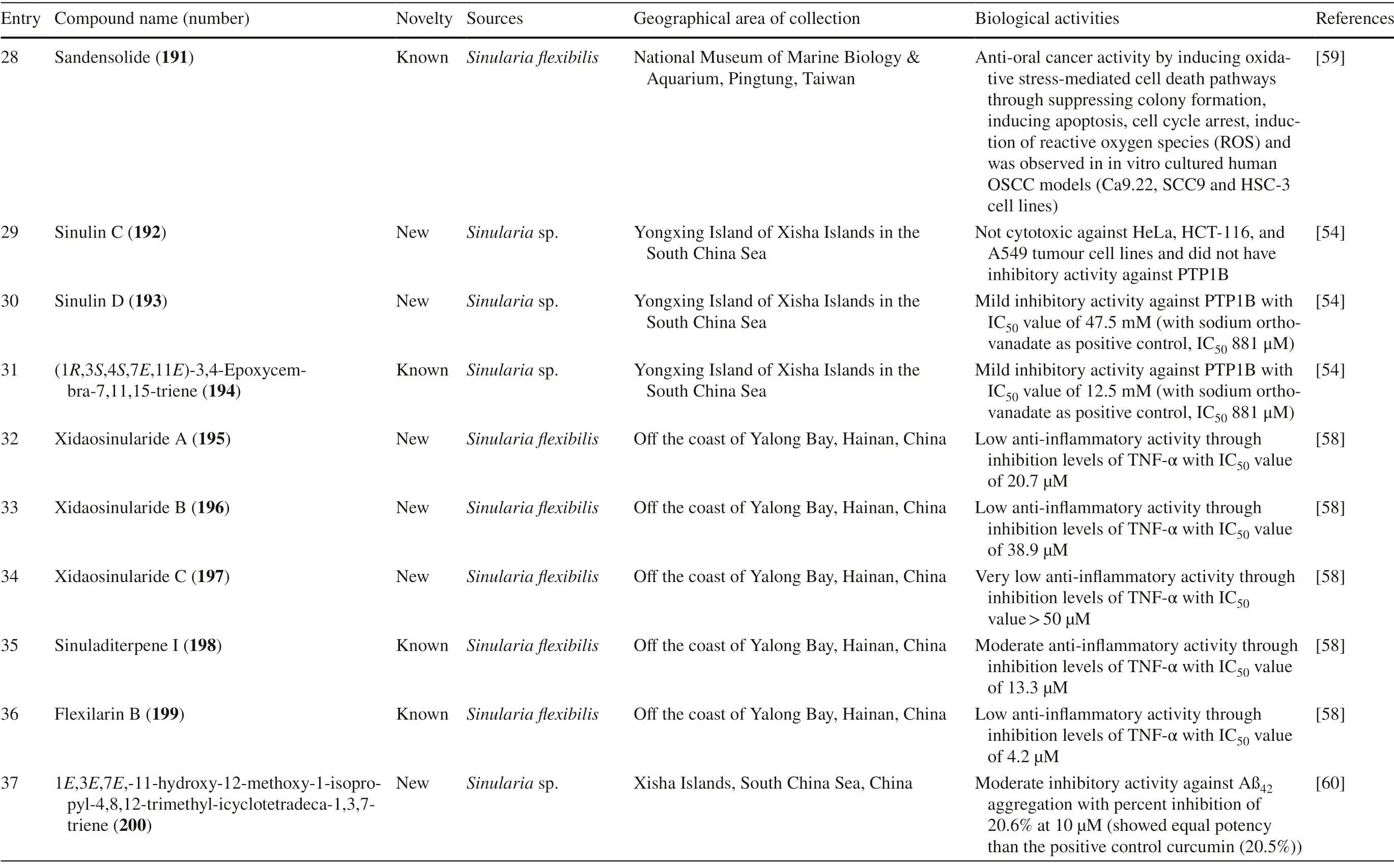
Table 2 (continued)
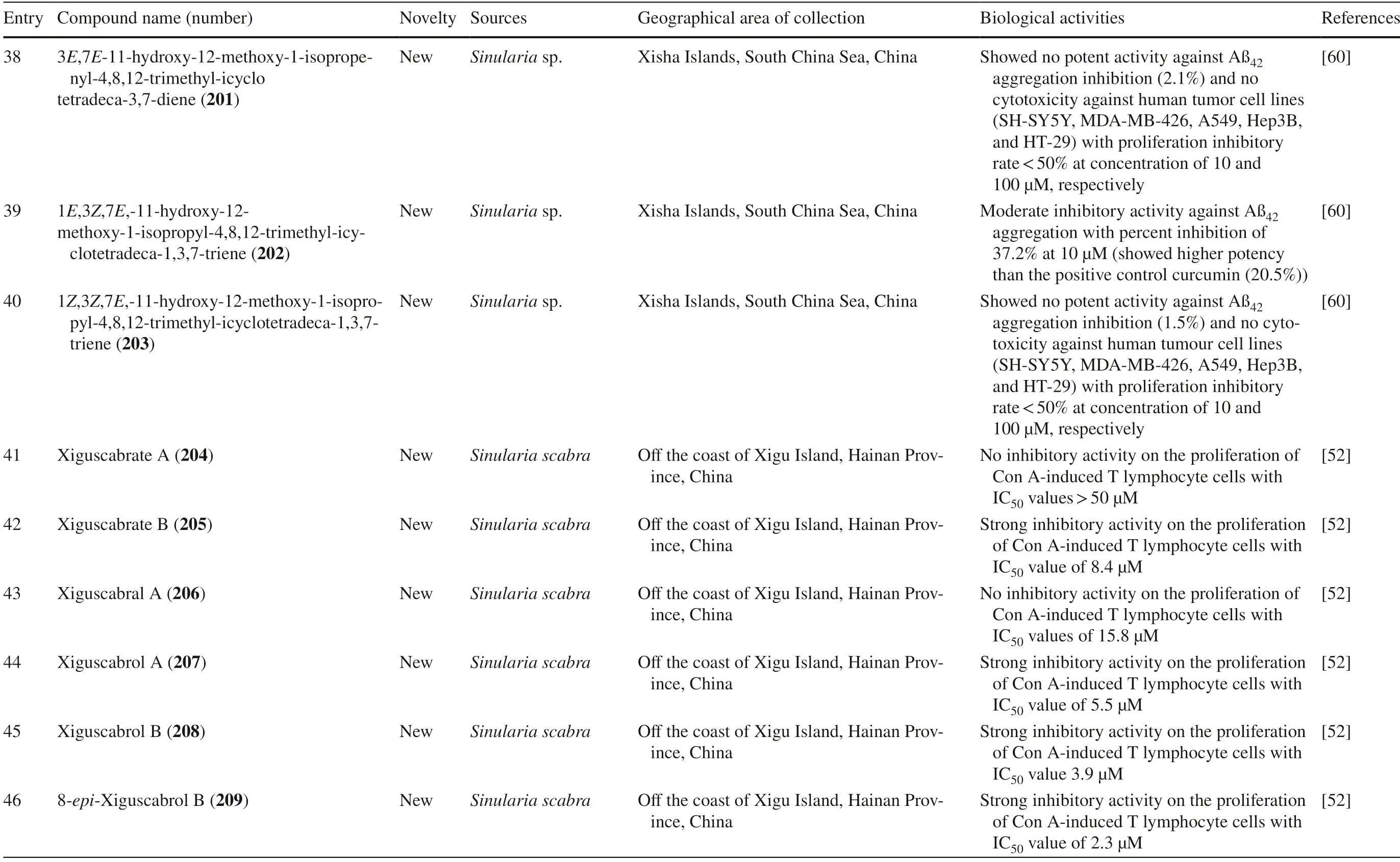
Table 2 (continued)
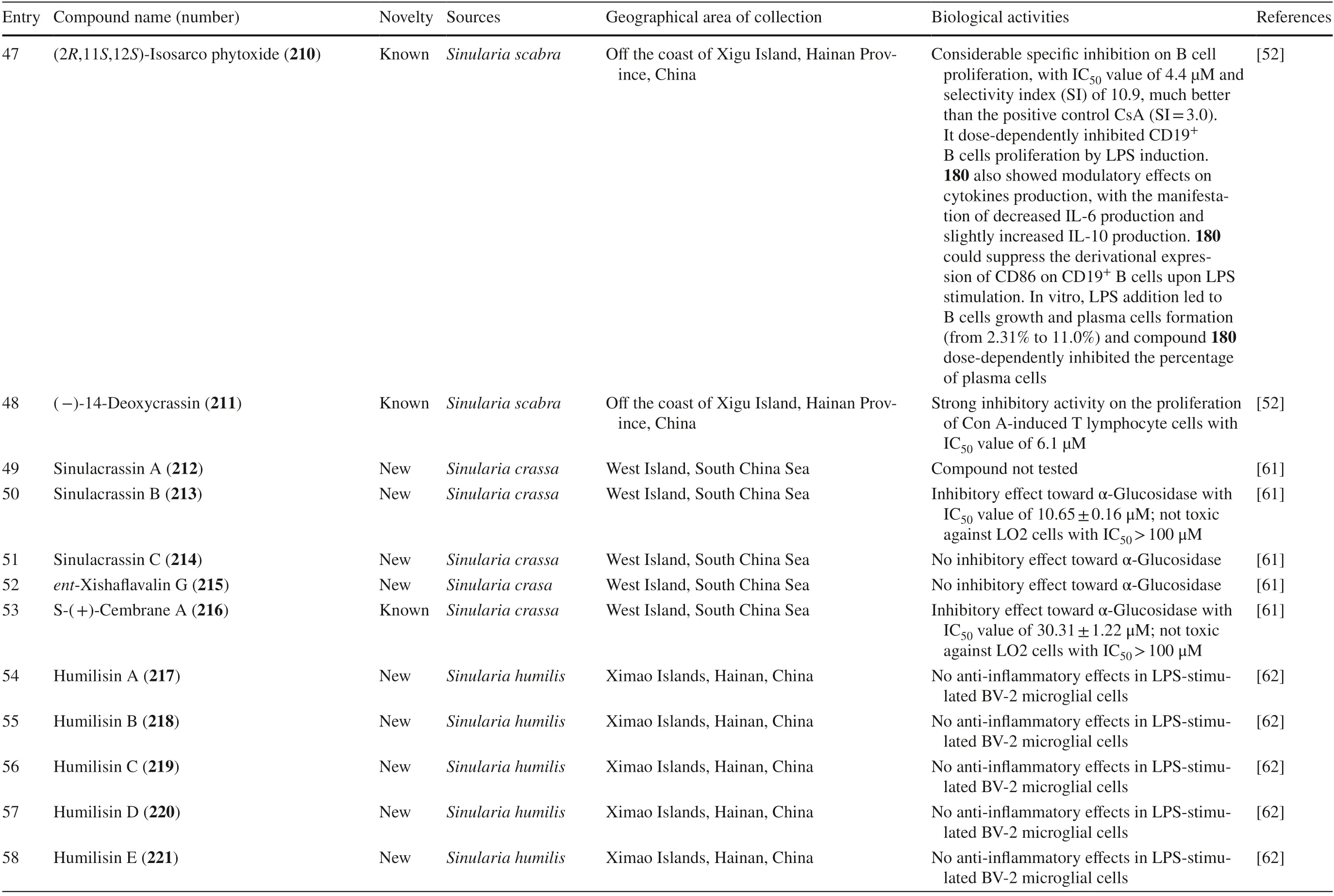
Table 2 (continued)

Table 2 (continued)
A known cembrane,sandensolide 191 was isolated from aquacultured S.fl exibilisin Pingtung,Taiwan [59].Qin et al.isolated two new and two known compounds from ChineseSinulariasp.,named sinulins C-D 192–193 and 5-epi-sinuleptolide 178,(1R,3S,4S,7E,11E)-3,4-epoxycembra-7,11,15-triene 194,with 192 being reported as not showing any biological activity as tested (Table 2,entries 29–31) [54].Eight cembranoids were isolated from ChineseS.fl exibilis,three of which were newly discovered.The three new compounds were categorized as polyoxygenated cembranoids (or fl exibilide-like cembranoids) and named xidaosinularides A-C 165–167.The known compounds were categorized as polyoxygenated cembranoids and included 11-dehydrosinulariolide 187,11-epi-sinulariolide acetate 188,sinulariolide 190,sinuladiterpene I 198,and fl exilarin B 199 [58] Tables 3 and 4.
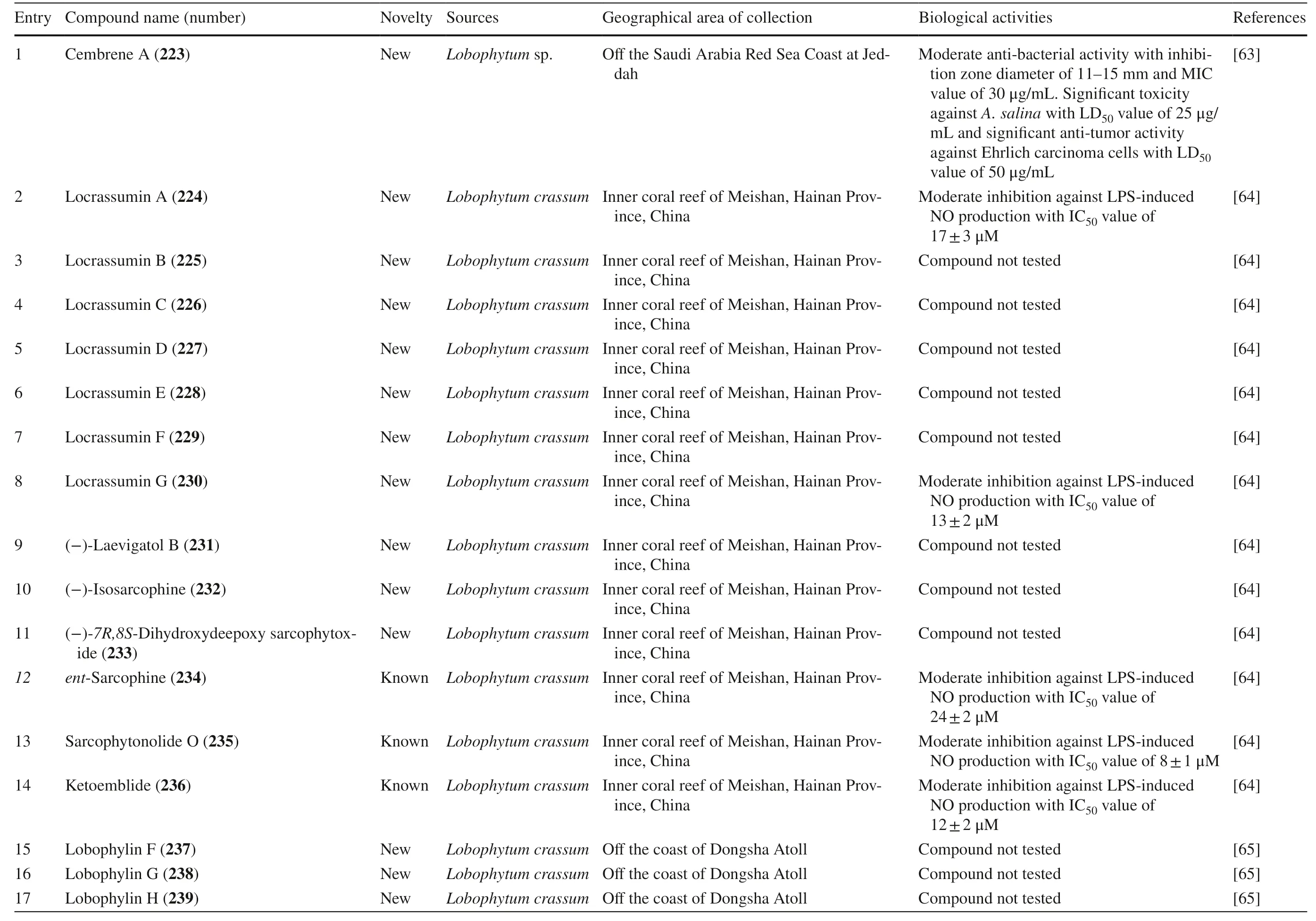
Table 3 The biological activities of cembranoid isolates from genera Lobophytum
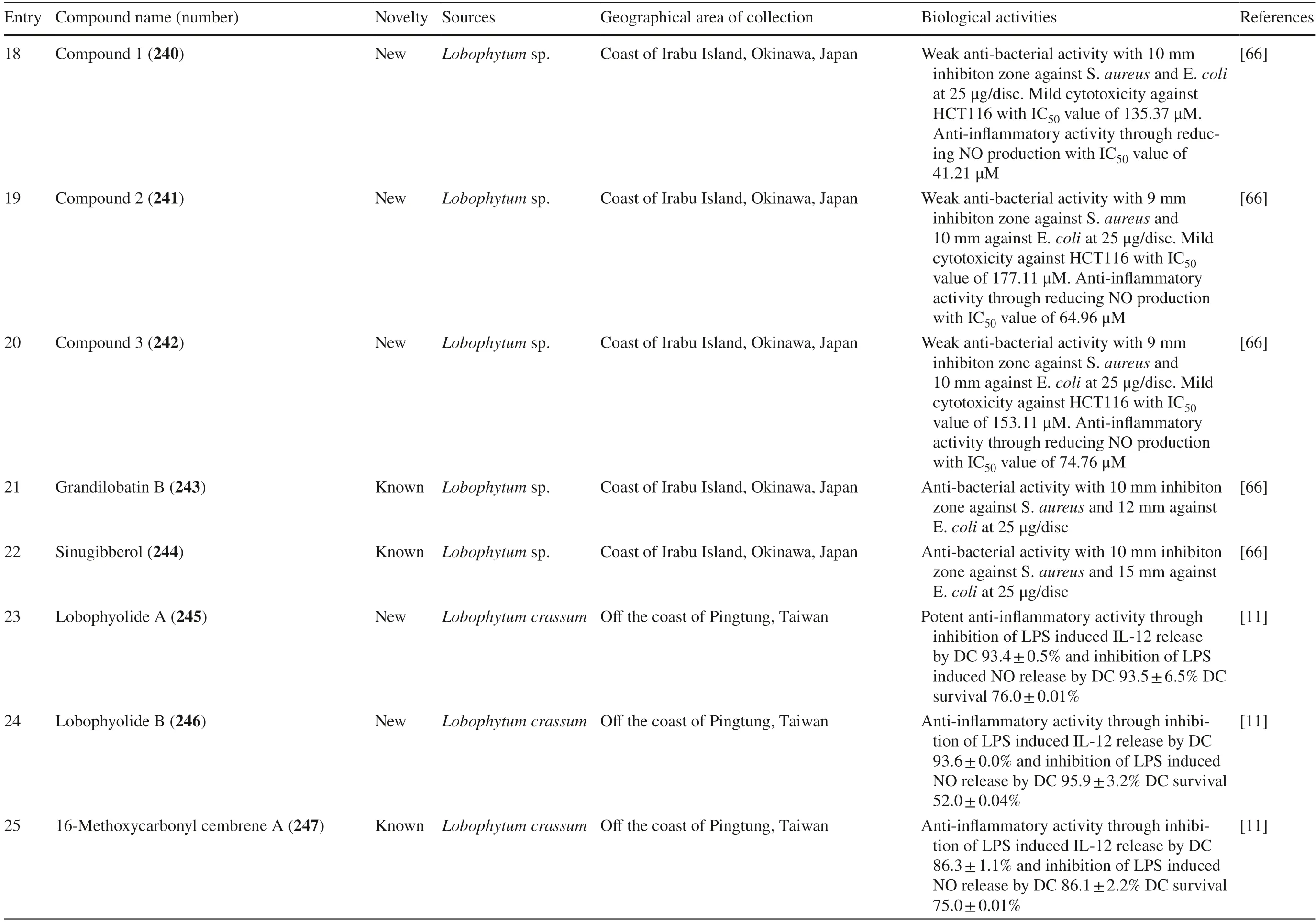
Table 3 (continued)
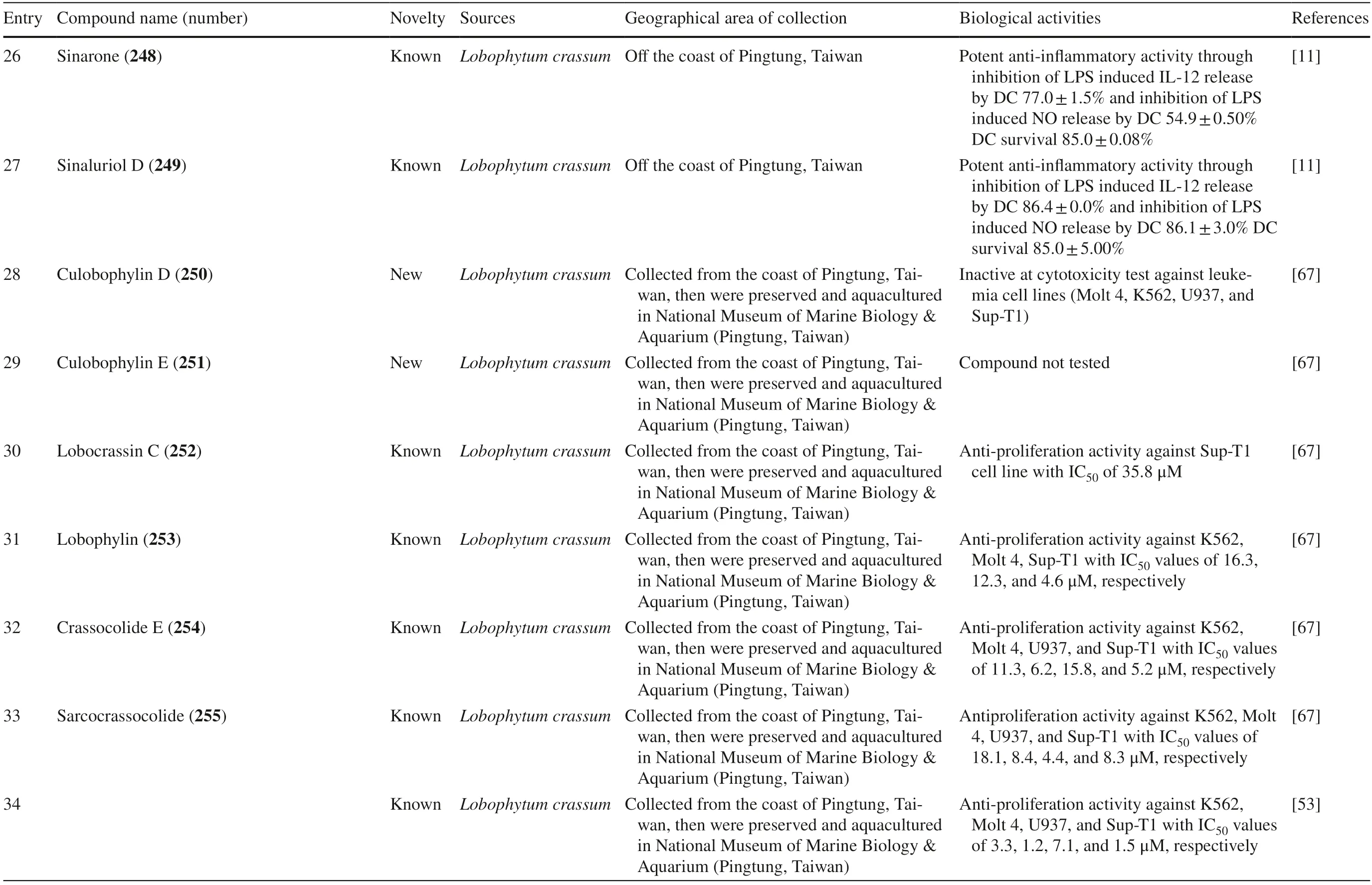
Table 3 (continued)
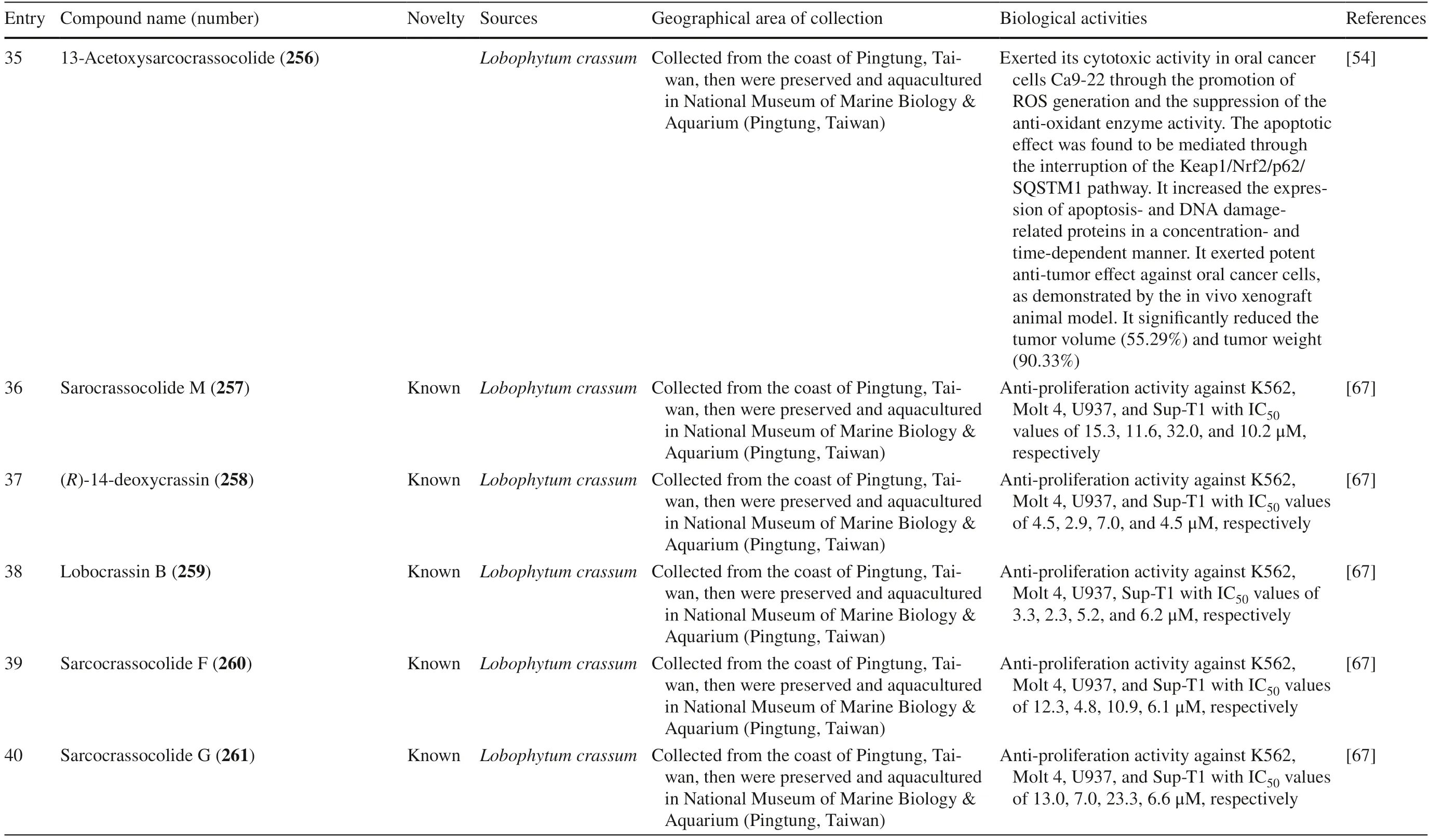
Table 3 (continued)
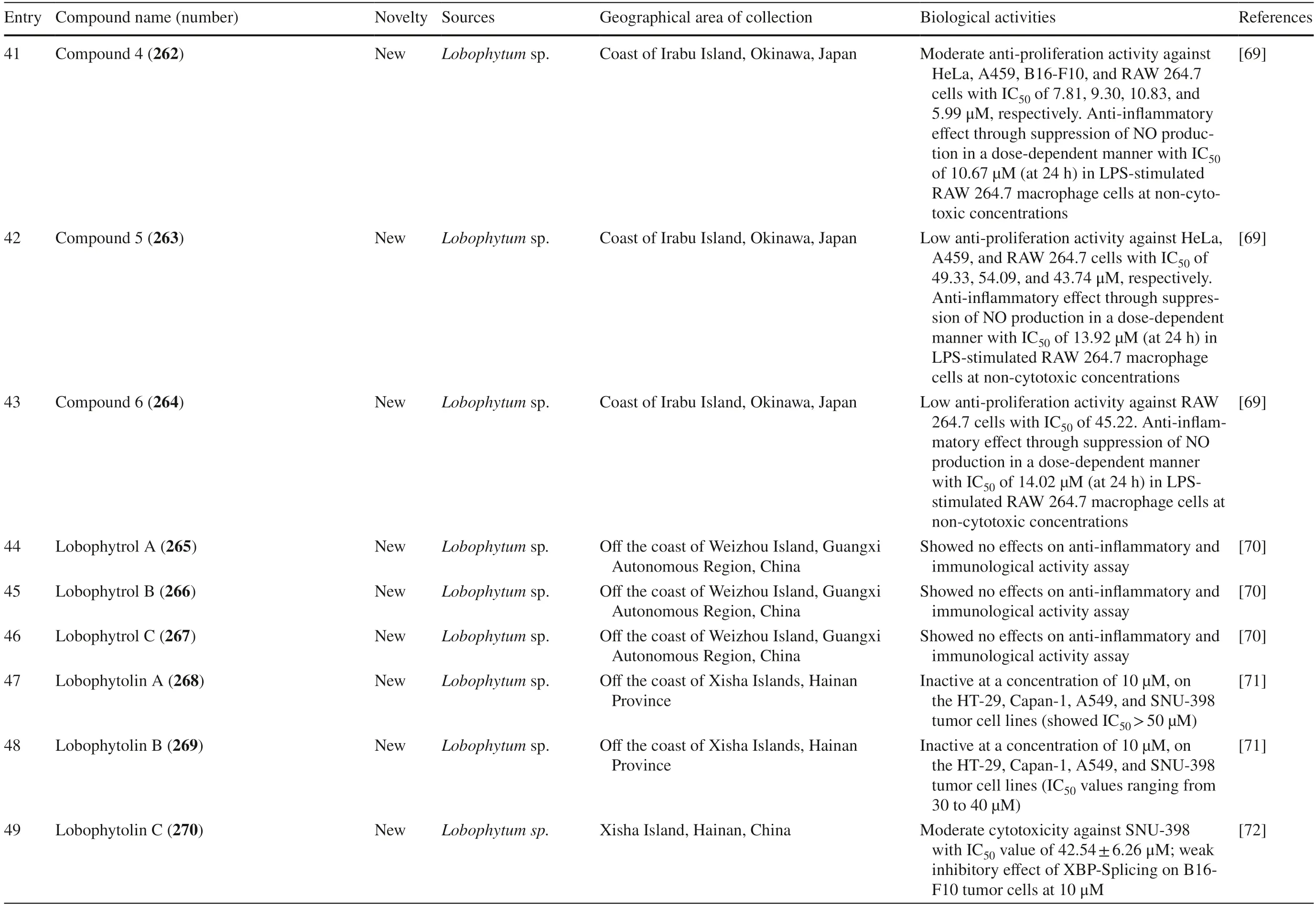
Table 3 (continued)
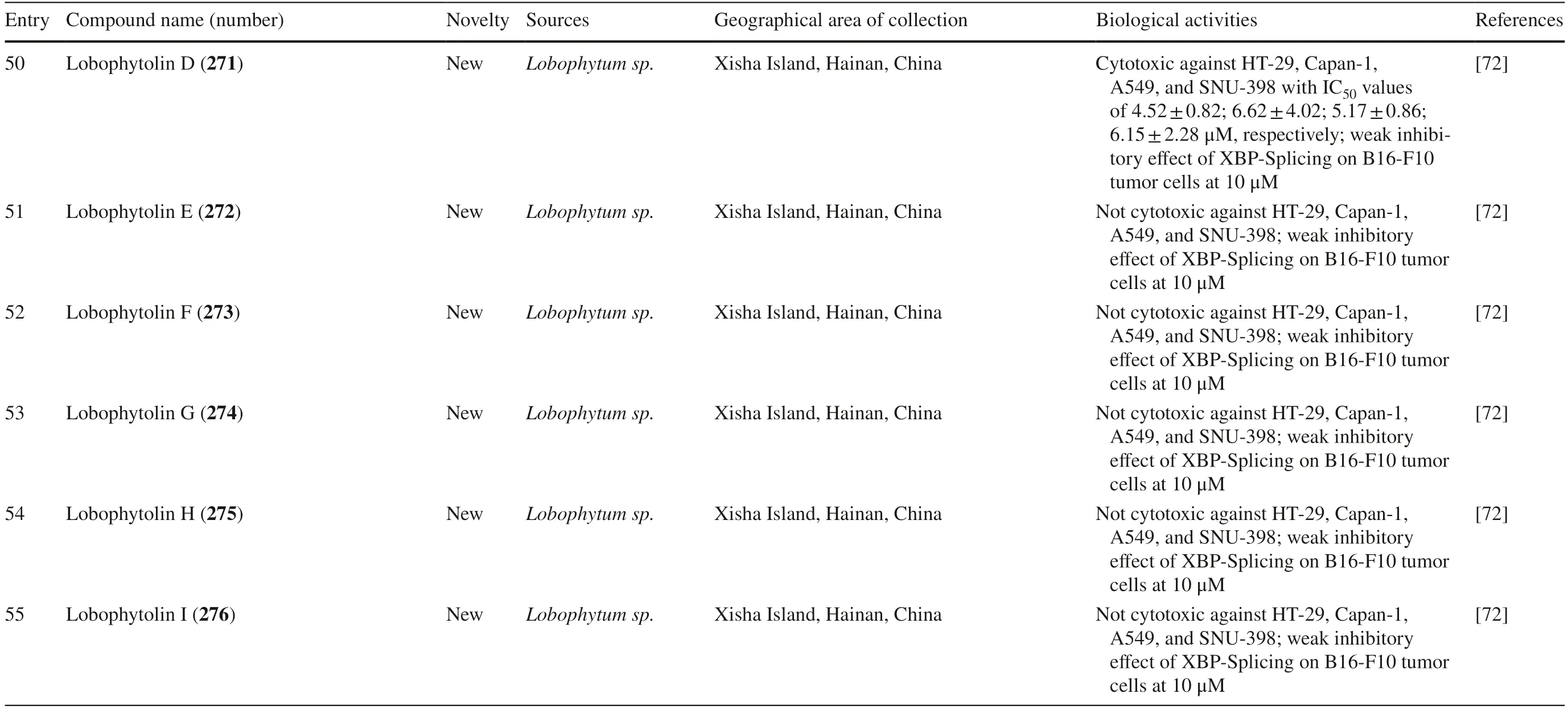
Table 3 (continued)

Table 4 The biological activities of cembranoid isolates from other soft coral species
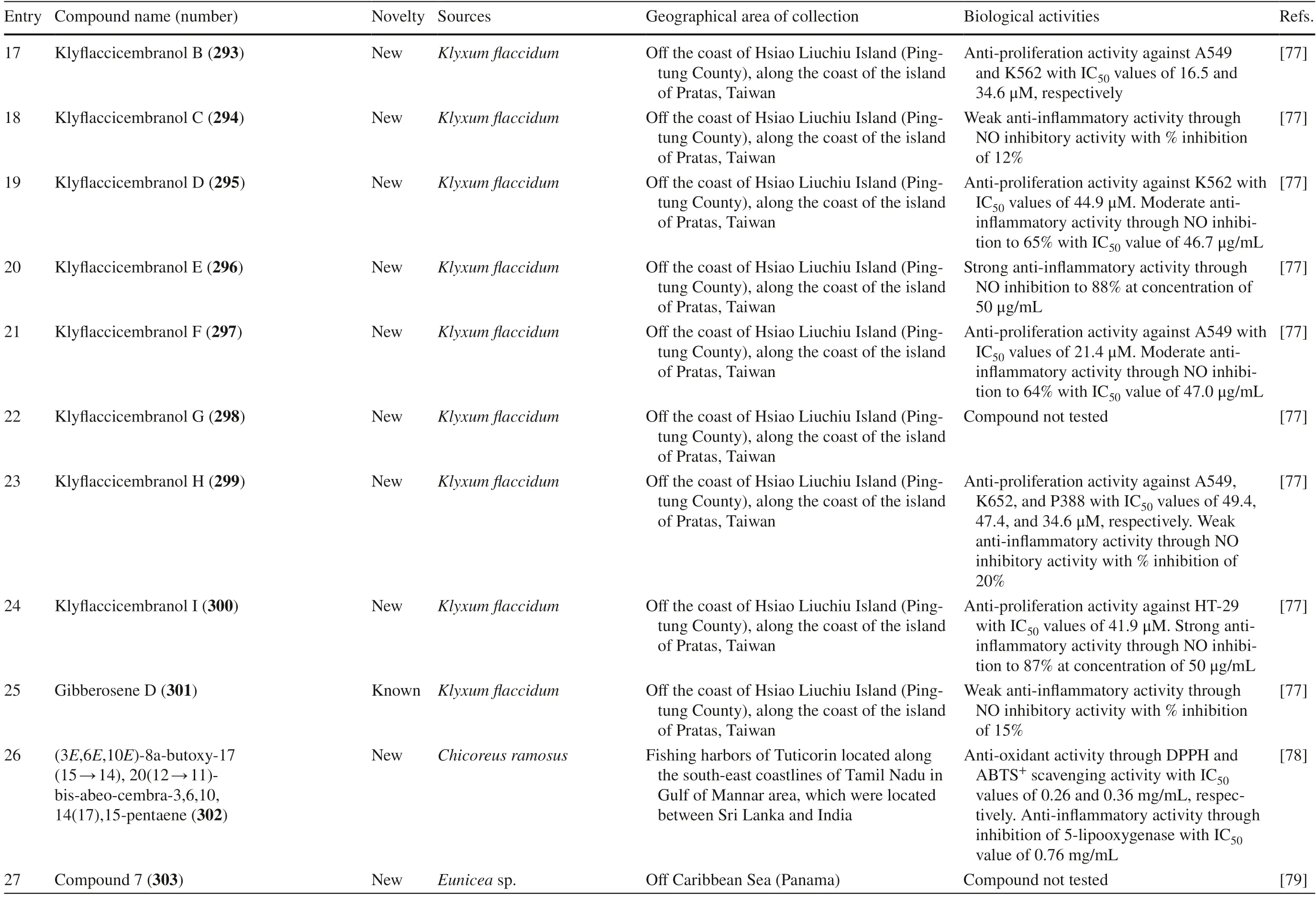
Table 4 (continued)
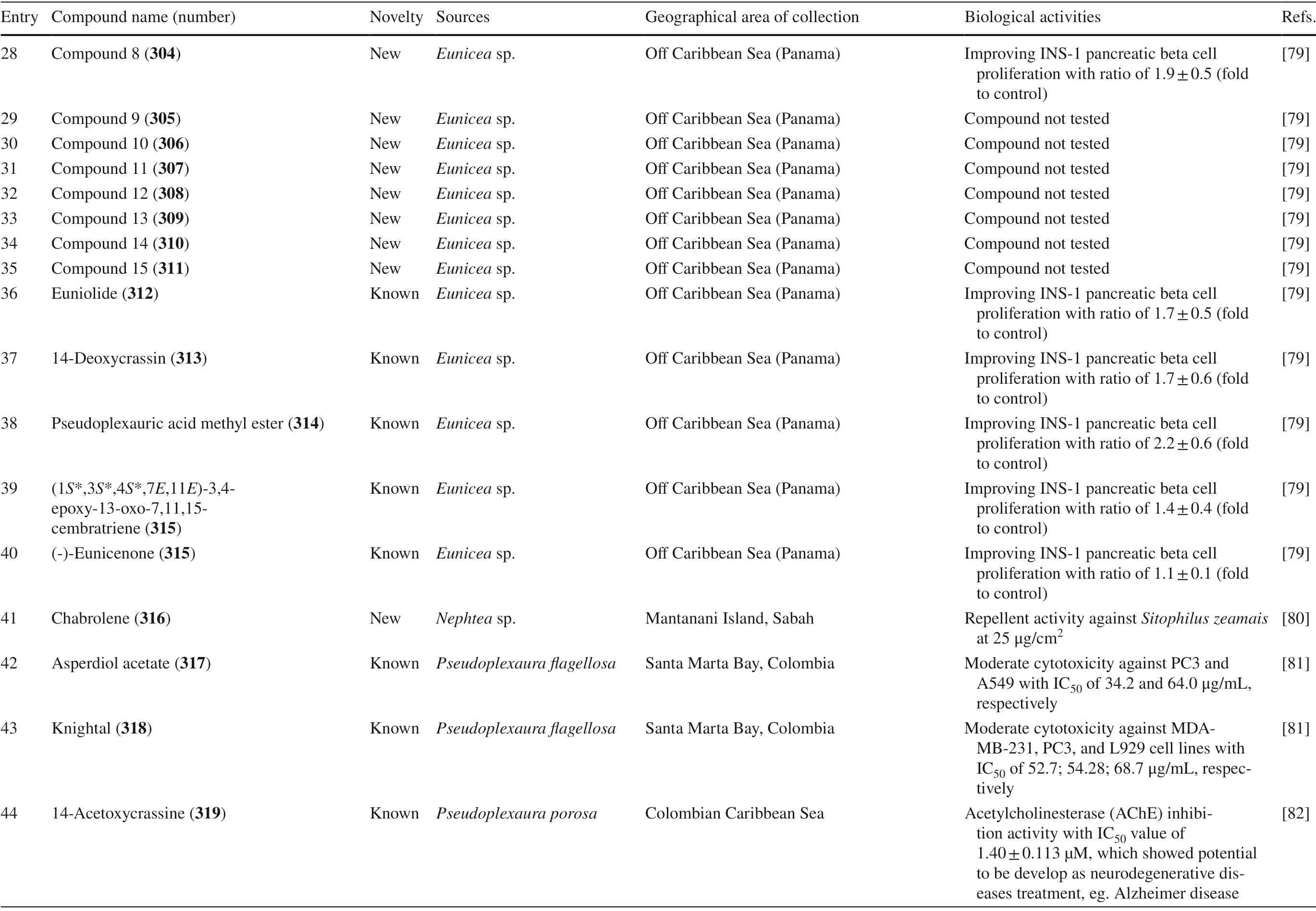
Table 4 (continued)
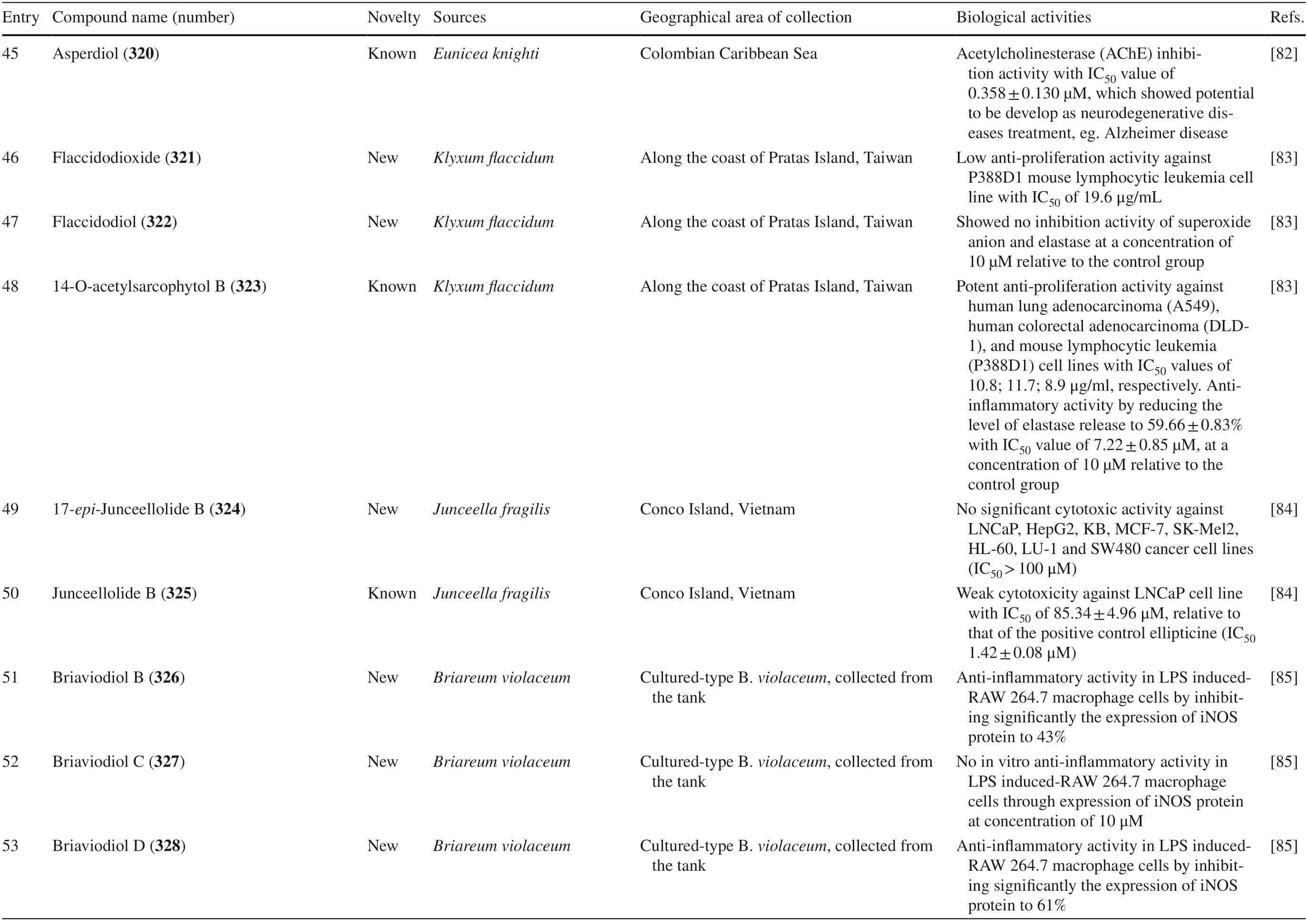
Table 4 (continued)
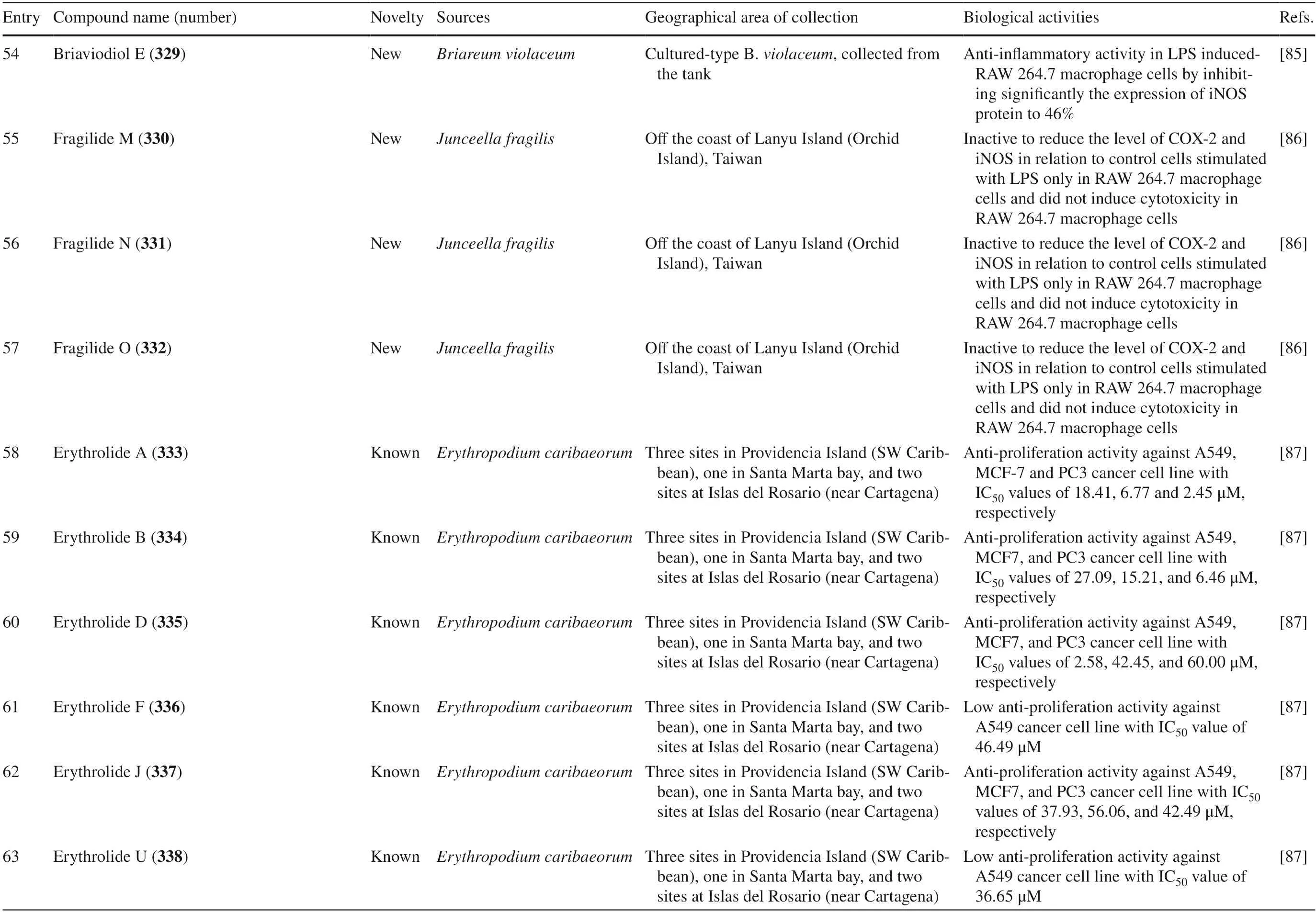
Table 4 (continued)
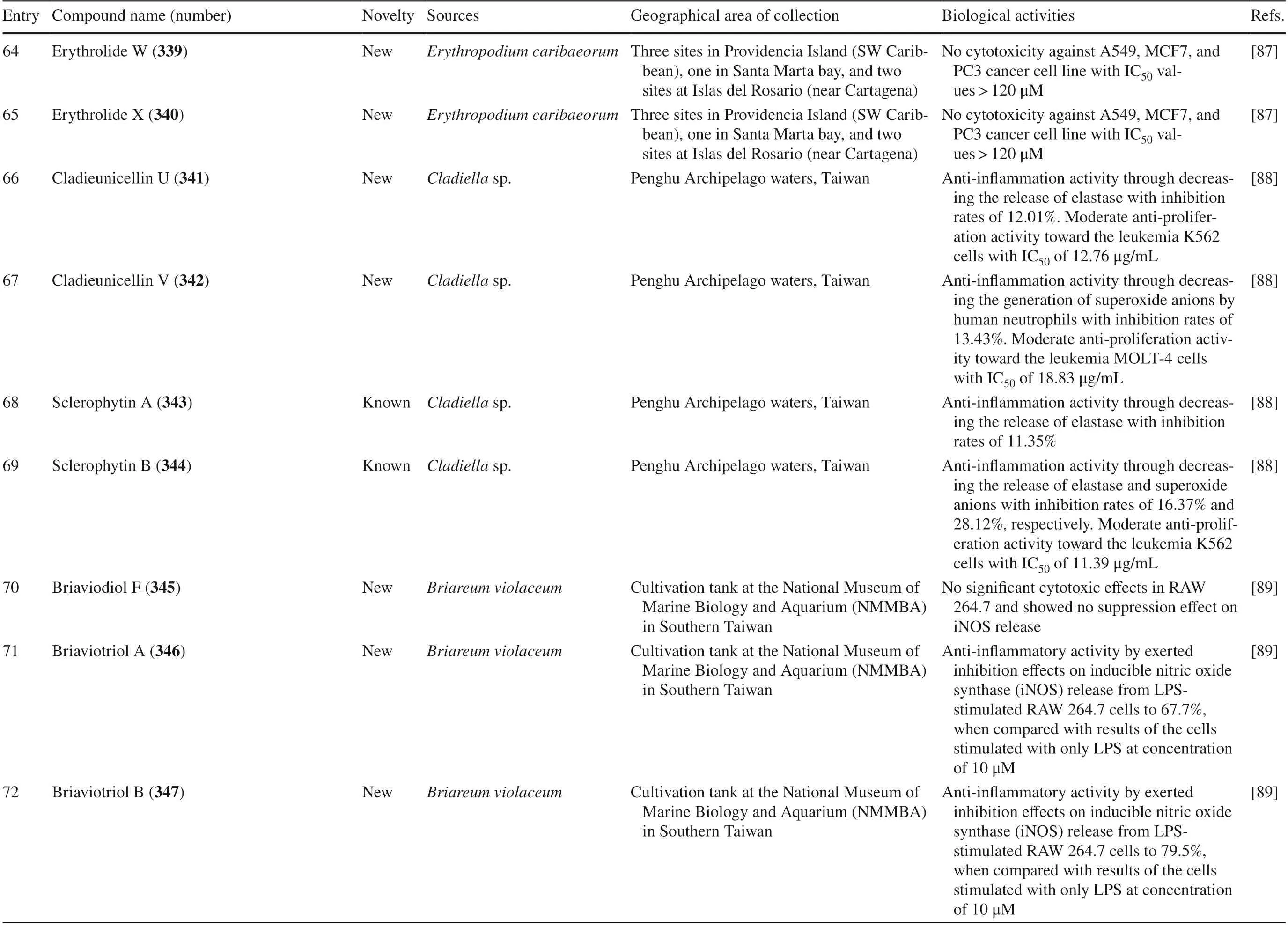
Table 4 (continued)
Table 4 (continued)
Table 4 (continued)
Sinulariasp.from Xisha Islands yielded four new cembranoids named 1E,3E,7E,-11-hydroxy-12-methoxy-1-isopropyl-4,8,12-trimethyl-icyclotetradeca-1,3,7-triene 200¸ 3E,7E-11-hydroxy-12-methoxy-1-isopropenyl-4,8,12-trimethyl-icyclotetradeca-3,7-diene 201,1E,3Z,7E,-11-hydroxy-12-methoxy-1-isopropyl-4,8,12-trimethyl-icyclotetradeca-1,3,7-triene 202,and 1Z,3Z,7E,-11-hydroxy-12-methoxy-1-isopropyl-4,8,12-trimethyl-icyclotetradeca-1,3,7-triene 203.The study showed that 201 and 203 had no biological activity [60].Sinularia scabrafrom Hainan,China,contained ten cembranoids.Six of them were novel compounds,namely,xiguscabrates A-B 204– 205,xiguscabral A 206,xiguscabrols A-B 207–208,and 8-epixiguscabrol B 209,with 204 and 206 not yet found to have biologically activity as tested.The known compound were sinulariol C 177,sinulariolide 190,(2R,11S,12S)-isosarcophytoxide 210,and (−)-14-deoxycrassin 211 [52].Figure 8 shows the structure of cembranoids isolated fromSinulariasp.
Sinularia crassafrom West Island,South China Sea contained four new and one known cembrane-type diterpenoids; sinulacrassins A-C 212–214,ent-xishafl avalin G 215,and S-(+)-cembrane A 216 (Fig.9).Compound 212 was not tested for its activity,while compound 213 and 216 showed a potential inhibitory eff ect towards α-Glucosidase [61].Lastly,six novel compounds were reported fromSinularia humiliscollected from Ximao Islands,Hainan,China namely humilisins A-F 217–222.Compound 222 was the only reported diterpenoid that possessed biological activity by decreasing NO level in anti-infl ammatory assay [62].
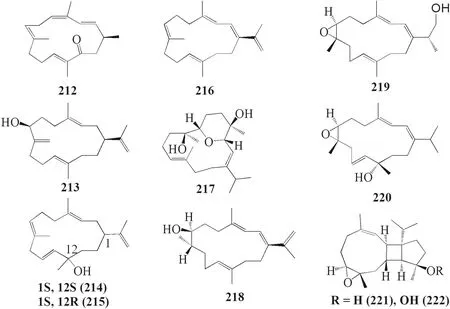
Fig.9 Cembranoids isolated from Sinularia crassa (212–216) and Sinularia humilis (217–222)
2.3 Cembranoids Reported from Genus Lobophytum
The present study reported 47 cembranoid compounds isolated fromLobophytumsp.collected from various geographical areas (Figs.10,11).Twenty-nine of those were new compounds and the other 18 were previously known compounds with newly discovered activities.Twelve of the new compounds were newly discovered and have not been thoroughly tested for their biological activities.
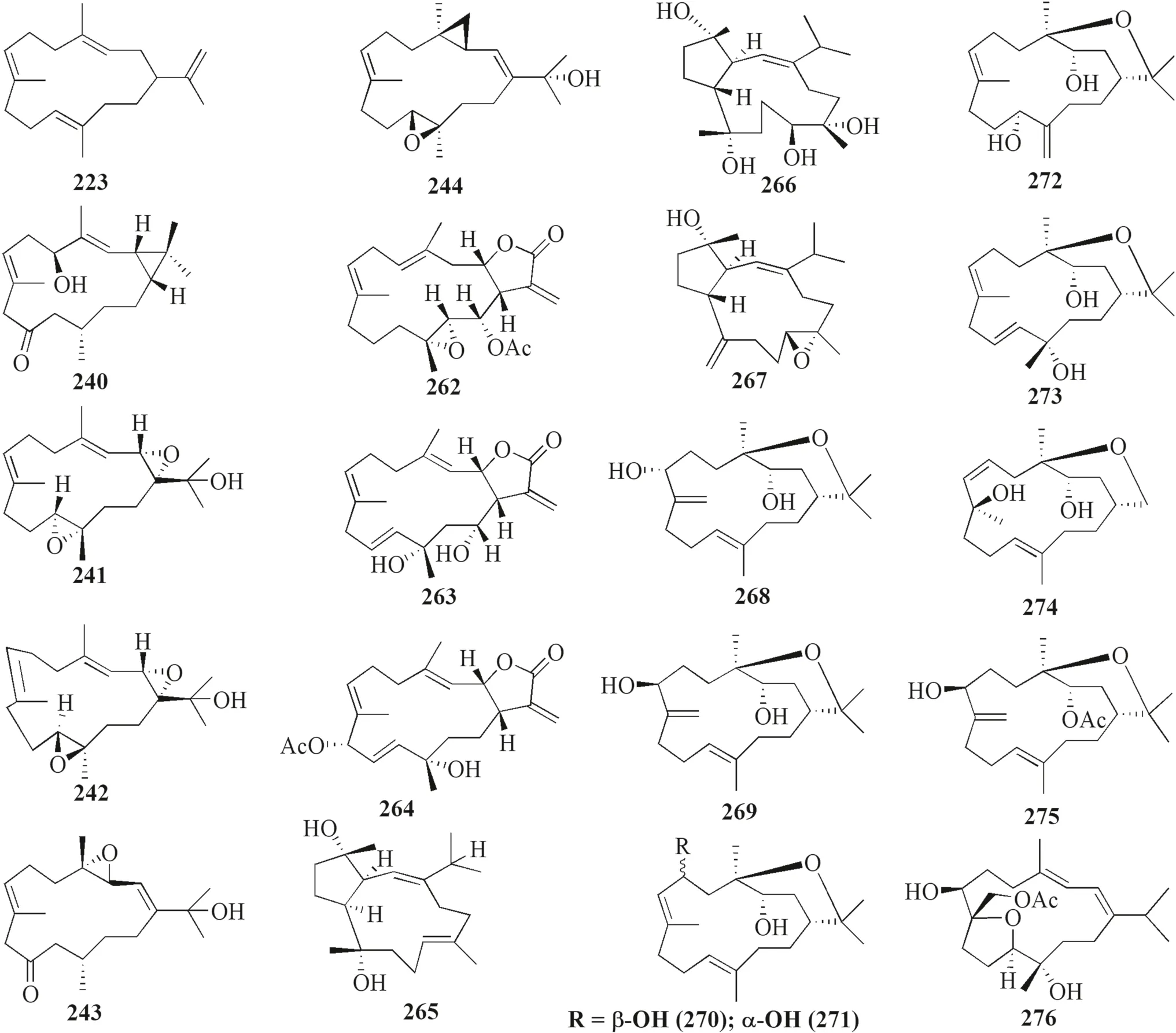
Fig.10 Cembranoids reported from Lobophytum sp
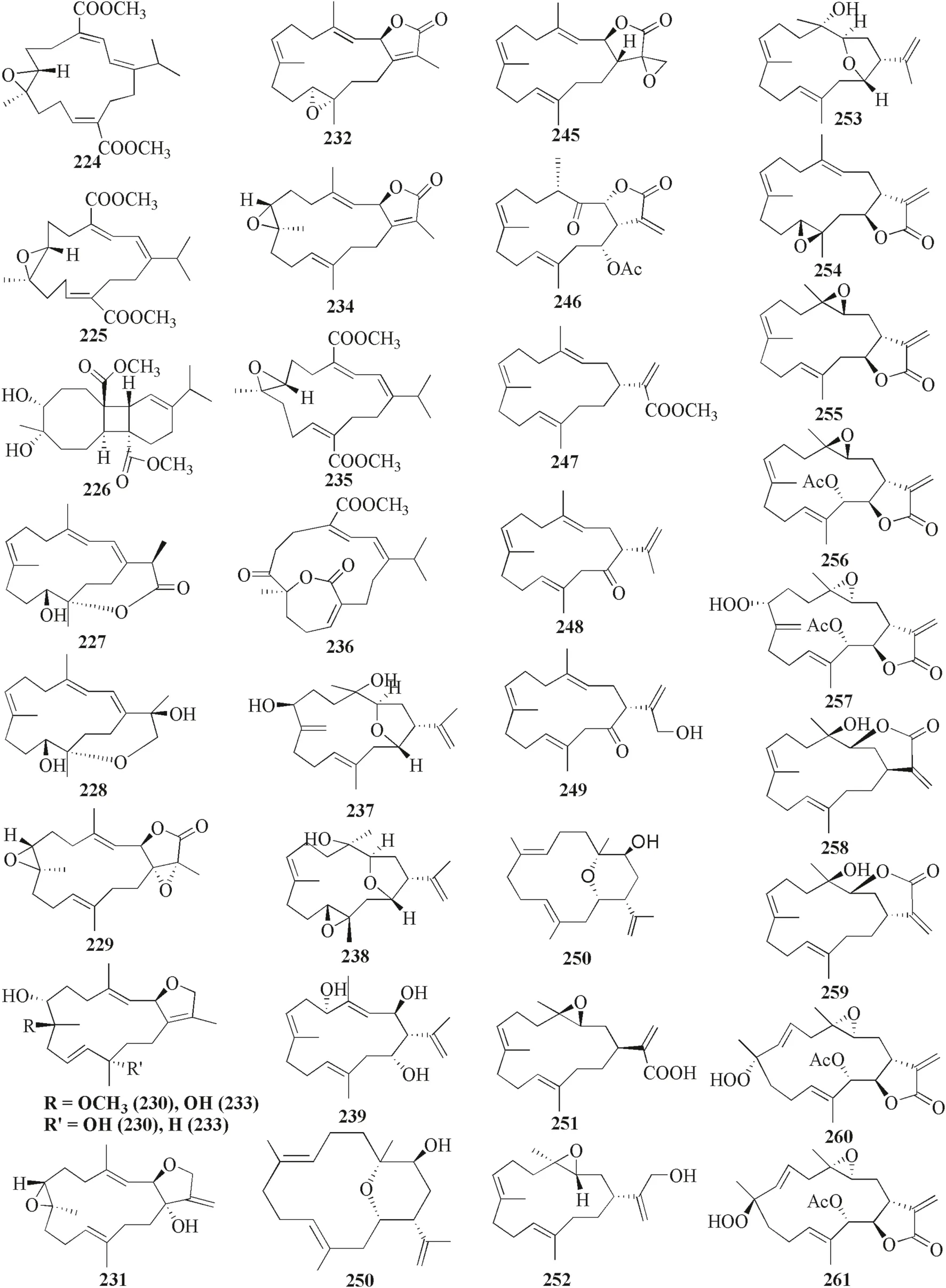
Fig.11 Cembranoids reported from Lobophytum crassum
Lobophytum crassumwas found to produce different cembranoid compounds.Cembrene A 223,a new cembranoid diterpene,was isolated from Red SeaLobophytumsp.in Jeddah [63].Ten new cembranoids and three known cembranoids were isolated from HainanLobophytum crassumin Meishan,China.Locrassumin A 224 and G 230 were the new compounds showing biological activities,whereas locrassumins B-F 225–229,(−)-laevigatol B 231,(−)-isosarcophine 232,and (−)-7R,8S-dihydroxydeepoxy sarcophytoxide 233 were the new compounds that have not been tested yet for their biological activity.Meanwhile,three known compounds with new activities wereent-sarcophine 234,sarcophytonolide O 235,and ketoemblide 236 [64].Three new-non tested compounds,lobophylins F–H 237–239,were isolated from Dongsha Atoll L.crassum[65].
Another study discovered a JapaneseLobophytumsp.that produced one new casbane-type diterpenoid and two new cembrane diterpenoids (compound 1–3 240–242) with various biological activities.Moreover,it also produced two known compounds,grandilobatin B 243 and sinugibberol 244 [66].The latter study reported that fi ve cembranoids was obtained from fromLobophytum crassumcollected from the coast of Pingtung,Taiwan.Two of them were new compounds named lobophyolides A-B 245–246,whereas three were known compounds called 16-methoxycarbonyl cembrene A 247,sinarone 248,and sinaluriol D 249 [11].In the same sampling area,twelve compounds were reported from the aquacultureLobophytum crassum.Two compounds were new (culobophylin D 250,and culobophylin E 251) while the others were known compounds including lobocrassin C 252,lobophylin 253,crassocolide E 254,sarcocrassocolide 255,13-acetoxysarcocrassocolide 256,sarocrassocolide M 257,(R)-14-deoxycrassin 258,lobocrassin B 259,sarcocrassocolides F-G 260–261 [67].Recently,a known compound 13-acetoxysarcocrassocolide 256 was also reported from the same aquaculturedLobophytum crassumby Liu et al.[68].
Three new unnamed cembranolide diterpenes (compound 4–6 262–264) with various biological activities were isolated from Irabu IslandLobophytumsp.which have [69].Furthermore,three new capnosane-type diterpenoids with no biological activities named lobophytrols A-C 265–267 were isolated fromLobophytumsp collected in Weizhou Island,China [70].Lastly,new macrocyclic cembranoids lobophytolins A-B 268–269 isolated fromLobophytumsp.were collected from Xisha Islands,China,with both compounds not showing any biological activities [71].Lastly,seven unreported cembranoid was isolated fromLobophytumsp.collected from the Xisha Island,Hainan,China.The new cembrane-type diterpenes,namely,lobophytolins C-I 270–276,displayed various anti-cancer activity towards HT-29,Capan-1,A549,and SNU-398 cancer cell line.Moreover,they also exhibited a weak inhibitory eff ect of XBP-Splicing on B16-F10 tumor cells [72].
2.4 Cembranoids from Other Soft Corals Species
The present study reported 80 cembranoid compounds isolated from other than the above-mentioned soft coral species collected from various geographical areas (Fig.10).Fiftyfi ve were new compounds and the other 25 were previously known compounds with newly discovered activities.Ten of the new compounds were newly discovered and have not been thoroughly tested for their biological activities.
In 2016,a known cembranoid named claudieunicellin S 277 was isolated fromCladiella tuberculosacollected from Penghu Archipelago waters,Taiwan [73].Six new briaranetype diterpenoids were isolated from TaiwaneseBriareumsp.named briarenolides ZI-ZVI 278–283.Among these,279 and 283 showed biological activities [74].Later in 2016,three new cembranoids were isolated fromNephtheasp.collected from Sabah,Malaysia.10-hydroxy-nephthenol acetate 284 and 7,8-epoxy-10-hydroxy-nephthenol acetate 285 were found to be biologically active,whilst 6-acetoxy-7,8-epoxy-10-hydroxy-nephthenol acetate 286 was not tested yet for its biological activity [75].Junceella fragilisfrom Hainan Island,China contained five new briarane diterpenoids named 3-deacetylpraelolide 287,13-α-acetoxyl-3-deacetylpraelolide 288,13-α-acetoxyl-2-deacetylpraelolide 289,13-α-acetoxyl-3-deacetyljunceellin 290,and 13-α-acetoxyl-2-deacetyljunceellin 291 [76].Several cembranoids were also isolated fromKlyxum fl accidumoriginated from Hsiao Liuchiu Island,Taiwan,named klyflaccicembranols A-I 292–300 and gibberosene D 301.Klyfl accicembranol G 298 was the only compound that has not been tested for its biological activities [77].
A novel cembrane has been isolated fromChicoreus ramosuscollected in fi shing harbors between Sri Lanka and India,namely (3E,6E,10E)-8a-butoxy-17(15 → 14),20(12 → 11)-bis-abeo-cembra-3,6,10,14(17),15-pentaene 302 [78].Meanwhile,nine new compounds were isolated fromEunicasp.collected from Caribbean Sea,namely compound 7–15 303–311.Among these compounds,304 was the only one showing biological activities,whilst the other compounds were not tested yet.Moreover,fi ve known cembranoids were also isolated this species,namely euniolide 312,14-deoxycrassin 313,pseudoplexauric acid methyl ester 314,(1S*,3S*,4S*,7E,11E)-3,4-epoxy-13-oxo-7,11,15-cembratriene 315,and (–)-eunicenone 316 [79].
The Bornean soft coralNephteasp.collected from Mantanani Island,Sabah,was found to produce new cembranoid norditerpene,i.e.chabrolene 317 [80].Pseudoplexaura fl agellosacollected from Colombia was reported to have two known cembrane diterpenes,namely asperdiol acetate 318 and knightal 319 [81].Known compounds 14-acetoxycrassine 320 and asperdiol 321 were successfully obtained fromPseudoplexaura porosaandEunicea knighticollected in the Caribbean sea,respectively[82].In 2019,Tseng and co-workers reported cembranoids fromKlyxum fl accidumcollected from Pratas Island,Taiwan.This species contained two new compounds,which are fl accidodioxide 322 and fl accidodiol 323.The later compound was reported to possess no activities.Moreover,a known compound 14-O-acetylsarcophytol B 324 was also reported from the same species [83].Junceella fragiliscollected from Vietnam was reported to produce new briarane-type diterpenoids,17-epijunceellolide B 325 and junceellolide B 326.While 325 did not possess any activities,the later showed new activities than before [84].
AquaculturedBriareum violaceumhas been reported to contain four novel hydroperoxyfurancembranoids,namely briaviodiols B-E 327–330 .One compound named briaviodiol C 328 did not possess any activity [85].In 2019,three new-non active triacetoxybriaranes were isolated fromJunceella fragilisin Lanyu Island,Taiwan,namely fragilides M–O 331–333 [86].Eight chlorinated briarane diterpenoids were isolated fromErythropodium caribaeorumoriginated from Providencia Island,Caribbean.Among these,six known compounds,namely erythrolides A-B 334–335,erythrolide D 336,erythrolide F 337,erythrolide J 338,and erythrolide U 339,were reported to have new activities.However,the new compounds erythrolides W-X 340–341 showed no biological activities [87].Cladiellasp.from Taiwan contained two new eunicellin diterpenoids cladieunicellins U-V 342–343 and two known eunicellin diterpenoids sclerophytins A-B 344–345 [88].
AquaculturedBriareum violaceumfrom Southern Taiwan was found to yield three new furanocembranoids and one known furanocembranoid.The new-non active compound was briaviodiol F 346,while the two other new compounds named briaviotriols A-B 347–348 were biologically active.Briaviodiol A 349 was the only compound that had been isolated before [89].Xishaflavalins G-H 350–351 were the new isolated cembrane from Chinese soft coralLemnalia flavawhich did not show any activities,whereas new activities were reported from the known cembrane nephthenol 352 [90].Stomopneustes variolarisfrom the Arabian Sea contained new cembrane named 4-hydroxy-1-(16-methoxyprop-16-en-15-yl)-8-methyl-21,22-dioxatricyclo [11.3.1.15,8] octadecane-3,19-dione 353 [91].Lastly,B.excavatumfrom Lanyu Island,Taiwan,contained two known briarane diterpenoids named briaviolides Y–Z 354–355 and one new-non active briarane diterpenoid named briarenol L 356 [92].
3 Biological Activities
Cembranoids and their analogues have been reported to have various biological activities such as anti-cancer,anti-bacterial,anti-infl ammation,anti-diabetic,neurological activity,anti-fouling,toxicity to brine shrimp,immunosuppressant,anti-Alzheimer’s,anti-oxidant,repellent activity againstSitophilus zeamais,and acetylcholinesterase (AChE) inhibition activity.The reported total numbers of cembranoid compounds from generaSarcophyton,Sinularia,Lobophytum,and other species that were successfully identifi ed were 139,42,47,and 80,respectively.Among them,221 were newly isolated compounds,and the other 87 compounds were previously known with newly discovered activities.The remaining 34 new compounds have not been tested for their biological activities.
3.1 Anti-bacterial
Compound 1 showed antibacterial activity againstStaphylococcus aureus,with minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC) values of 75 and 25 μM,respectively [17].Compound 2 is olated fromSarcophyton trocheliophorumalso possessed moderate antibacterial activity againstBacillus subtilis,Staphylococcus aureus,andVibrio choleraewith MIC values of 125,100 and 125 mg/mL,respectively,but it did not have activity againstEscherichia coli[18].Compound 8,exhibited antibacterial activity against several bacteria,viz.Acinetobacter baumannii(MIC = 4.2 μM),Escherichia coli(MIC = 6.0 μM),Klebsiella pneumoniae(MIC = 5.8 μM),Pseudomonas aeruginosa(MIC = 5.2 μM),Staphylococcus aureus(MIC = 4.0 μM),Staphylococcus epidermidis(MIC = 5.7 μM),andStreptococcus pneumoniae(MIC = 6.0 μM).While,9 and 10,which were also tested against the bacteria mentioned above,showed weak antibacterial activity.Compound 9 was reported to have inhibition zones of 7,8,7,and 7 mm zones of 11,11,and 6 mm againstKlebsiella pneumonia,Staphylococcus aureus,and Staphylococcus epidermidis,respectively [2 1].The compound fromStaphylococcus trocheliophorum,84,exerted moderate antibacterial activity againstStaphylococcus aureuswith MIC value of 250 μM [36].Additionally,85 exhibited anti-fungal activity towardsOchroconis humicolaandHaliphthoros milfordensiswith MIC value of 6.25 μg/mL [37].
Compound 223 isolated fromLobophytumsp.showed moderate anti-bacterial activity againstAcinetobactersp.,Escherichia coli,Klebsiella pneumonia,Pseudomona aeruginosa,Staphylococcus aureus,Staphylococcus epidermidis,andStreptococcus pneumonia.It had inhibition zone diameters of 14,13,13,13,11,11,11 mm,respectively and MIC value of 30 μg/mL against those bacteria [63].The OkinawanLobophytumsp.produced five cembranoid compounds (240–244) that exhibited antibacterial activity againstStaphylococcus aureusandEschericia coli.At a concentration of 25 μg compound 199–203 had an inhibition zone of 10,9,9,10,10 mm,respectively againstStaphylococcus aureusand 10,10,10,12,15 mm,respectively againstEscherichia coli[66].Furthermore,cembranoids isolated fromNephtheasp.,284 and 285,exerted anti-bacterial activity againstStaphylococcus aureuswith MBC of 180 and 150 μg/mL,respectively andEschericia coliwith MBC of 75 and 75 μg/mL,respectively [75].
3.2 Anti-cancer
New compounds 24,25 and 26 isolated fromSarcophyton ehrenbergishowed low to moderate anti-proliferation activity against A549 human lung carcinoma cells with inhibition concentr ation 50 (IC50) values of 50.1,76.4,and 50.8 μM,respectively,but inactive towards Caco-2 human colorectal adenocarcinoma cells.Compounds 24 and 26 also exhibited low to moderate cytotoxicity to HepG2 human liver carcinoma cells with IC50values of 98.6 and 53.8 μM [17].In addition,the known compounds 27–31 isolated fromSar-cophyton glaucomalso exerted moderate to potent activity against HepG2.Compound 27 and 28 were tested together and exerted effective concentration 50 (EC50) value of 11.32 μg/mL,while compound 29– 31 possessed EC50values of 17.84; 9.97; and 10.32 μg/mL,respectively [26].Another study reported anti-cancer activity against MCF-7 human breast cancer cells from compounds 30 and 31 with IC50values of 24.97 and 22.39 μg/mL,respectively [32].
Compounds 42– 47 also showed cytotoxic activity towards MCF-7,with growth inhibition 50 (GI50) values of 18.13; 12.22; 24.2; 22.27; 18.88; and 20.041 ppm,respectively [29].Compound 62 extracted fromSarcophyton mililatensiswas reported to have strong cytotoxicity towards HL-60 human leukemia cells and A549 cells,with IC50values of 0.78 μmol/mL and 1.26 μmol/mL,respectively [31].New compounds 92–96 showed cytotoxicity towards MCF-7 cells with IC50values of 23.84; 26.22; 26.81; 25.28; and 27.2 μg/mL,respectively [32].New potent anti-cancer activity from known compounds 103,104 and 105 isolated fromSarcophyton ehrenbergiwas reported against A549 cells,with inhibition concentration 25 (IC25) values of 23.3,27.3,and 25.4 μM,respectively.However,they were not active against Caco-2 cells.Additionally,104 and 105 exhibited weaker activity against HepG2 cells with IC25values of 22.6 and 31.8 μM,respectively [40].Finally,from genusSarcophyton,139,a known compound isolated fromSarcophyton glaucumexhibited anti-proliferation activity against HEK293 human embryonic kidney cells with lethal dose 50 (LD50) of 123.5 mM [44].
Compound 141,145–147 isolated fromSarcophyton digitatumshowed anti-cancer activity towards various cancer cell line.Compound 141 showed cytotoxicity against MCF-7 and MDA-MB-231 with IC50of 9.6 ± 3.0 and 14.8 ± 4.0 μg/mL,respectively.Moreover,145 showed cytotoxicity towards MCF-7,HepG2,and HeLa with IC50values of 10.1 ± 3.3; 14.9 ± 3.5; and 17.1 ± 4.5 μg/mL,respectively.In addition,146 exhibited cytotoxicity towards MCF-7,MDAMB-231,and HepG2 with IC50value of 9.4 ± 3.0; 17.8 ± 4.5; 14.9 ± 4.2 μg/mL,respectively.Lastly,147 showed cytotoxicity towards MCF-7 with an IC50value of 10.9 ± 4.3 μg/mL [45].
Another study reported that cembranoid isolated fromSarcophyton tenuispiculatumalso possessed anti-cancer activity including compound 148,151–155.Compound 148,151–155 showed cytotoxicity against MCF-7 with IC50value of 34.3 ± 3.7; 37.6 ± 4.2; 33.3 ± 3.5; 30.1 ± 3.1; 24.3 ± 3.0; 27.2 ± 4.0 μm,respectively.Whilst compound 151–152,154–155 showed cytotoxicity against HepG2 with IC50value of 35.2 ± 4.4; 28.6 ± 3.4; 34.5 ± 4.2 and 36.4 ± 5.3 μm,respectively.Furthermore,compound 153 showed cytotoxicity towards MDA-MB-231 cell line with an IC50value of 38.6 ± 5.0 μm [46].
Several compounds from the genus Sinularia were also reported to have anti-cancer activity.Compound 172 fromSinularia erectashowed anti-proliferation activity against K562 human leukimia cell line with an IC50value of 9.2 μM [49].Compound 178 fromSinularia compactashowed anti-proliferation activity against HCT-116 human colorectal carcinoma cell and A549,with IC50values of 10.1 and 14.7 μM,respectively [53].The cembranoid compound,178,isolated fromSinulariasp.found in Yongxing Island,South China Sea had anti-cancer activity was towards HeLa human cervical cancer and HCT-116 with IC50values of 11.6 and 33.3 μM,respectively [54].
In 2018,Tsai et al.isolated 181 from aquaculturedSinularia sandensis.The compound exerted a concentrationdependent anti-proliferative effect on NCI-N87 human gastric carcinoma cells and promoted apoptosis induction.The anti-proliferation activity was associated with the release of cytochrome c from mitochondria,activation of pro-apoptotic proteins,e.g.cysteine-aspartic proteases(caspase)-3/-9,Bcl-2-associated X protein (Bax) and Bcl-2-associated agonist of cell death (Bad),and inhibition of the anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2),B-cell lymphoma-extra large (Bcl-xL),and myeloid cell leukemia 1 (Mcl-1).This compound also triggered endoplasmic reticulum (ER) stress,leading to activation of the PERK/elF2α/ATF4/CHOP apoptotic pathway.Further,181 also initiated autophagy in NCIN87 cells and induced the expression of autophagy-related proteins,including Autophagy related (Atg)3,Atg5,Atg7,Atg12,microtubule-associated protein light chain (LC)3-I,a nd LC3-II [55].
Compounds 182 and 183 isolated fromSinulariasp.found in Sabah,Malaysia possessed anti-proliferation activity against HL-60 cancer cell line through apoptosis mechanism that involved the up-regulation of Bax,the downregulation of Bcl-xL,and the activation of caspase-3 [56].Wu et al.isolated 7 cembranoids,184–190,fromSinularia flexibiliswhereas four of them (187–190) exhibited anti-proliferation activity.Compound 187 showed anti-proliferation activity against P388 mouse leukimia cells,K562,HT-29 human colon cancer cell lines,with IC50values of 9.3,23.4,and 15.9 μM,respectively.Compound 188 exhibited antiproliferation activity against P388,K562,HT-29 cancer cell lines,with IC50values of 6.9,12.2,and 9.6 μM,respectively.Compound 189 showed anti-proliferation activity against P388 and K562 cancer cell lines,with IC50values of 16.0 and 26.7 μM,respectively.Compound 190 exerted anti-proliferation activity against K562 and HT-29 cancer cell lines,with IC50values of 21.7 and 27.1 μM,respectively [57].Cembranoid 187 isolated fromSinularia flexibiliscollected in Hainan exerted broad anti-proliferation activity against A549,HT-29,SNU-398 human hepatocellular carcinoma,and Capan-1 human pancreatic ductal adenocarcinoma cell line,with IC50values of 27.4,22.7,8.9,and 9.4 μM,respectively [58].188 and 190 isolated from the same species in Hainan,China,showed moderate anti-proliferation activities against HT-29,SNU-398,and Capan-1,with IC50values of 32.6; 24.9; 28.7 μM and 33.6; 24.7; 26.1 μM,respectively [58].Compound 191 isolated from aquacultureSinularia flexibilisin Taiwan exerted anti-oral cancer activity by inducing oxidative stress-mediated cell death pathways through suppressing colony formation,inducing apoptosis and cell cycle arrest,as well as inducing reactive oxygen species (ROS) as observed in three in vitro cultured human oral squamous cell carcinoma (OSCC) models (Ca9.22,SCC9 and HSC-3 cell lines) [59].
Compound 223 fromLobophytumsp.exerted significant anti-tumor activity against Ehrlich ascites carcinoma cells with LD50of 50 μg/mL [63].Roy et al.isolated 240– 242 from the Okinawan soft coralLobophytumsp.These compounds showed mild cytotoxicity against HCT-116,with IC50values of 135.37,177.11,and 153.11 μM,respectively [66].Out of the twelve new cembranoids isolated from aquaculturedLobophytum crassumcollected from the coast of Pingtung,Taiwan,250–261,ten showed anti-proliferation activitiy [67].Compound 211 had IC50of 35.8 μM against SUP-T1 human T-cell lymphoblastic lymphoma cell,compound 212 had activity against K562,MOLT-4 human acute T lymphoblastic leukaemia A,SUP-T1,with IC50values of 16.3,12.3,and 4.6 μM,respectively,while compounds 254–261 was active against K562,Molt 4,U937 human myeloid leukaemia cell line,and SUP-T1.The IC50of compounds 254– 261 against K562 were 11.3,18.1,3.3,15.3,4.5,3.3,12.3,and 13.0 μM,respectively; against MOLT-4 were 6.2,8.4,1.2,11.6,2.9,2.3,4.8,and 7.0 μM,respectively; against U937 were 15.8,4.4,7.1,32.0,7.0,5.2,10.9,and 23.3 μM,respectively; and against SUP-T1 were 5.2,8.3,1.5,10.2,4.5,6.2,6.1,and 6.6 μM,respectively [67].
Compound 256 from aquaculturedLobophytum crassumshowed cytotoxic activity against Ca9-22 human oral cancer cells through ROS generation and the suppression of the anti-oxidant enzyme activity.The apoptotic effect was found to be mediated through the interruption of the Keap1/Nrf2/p62/SQSTM1 pathway.It increased the expression of apoptosis and DNA damage-related proteins in a concentration and time-dependent manner.It also exerted potent antitumor effect against oral cancer cells,as demonstrated by the in vivo xenograft animal model.This compound reduced the tumor volume by 55.29% and tumor weight by 90.33% [68].In 2019,Roy et al.,isolated three cembranoids,262– 264,from Okinawa,Japan,which showed anti-proliferation activity against various cancer cell lines.Compound 262 showed moderate anti-proliferation activity against HeLa,A459,B16-F10 mouse skin melanoma,and RAW 264.7 mouse macrophage cells,with IC50of 7.81,9.30,10.83,and 5.99 μM,respectively.Compound 263 exerted low anti-proliferation activity against HeLa,A459,and RAW 264.7 cells,with IC50of 49.33,54.09,and 43.74 μM,respectively.Compound 264 possessed low anti-proliferation activity against RAW 264.7 cells,with IC50of 45.22 μM [69].
Lobophytumsp.collected from Xisha Island contained two compounds that exhibited anti-cancer activity.Compound 270 showed moderate cytotoxicity against SNU-398 with an IC50value of 42.54 ± 6.26 μM.Besides,271 exhibited anti-cancer activity towards various cancer cell line including HT-29,Capan-1,A549,and SNU-398 with IC50values of 4.52 ± 0.82; 6.62 ± 4.02; 5.17 ± 0.86; 6.15 ± 2.28 μM,respectively [72].Compound 277 isolated fromCladiella tuberculosapossessed moderate anti-proliferation activity against MOLT-4,K562,SUP-T1,with IC50values of 6.04,6.80,6.90 μg/mL,respectively [73].In 2016,Ishii et al.,isolated 284 and 285 from the Bornean soft coralNephtheasp.They possessed anti-proliferation activity against HeLa with IC50values of 40 and 125 μg/mL,respectively,and against MCF-7 with IC50values of 25 and 75 μg/mL,respectively [75].In 2017,Ahmed et al.,isolated 293 which showed anti-proliferation activity against A549 and K562 with IC50values of 16.5 and 34.6 μM,respectively [77].Four new compounds,namely 295,296,299 and 300,isolated fromKlyxum flaccidumexerted anti-cancer activity towards various cancer cell lines.295 possessed anti-proliferation activity against K562 with IC50of 44.9 μM.297 showed anti-proliferation activity against A549 with IC50of 21.4 μM.298 exerted anti-proliferation activity against A549,K652,and P388 with IC50values of 49.4,47.4,and 34.6 μM,respectively.300 displayed anti-proliferation activity against HT-29 with IC50values of 41.9 μM [77].Two known compounds,317 and 318 were isolated from ColombianPseudoplexaura flagellosa.317 showed moderate cytotoxicity against PC3 human prostate cancer cell line and A549 with IC50of 34.2 and 64.0 μg/mL,respectively.Further,318 exerted moderate cytotoxicity against MDAMB-231 human breast cancer cell,PC3,and L929 mouse fibroblast cell lines with IC50of 52.7,54.28 and 68.7 μg/mL,respectively [81].Tseng et al.(2019) isolated 321 and 3 23 from TaiwaneseKlyxum flaccidumwhich showed anticancer activity.321 displayed low anti-proliferation activity against the P388D1 mouse lymphocytic leukemia cell line with IC50of 19.6 μg/mL,while 323 showed a broad range of anti-cancer activities against A549,DLD-1 human colorectal adenocarcinoma,and P388D1 cell lines with IC50values of 10.8,11.7 and 8.9 μg/ml,respectively [83].The known compound,325,isolated from VietnameseJunceella fragilisshowed weak cytotoxicity against the LNCaP human prostate adenocarcinoma cells with IC50of 85.34 μM,as compared with that of the positive control ellipticine (IC50of 1.42 μM) [84].
In 2019,Molina et al.isolated six novel cembranoids (333–338) which possessed anti-cancer activity towards various cancer cell lines.333,334,335 and 337 showed cytotoxicity against A549,MCF-7 and PC3 cancer cell lines.333 possessed anti-tumor activity against A549,MCF-7 and PC3 with IC50values of 18.41,6.77 and 2.45 μM,respectively.334 exerted anti-proliferation activity against A549,MCF7,and PC3 cancer cell lines with IC50values of 27.09,15.21,and 6.46 μM,respectively.335 possessed anti-proliferation activity against A549,MCF7,and PC3 cancer cell lines with IC50values of 2.58,42.45,and 60.00 μM,respectively.337 exerted anti-proliferation activity against A549,MCF7,and PC3 cancer cell lines with IC50values of 37.93,56.06,and 42.49 μM,respectively.3 37 and 338 showed low anti-proliferation activity against the A549 cancer cell line with IC50of 46.49 and 36.65 μM,respectively [87].Cladiellasp.from Penghu Archipelago contained three new cembranoids (341,342 and 344) which possessed anti-cancer activity.341 and 344 exhibited moderate anti-proliferation activity toward the leukemia K562 cells with IC50of 12.76 and 11.39 μg/mL,respectively while 342 showed moderate anti-proliferation activity toward the leukemia MOLT-4 cells with IC50of 18.83 μg/mL [88].
3.3 Anti-infl ammation
Two novel compounds isolated fromSarcophyton elegans,18 and 19,showed anti-infl ammatory activity by inhibition oflipopolysaccharide (LPS)-induced nitrite oxide (NO) production by RAW 264.7 macrophages with IC50values of 18.2 and 32.5 μM,respectively [24].Compound 31 isolated fromSarcophyton glaucomhad inhibition activity towards the expression ofinducible nitrite oxide synthase (iNOS) at 50 and 100 μM.This compound also showed activity against the expression of cyclooxygenase-2 (COX-2) at 25,50,and 100 μM in RAW 264.7 [33].Other anti-inflammatory activities were also reported from a new compound,57,and a known compound,62.These two compounds showed inhibitory activity towards Tumor Necrosis Factor α (TNFα)-induced nuclear factor kappa B (NF-κB) activation (a therapeutical target in cancer),with IC50values of 35.23 and 22.52 μmol/mL,respectively [31].
Novel compounds 69–73,and known compound 75,isolated fromSarcophyton cherbonnieriexhibited anti-inflammatory activity by the inhibition of N-formylmethionineleucyl-phenylalanine/cytochalasin B (fMLF/CB)-induced superoxide anion generation and estalase release in human neutrophils at various potentials.Moderate inhibition activities were shown by 69,71 and 74 with respective values of 32.1,44.5,and 64.6% superoxide anion generation,and 37.6,35.6,and 42.6% elastase release at 30 μM were reported.Weaker activities were exerted by 70,72,73 and 75 with inhibitory effects of 4.0,6.4,2.6,and 3.5% on superoxide anion generation,and inhibition by 23.5,27.6,30.5,and 20.7% on elastase release have been reported [34].Three known compounds 97,98,and 102,as well as the newly discovered compound,100,isolated fromSarcophyton ehrenbergi,exerted anti-inflammatory activity by TNF-α secretion inhibition in RAW 264.7.The most potent activity was exhibited by 98 with IC50similar to dexamethasone as the positive control (8.5 μM vs.8.7 μM,respectively).Meanwhile,the other three had moderate effects,with IC50values of 28.5,24.2,and 27.3 μM [39].Other studies also reported several new and known compounds with similar activity.The IC50of the three new compounds 129,130,and 133 were 21.3,30.8,and 38.6 μM,respectively,while those of the five known compounds 134–138 were 9.1,15.4,29.5,12.5,and 7.2 μM,respectively [43].
Compounds isolated from the soft coralSinularia erecta,170 and 171,exhibited anti-inflammatory activity through the inhibition of superoxide generation and elastase release in fMLP/CB-induced human neutrophils,with IC50values of 2.3 and 8.5 μM,respectively [49].TaiwaneseSinularia nanolobatacontained four new cembranoids,174–177.Only 177 showed anti-inflammatory activity in RAW 264.7 cells induced by LPS and it effectively reduced the levels of NO to 2.3% at a concentration of 100 μM.Moreover,177 at a concentration of 50 μM also exhibited good inhibitory activity against iNOS compared to the positive control aminoguanidine (AG).The level of NO was also reduced significantly to 19.6% while giving a 104.6% retention of cell viability [51].The Bornean soft coralSinulariasp.contained 182 and 183 which showed anti-inflammatory activity through inhibition of NO,prostaglandin E2(PGE2),Interleukin (IL)-1β,IL-6,and iNOS in LPS-induced RAW 264.7 macrophages.Compounds 182 and 183 showed the most potent activity on the inhibition of NO production at 12.5 and 25.0 μg/mL compared to that of the negative control.The inhibition against PGE2in LPS-induced RAW 264.7 macrophages of 182 and 183 were shown in a dose-dependent manner.Both compounds also showed significant inhibition against the accumulation ofinterleukin (IL-1β and IL-6) production at 25.0 μg/mL,with a reduction ofless than 10% to both interleukins.The inhibition of NO,IL-1β,and IL-6 shown by 182 and 183 through the downregulation ofiNOS expression.Weak inhibition was displayed against PGE2by slight suppression of COX-2 expression [56].Compound 188 isolated from species collected in Hainan,China,showed high anti-inflammatory activity through inhibition of TNF-α,with an IC50of 2.7 μM [58].
Among several compounds isolated fromSinularia flexibiliscollected in Liuqiu,only compound 189 showed anti-inflammatory properties by significantly inhibiting the release of superoxide anion generation and elastase with IC50values of 10.8 and 11.0 μM,respectively [94].Seven of eight cembranoids successfully isolated fromS.flexibilis(188,190,195,196,197,198,and 199) showed anti-inf almmatory activity through the inhibition of TNF-α,with IC50values of 2.7,4.7,20.7,38.9,> 50,13.3,and 4.2 μM,respectively [58].
Hainan soft coralLobophytum crassumcontained 13 cembranoids (224–236),five of which (224,230,234,235,236) showed moderate anti-inflammatory activity through inhibition against LPS-induced NO production,with IC50values of 17,13,24,8,and 12 μM,respectively [64].The Okinawan soft coralsLobophytumsp.were found to contain the cembranoids 240,241 and 242 that exhibited antiinflammatory activity through reducing NO production,with IC50values of 41.21,64.96,and 74.76 μM,respectively [66].Lai et al.[11] isolated 245 to 249 fromLobophytum crassum,which showed potent anti-inflammatory activity through inhibition of LPS induced IL-12 release by dendritic cells (DC),with inhibition potency of 93.4,93.6,86.3,77.0 and 86.4%,respectively.At the same time,inhibition of LPS induced NO release by DC of these five compounds (245–249) were recorded at values of 93.5% with DC survival at 76.0%,95.9% with DC survival at 52.0%,86.1% with DC survival at 75.0%,54.9% with DC survival at 85.0%,and 86.1% with DC survival at 85.0% [11].Cembranoids 262,263 and 264 from the Okinawan soft coralLobophytumsp.displayed anti-inflammatory ef efcts through the suppression of NO production in a dose-dependent manner with IC50of 10.67,13.92,and 14.02 μM,respectively after 24 h in LPS-stimulated RAW 264.7 macrophage cells,at non-cytotoxic concentrations [69].
Two new compounds isolated fromBriareum sp.(279 and 283) displayed anti-infl ammatory activity by reducing iNOS level to 47.2% and 55.7%,respectively,at a concentration of 10 μM [74].From a collection of HainanJunceella fragilis,fi ve cembranoids (287,288,289,290,291) were isolated that exerted anti-infl ammatory activity through the inhibition of NO production by 39.4,42.7 (288 and 289 were tested together) and 36.3% (290 and 291 were tested together),respectively (at 50 μM) in RAW 264.7 cells [76].Ten cembranoids (292–301) isolated in 2017 fromKlyxum fl accidum,of which 8 (292,294–297,299,300,301) possessed various anti-infl ammatory activities.292,294,299,301 showed weak NO inhibitory activity with 25,12,20,15% inhibition,respectively,while 295 exerted moderate NO inhibition up to 65% (IC50of 46.7 μg/mL) and 297 up to 64% (IC50value of 47.0 μg/mL).Furthermore,296 and 300 strongly inhibited 88% and 87% of NO production at 50 μg/ml,respectively [77].
A novel cembranoid fromChicoreus ramosus,302,showed anti-infl ammatory activity through the inhibition of 5-lipooxygenase,with IC50of 0.76 mg/mL [78].Antiinfl ammatory activity was evident in 323 isolated fromK.fl accidum,predicted to occur by a reduction in the level of elastase release to 59.66%,with IC50of 7.22 μM at a concentration of 10 μM relative to the control group [83].Three out offour new cembranoids (326,328,329) isolated from cultured typeBriareum violaceumpossessed antiinfl ammatory activity in LPS-induced RAW 264.7 macrophage cells by signifi cantly inhibiting the expression ofiNOS protein to 43,61,46%,respectively [85].Four new compounds (341–344) isolated in 2019 displayed various anti-infl ammatory activities.Compounds 341 and 343 decreased the release of elastase with inhibition rates of 12.01% and 11.35%,respectively,while 342 decreased the generation of superoxide anions by human neutrophils with the inhibition rate of 13.43%,and 344 had an inhibition rate of 28.12%.Additionally,344 also decreased the release of elastase with the inhibition rate of 16.37% [88].Three new cembranoids (346–348) isolated from aquaculturedB.violaceumpossessed anti-infl ammatory activity by suppressing the release ofinducible nitric oxide synthase (iNOS) in LPS-stimulated RAW 264.7 cells with values of 67.7,79.5,and 61.9%,respectively,compared to the results of the cells stimulated with only LPS at a concentration of 10 μM [89].Anti-infl ammatory activity was also shown by the Arabian soft coralStomopneustes variolaris,which produced the novel compound 352 that inhibited 5-lipoxygenase with IC50of 2.01 mM,as compared to positive control ibuprofen (IC 50 4.50 mM).The selectivity ratio of cyclooxygenase-1 (COX-1) to COX-2 for the studied compound was found to be greater (1.25) than that ofibuprofen (0.43) [91].Two known compounds isolated fromBriareum excavatum,353 and 354,displayed an anti-infl ammatory eff ect,where 353 signifi cantly reduced the release of COX-2 to 65.30% at 10 μM in RAW 264.7 macrophages stimulated by LPS.In comparison,354 showed anti-infl ammatory activity through signifi cantly reducing the release ofiNOS to 60.29% at 10 μM using the same model [92].
Known cembranoid 145 and 147 isolated fromSarcophyton digitatumshowed anti-infl ammatory activity through inhibiting the production of IL-1β to 68 ± 1 and 56 ± 1%,respectively in LPS-stimulated murine macrophages J774A.1 at a concentration of 10 μg/mL with IC50values of 10.7 ± 2.7 and 14.9 ± 5.1 μg/mL.[45].In addition,Sarcophyton tenuispiculatumcontained 156 which possessed anti-infl ammatory activity through inhibiting the production of IL-1β to 56 ± 1% in LPS-stimulated murine macrophage J774A.1 cell at a concentration of 30 μm [46].New briaranes 357 and 360 exhibited anti-infl ammatory activity by enhancing the release ofiNOS (142.03 and 134.11%,respectively) and COX-2 (159.21 and 196.03%,respectively) in LPSstimulated RAW 264.7 macrophage cells at concentration of 10 μM [93].Sarcophyton roseumcollected from Egypt contained 158 which possessed anti-infl ammatory activity via iNOS inhibition with IC50of 50 μM.Whilst,from the same species,161 was isolated and showed anti-infl ammatory activity via Nrf-2 induction at 100 μM (2.1-fold),50 μM (1.4-fold),and 25 μM (0.9-fold).Furthermore,162 exhibited anti-infl ammatory activity via iNOS inhibition with IC 50 of 39 μM and Nrf-2 induction at 100 μM (1.8-fold),50 μM (1.5-fold),and 25 μM (1.5-fold) [47].
Sarcophyton cherbonniericontained cembranoids which possessed anti-inflammatory activity namely 163–169.Compound 163–169 showed inhibition on superoxide anion generation to 11.0 ± 8.7; 29.8 ± 9.8; 44.5 ± 7.9; 6.4 ± 7.3; 6.2 ± 5.5; 12.9 ± 11.4; and 17.1 ± 11.6%,respectively,at concentration of 30 μM.Furthermore,those compounds also inhibited the release of elastase to 35.1 ± 10.6; 48.2 ± 12.5; 35.6 ± 10.7; 27.6 ± 12.8; 29.7 ± 11.1; 16.7 ± 10.2; and 27.6 ± 12.0%,respectively,at concentration of 30 μM [48].Lastly,diterpenoid 222 isolated fromSinularia humiliscollected in Ximao Islands have signifi cant anti-infl ammatory eff ects in LPS-stimulated BV-2 microglial cells with 83.96% ± 2.02% and 65.70% ± 2.76% NO level decrease at 10 and 20 μM,respectively [62].
3.4 Other Biological Activities
Other reported biological activities of cembranoids include induction of T lymphocyte proliferation.Three new compounds and a known compound isolated fromSarcophyton trocheliophorum,86–89,were reported to be active on T lymphocyte cells from mice splenocytes.Compounds 8 6,88,and 89 significantly induced cluster of differentiation 3 (CD3+) T lymphocyte cells proliferation at 3 μM.In addition,86 increased the CD4+/CD8+T lymphocyte cells ratio on mice splenocytes.In contrast,compound 87 exhibited decreased the CD4+/CD8+ratio [38].Other active agents that exhibited activities related to T lymphocyte cell proliferation were two new compounds,118 and 119,and also a known compound,122,which were obtained fromSarcophyton mililatensis.Those compounds showed antiproliferation activity against Concanavalin A (ConA)-induced T lymphocyte cell proliferation with IC50values of 49.8,38.9,and 11.4 μM,respectively.Additionally,the three compounds also exerted anti-proliferation activity on LPS-induced B lymphocyte cells,with IC50values of 20.2,22.1 and 4.9 μM,respectively.In the same report,a known compound,121,also exhibited anti-proliferation activity on LPS-induced B lymphocyte cell proliferation,with IC50of 4.8 μM [42].
One study reported that two compounds,81 (a new compound) and 84 (a known compound),extracted fromSarcophyton trocheliophorumshowed inhibitory effect towards protein-tyrosine phosphatase 1B (PTP1B),with IC50values of 19.9 and 15.4 μM,respectively [36].This inhibitory effect is one ofinterest in the development of type 2 diabetes mellitus treatment as PTP1B is known as a negative regulator of the insulin signaling pathway [95].Two new compounds,107 and 110,isolated fromSarcophyton glaucumexhibited anti-larval settlement activity with an adhesive rate of 6.52 and 4.60% at 25 ppm,respectively.In the same study,three other known compounds,115,116 and 117,were shown to have anti-fouling activity againstBalanus amphitrite,with adhesive rates of 8.19,14.14,and 7.78% at 25 ppm,respectively [41].One of the known compounds from Sarcophytonglaucum,139,possessed neurological activity by competitive inhibition of neuronal glycine with inhibitory constant (KI) = 109 μM.It did not have any effect on strychnine toxicity in a mouse experiment model [44].
Compounds 179 and 180 exhibited lethality against brine shrimpArtemia salinawith lethal ratios of 90.5 and 90.0%,respectively at a concentration of 50 μg/mL [53].Several of the ten cembranoids (177,190,204–211) isolated in 2019 were found to possess immunosuppressive activity.Cembranoid 160 showed significant inhibitory effects on the proliferation of LPS induced B lymphocyte cells,with an IC50value of 9.2 μM.177,205,207,208,209,211 possessed immunosuppressive activities through potent inhibition on the proliferation of Con A-induced T lymphocyte cells,with IC50values of 4.5,8.4,5.5,3.9,2.3,and 6.1 μM,respectively.Compound 210 had considerable specific inhibition on B lymphocyte cell proliferation,with an IC50value of 4.4 μM and selectivity index (SI) of 10.9.This performance was much better than that of the positive control cyclosporin A (CsA) (SI = 3.0).210 dose-dependently inhibited CD19+B lymphocyte cells proliferation by LPS induction,while it also showed modulatory ef efcts on cytokine production,with the manifestation of decreased IL-6 production and slightly increased IL-10 production.210 could suppress the derivational expression of CD86 on CD19+B lymphocyte cells upon LPS stimulation.In vitro,LPS addition led to B lymphocyte cell growth and plasma cell formation (from 2.31% to 11.0%) and compound 210 dose-dependently inhibited the plasma cell proliferation [52].193 and 194 isolated from Yongxing IslandSinulariasp.possessed anti-diabetic activity through mild inhibitory activity against PTP1B with IC50values of 47.5 and 12.5 mM,respectively,measured against sodium orthovanadate as the positive control (IC50881 μM) [54].Cembranoids 200 and 202 isolated from Xisha IslandsSinulariasp.can inhibit Alzheimer’s amyloid-beta 42 (Aß42) aggregation at a concentration of 10 μM,with inhibition of 20.6 and 37.2%,respectively.This potency was comparable to that of the positive control curcumin (20.5%) [60].Cembranoid 223 showed significant toxicity againstA.salinawith an LD50value of 25 μg/mL [63].
A new cembranoid,302,isolated fromChicoreus ramosuspossessed anti-oxidant activity through 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) scavenging activity,with IC50values of 0.26 and 0.36 mg/mL,respectively [78].Fourteen cembranoids have been isolated from the Caribbean SeaEuniceasp.,with some of them possessing antidiabetic activity.304,312–315 improved INS-1 pancreatic β cell proliferation with a ratio of 1.9,1.7,1.7,2.2,1.4,and 1.1,respectively compared with control.In this regard,303 and 305–311 have not been tested for biological activity [79].The Bornean soft coralNephtheasp.contained 316,which showed insecticidal activity through repellent activity against maize weevilSitophilus zeamais(grains pest) at 25 μg/cm2[80].In 2019,Castellanos et al.isolated 319 fromPseudoplexaura porosaand 320 fromEunicea knighti,which possessed AChE inhibition activity with IC50of 1.40 and 0.358 μM,respectively.These compounds have the potential to be developed for neurodegenerative disease treatment,e.g.Alzheimer’s disease [82].The known compound,351,isolated fromL.flavapossessed immunosuppressive activity through inhibiting the proliferation of ConA-induced T lymphocyte cells and/or LPS-induced B lymphocyte cells in vitro,with IC50of 10.7 and 38.6 μM,respectively [90].Compound 3 52 isolated fromS.variolarispossessed potent anti-oxidant activity through DPPH and ABTS+scavenging activity with IC50values of 1.41 and 1.61 mM,respectively,which were greater than that of the standard agent α-tocopherol (IC50of 1.51 and 1.70 mM,respectively) [91].Cembrane-type diterpenoid 213 and 216 showed inhibitory effect toward α-Glucosidase with IC50value of 10.65 ± 0.16 and 30.31 ± 1.22 μM,respectively [61].Furthermore,Lobophytumsp.from Xisha Island contained seven compounds namely 270–276 which exhibited a weak inhibitory effect of XBP-Splicing on B16-F10 tumor cells at a concentration of 10 μM [72].
Many compounds reviewed in this paper were found to have no biological activity ofinterest reported in the respectively published article,such as 174–176 isolated fromS.nanolobata[51].The same was true to 184–186 isolated fromS.flexibilis[94]; 192 isolated from the South China Sea soft coralSinulariasp.[54],201 and 203 isolated from Xisha IslandsSinulariasp.[60],204 and 206 isolated from Xigu IslandS.scabra[52],and neither did for 278,280,281 and 282 isolated from the soft coralBriareumsp.did not possess any biological activity [74].Cembranoids 225–229 and 231–233 isolated by Zhao et al.in 2016 did not exhibit the activity ofinterest [64].The same goes for three new cembranoids isolated by Zhang et al.[49],namely 265,266 and 267 [70] as well as two new cembranoids,268 and 269,isolated by Li et al.[43] from the Hainan soft coralLobophytumsp.[71].No biological activity was detected in another new compound,322,fromK.flaccidum[83],as was the case with a new cembranoid,324,isolated fromJ.fragilis[84],and with 327 derived from aquaculturedB.violaceum[85].Similarly,no biological activities ofinterest were recorded in the original published papers of the three novel cembranoids (330,331 and 332) isolated fromJ.fragilis[86],two new cembranoids (339 and 340) fromE.caribaeorum[87],345 from aquaculturedB.violaceum[89],two novel cembranoids,349 and 350,fromL.flavathat originated from Xisha Islands [90],as well as a novel compound,3 55,isolated from the Taiwanese soft coralB.excavatum[92].
Several cembranoid compounds have been recently discovered and have not been thoroughly tested for their biological activities [27,28,39].Rahelivao et al.[59],isolated a new compound,173,from the Madagascar soft coralS.gravis,but no biological activity was reported [50].Dongsha Atoll soft coralsL.crassumcontained three novel compounds,237,238 and 239,which have not yet been explored for their biological activities [65].250 isolated from aquaculturedL.crassumdid not possess any biological activity ofinterest,while 251 has not been thoroughly tested [67].286 isolated from the Bornean soft coralNephtheasp.[75] and 298 extracted fromKlyxum flaccidum[77] have not been tested yet.Lastly,five briaranes were isolated fromBriareum stecheiwhich cultured in the National Museum of Marine Biology and Aquarium,Pingtung,Taiwan.briarenols W-Z 356–359 were the new reported compound and solenolide A 360 was the only known compound being isolated.Compound 357 and 360 were the only compound which exhibited anti-inf almmatory activity by enhancing the release ofiNOS and COX-2 [93].
4 Conclusions
Soft corals or Alcyonacea are rich potential sources of uniques compounds,particularly cembranoid diterpenes.These compounds have been demonstrated to display a spectrum of pharmacological activities such as anti-tumor,antibacterial and anti-infl ammatory.Discoveries are being reported continually in the literature for cembranoid compounds isolated from soft corals as technologies for chemical extraction and characterization of secondary metabolites become more advanced.
This review provides an update on recent studies that encompass the isolation of up to 360 cembranoids from marine soft corals and brief accounts of their biological activities reported in the span of the recent fi ve years.Most of the studied compounds were isolated from Sarcophyton sp.(45%),followed by Lobophytum sp.(15%),and Sinularia sp.(14%).Other marine soft corals made up the remaining 26% of species.It is known that cembranoids from marine soft corals possess various biological characteristics.Antiinfl ammatory (38%) was found to be the most common biological activity exhibited by cembranoids reported in this review,followed by anti-cancer (35%),and anti-bacterial (7%),whereas other activities encompassed the remaining 20% (Fig.12).These early fi ndings can lead to more detailed studies for marine cembranoid-based drug discovery and development.
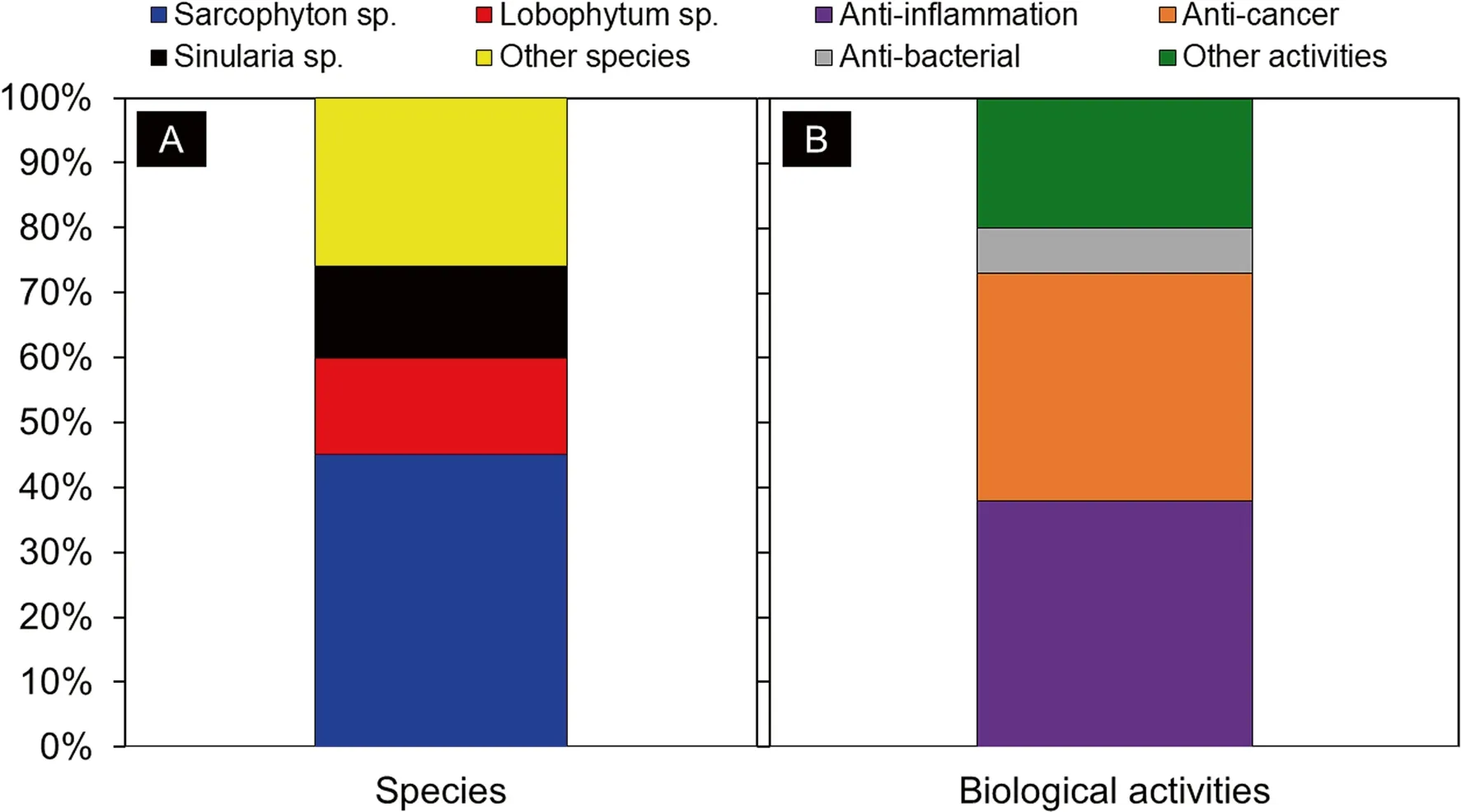
Fig.12 The percentages of cembranoid-producing soft coral species (a).The percentage of different biological activities exhibited by cembranoids (b)
Despite the abundance of unique cembranoids identifi ed,the low quantitiy ofisolated compounds may be a big challenge for drug applications’ evaluation and development.We consider such approaches like synthesis and biosynthesis studies to be developed for applications of these cembranoids for drug discovery.Furthermore,with the recent advanced technology,various types of specifi c soft corals are becoming possible in aquaculture.This technology provides more abundant organisms to be extracted and a considerable quantity of molecules to be assessed for in vitro and in vivo study.
AcknowledgementsThis research was funded by the Indonesian Institute of Sciences (LIPI) through the DIPA 2021 Research Fund B-10405/IPH/HK.01.03/XI/2020.Authors greatly acknowledge to the Head of Research Center for Biotechnology,Indonesian Institute of Sciences,as well as the research and administration staff members for their support.
Author ContributionsResources,MYN,DS,AYN,SIR,AB,LS,AA,AP,FI,MFW; data curation,MYN and DS; writing—original draft preparation,MYN,DS,AYN,FI,AB,MYP; writing—review and editing,MYN,DS,MYP,AA,MF,FI,LS,and AP AB; visualization,AYN.MYP; supervision,MYP,AB; project administration,MYP,SIR,MFW,DAW,FI,AB; funding acquisition,MYP.All authors have read and agreed to the published version of the manuscript.
Declarations
Conflicts ofinterestThe authors declare no confl ict ofinterest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
杂志排行
Natural Products and Bioprospecting的其它文章
- Jatrophane Diterpenoids from the Seeds of Euphorbia peplus with Potential Bioactivities in Lysosomal-Autophagy Pathway
- Reversal of Tetracycline Resistance by Cepharanthine,Cinchonidine,Ellagic Acid and Propyl Gallate in a Multi-drug Resistant Escherichia coli
- Sesamol Alleviates the Cytotoxic Eff ect of Cyclophosphamide on Normal Human Lung WI-38 Cells via Suppressing RAGE/NF-κB/Autophagy Signaling
- Structurally Diverse Sesquiterpenoids with Anti-neuroinfl ammatory Activity from the Endolichenic Fungus Cryptomarasmius aucubae
- Repression of Polyol Pathway Activity by Hemidesmus indicus var.pubescens R.Br.Linn Root Extract,an Aldose Reductase Inhibitor:An In Silico and Ex Vivo Study
- Evaluation of Terpenes’ Degradation Rates by Rumen Fluid of Adapted and Non-adapted Animals
