Plasma-assisted Co/Zr-metal organic framework catalysis of CO2 hydrogenation:influence of Co precursors
2021-05-22YanqinLI李艳琴JingZHAO赵静DecaiBU部德才XuleiZHANG张旭磊TengPENG彭腾LanboDI底兰波andXiulingZHANG张秀玲
Yanqin LI(李艳琴),Jing ZHAO(赵静),Decai BU(部德才),Xulei ZHANG(张旭磊), Teng PENG (彭腾), Lanbo DI (底兰波) and Xiuling ZHANG (张秀玲)
College of Physical Science and Technology, Dalian University, Dalian 116622, People’s Republic of China
Abstract In this study, Co/Zr-metal organic framework (MOF) precursors were obtained by a roomtemperature liquid-phase precipitation method and the equivalent-volume impregnation method,respectively, using a Zr-MOF as the support, and Co/Zr-MOF-M and Co/Zr-MOF-N catalysts were prepared after calcination in a hydrogen–argon mixture gases (VA r : VH2=9: 1) at 350 °C for 2 h.The catalytic activities of the prepared samples for CO2 methanation under atmosphericpressure cold plasma were studied.The results showed that Co/Zr-MOF-M had a good synergistic effect with cold plasma.At a discharge power of 13.0 W,VH 2: VCO2=4: 1and a gas flow rate of 30 ml·min−1,the CO2 conversion was 58.9%and the CH4 selectivity reached 94.7%,which was higher than for Co/Zr-MOF-N under plasma (CO2 conversion 24.8%, CH4 selectivity 9.8%).X-ray diffraction, scanning electron microscopy, transmission electron microscopy, N2 adsorption and desorption (Brunauer–Emmett–Teller) and x-ray photoelectron spectroscopy analysis results showed that Co/Zr-MOF-M and Co/Zr-MOF-N retained a good Zr-MOF framework structure, and the Co oxide was uniformly dispersed on the surface of the Zr-MOF.Compared with Co/Zr-MOF-N, the Co/Zr-MOF-M catalyst has a larger specific surface area and higher Co2+/Cototal and Co/Zr ratios.Additionally, the Co oxide in Co/Zr-MOF-M is distributed on the surface of the Zr-MOF in the form of porous particles,which may be the main reason why the catalytic activity of Co/Zr-MOF-M is higher than that of Co/Zr-MOF-N.
Keywords: atmospheric-pressure cold plasma, CO2, supported Co catalytic materials, metal organic framework
1.Introduction
CO2is an inexpensive C1 resource with the most abundant storage in the world.Under certain conditions, CO2can be converted into high-value-added compounds such as hydrocarbons,alcohols and organic acids,which not only solves the problem of the effective utilization of C1 resources but could also alleviate the global warming caused by CO2.Therefore,finding an effective method to convert CO2into high-valueadded chemicals has attracted attention all over the world[1].The plasma-assisted catalytic CO2hydrogenation reaction is an effective method for preparing hydrocarbons.Compared with traditional heat-assisted catalytic reactions, high-energy particles in plasma not only effectively activate CO2and H2but can also change the surface morphologies of the catalyst and the valence of the active component [2].
Metals are the main catalysts for plasma-assisted catalytic CO2hydrogenation reactions.Noble metals exhibit excellent catalytic performances; however, the application of noble metals faces many difficulties due to their high cost and lower reserves [3].Transition metal catalysts such as Co can solve the problems of poor catalyst stability and high noble metal catalytic material costs [4–7].Supported metal Co and its oxides have received more attention in the fields of catalysis and energy due to their excellent catalytic activities [8, 9].
Metal organic framework (MOF) materials are widely used in catalysis, drug delivery and other fields due to their high specific surface areas, uniform pore sizes, periodic structures and other characteristics.Many studies have found that MOFs can be used as templates to synthesize catalysts with unique morphologies[10–13],and some of them can be used as supports in catalysis because of their high specific surface areas and thermal stabilities[14–18].Zhanget al[19]synthesized a Co–formic acid MOF (Co-MOF) by a liquidphase precipitation method.This material could be calcined at low temperatures to prepare a porous Co3O4catalytic material which exhibited excellent catalytic activity for CO oxidation.The research of Xuet al[20] showed that a Zr-MOF could exist stably in an air environment at a temperature lower than 400 °C.Zhaoet al[21] reported a catalytic material with a single copper nanoparticle embedded in a Zr-MOF.Compared with traditional Cu/ZnO/Al2O3catalytic material, the methane yield in the CO2hydrogenation reaction increased by eight times.
In this study, a Zr-MOF prepared by a solvothermal method was used as a support, and the precursors of Co-MOF/Zr-MOF and Co(NO3)2/Zr-MOF were prepared by a liquid-phase precipitation method and equivalent-volume impregnation, respectively.They were placed in a tube furnace, and the temperature was held at 350 °C for 2 h in an Ar/H2gas mixture.After natural cooling,the Co/Zr-MOF-M and Co/Zr-MOF-N catalysts were prepared, and x-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and x-ray photoelectron spectroscopy (XPS) were used to characterize the structures,morphologies and elemental compositions of the catalyst.Furthermore, the catalytic activities of the prepared material for CO2hydrogenation were evaluated in an atmosphericpressure cold plasma.
2.Experimental
2.1.Materials and synthesis of Zr-MOF
All chemical reagents were commercially purchased and used without further purification.Zr-MOF was synthesized by a solvothermal method[20].In brief,5 mmol(1.165 g)of ZrCl4and 5 mmol (0.831 g) of H2BDC (terephthalic acid) were dissolved in 30 ml of DMF (N, N-dimethylformamide).The liquid mixture was placed in an ultrasonic reactor for 20 min and heated at 120 °C for 24 h in a Teflon-lined steel autoclave.The obtained solid powder was washed three times with DMF and anhydrous methanol, after which the filtered white solid was dried in a vacuum drying oven at 100°C for 7 h to obtain the Zr-MOF.
2.2.Preparation of catalyst
The Co-MOF/Zr-MOF precursor was prepared by a liquidphase precipitation method.The process was as follows.First,0.50 g PEG (polyethylene glycol, average Mn = 2000) and 0.58 g cobalt nitrate were dissolved in 10 ml of methanol to form Solution A.Then, 0.50 g PEG, 0.3 ml formic acid and 1.01 g ammonium formate were dissolved in 10 ml methanol,and 1.00 g of Zr-MOF was quickly added to the above solution.The mixture was stirred on a magnetic stirrer for 20 min to obtain Solution B.Finally, Solution A was slowly added to Solution B under continuous stirring at room temperature for 1 h and aged for 24 h.The pink solid powder was centrifuged (9000 r.p.m., 10 min) and washed five times with ethanol, after which it was dried in a vacuum drying oven at 55 °C for 7 h to obtain the Co-MOF/Zr-MOF precursor.
The Co(NO3)2/Zr-MOF precursor was prepared by the equivalent-volume impregnation method.The process was as follows.First, 5.80 g of Co(NO3)2·6H2O was added to a 25 ml volumetric flask to prepare a 15% Co(NO3)2·6H2O aqueous solution.Then, 2.15 ml of the aqueous solution was added to 1.00 g of Zr-MOF and evenly mixed.Finally, after being allowed to stand at room temperature for 12 h, the mixture was dried at 120 °C for 2 h to obtain the Co(NO3)2/Zr-MOF precursor.
The precursors of Co-MOF/Zr-MOF and Co(NO3)2/Zr-MOF were placed separately in a tube furnace,and a mixture gas of Ar/H2(VAr:VH2=9: 1)was introduced.The temperature was maintained at 350°C for 2 h(according to the results of figure S1, available online at stacks.iop.org/PST/23/055503/mmedia) and then cooled naturally to obtain Co/Zr-MOF-M and Co/Zr-MOF-N catalysts.Inductively coupled plasma atomic emission spectroscopy measurements showed that the Co contents of the two catalysts were 10.84%and 11.20%,respectively.The synthesis process for Co/Zr-MOF-M and Co/Zr-MOF-N is shown in figure 1.
2.3.Characterization of catalyst
The crystal structure of the catalytic material was analyzed by an XRD system (Dandong Fangyuan Co., Ltd, China; Cu target, Kα radiation, λ=0.1506 nm).The tube voltage was 40 kV, the tube current was 40 mA, the scanning range was 5°–90°and the step size was 0.03°.The specific surface areas,pore sizes and pore volumes of catalytic material were determined using a NOVA 2200e nitrogen physical adsorption–desorption (Brunauer–Emmett–Teller, BET) instrument(Quantachrome, USA).The surface morphologies of the catalyst were determined by SEM (Sigma 500, Zeiss).The average size and distribution of the nanoparticles were statistically analyzed using TEM (HT7700, Hitachi, Japan;acceleration voltage 120 kV).The elemental compositions of the catalyst were characterized by an XPS system(ESCALAN250, Thermo VG, USA; Al/Kα was the x-ray source).All the binding energy values were calibrated based on the C 1s(binding energy 284.6 eV)peak for each element.Inductively coupled plasma optical emission spectroscopy(Agilent 700) was used to determine the Co content in the catalyst.
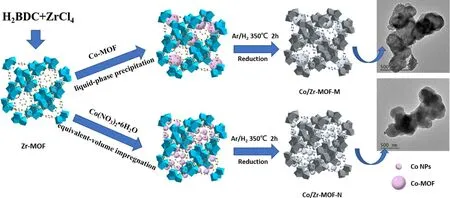
Figure 1.Schematic illustration for the synthesis of Co/Zr-MOF-M and Co/Zr-MOF-N.
2.4.Evaluation of catalyst performance
The performance of the prepared catalysts was evaluated by placing 0.30 g of catalyst into an atmospheric-pressure cold plasma quartz reactor (inner diameter 8 mm, outer diameter 10 mm) [21].A copper wire with a diameter of 1 mm was wound on a copper rod with a diameter of 2 mm as the inner electrode (high-voltage electrode), a layer of aluminum foil was wrapped around the quartz reactor as the outer electrode(ground electrode); the discharge gap was 2 mm.The discharge voltage and discharge frequency of the plasma power supply (CTP-2000 K, Nanjing Suman Electronics Co., Ltd)were 13.0 kV and 7.1 kHz,respectively.The total flow rate of CO2and H2was 30 ml·min−1(VH2:VCO2=4: 1) and a GC7890 gas chromatography system was used for online analysis.The CO2conversion, CO selectivity and CH4selectivity were calculated, respectively, as follows:
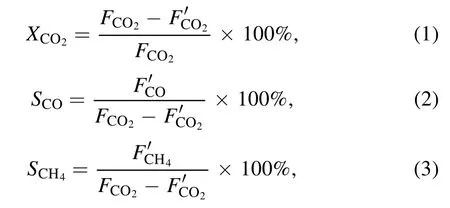
whereFandF′ are the inlet and outlet gas flow rates,respectively.
3.Results and discussion
Zr-MOF, Co/Zr-MOF-M and Co/Zr-MOF-N were placed separately into an atmospheric-pressure plasma reactor to study the CO2hydrogenation conversion under the combined action of the catalytic material and plasma.As shown in figure 2(a), when Zr-MOF was added to the plasma reactor,the CO2conversion was 20.2%, and the CO and CH4selectivities were 95.1% and 4.9%, respectively.When Co/Zr-MOF-N was added to the plasma reactor,the CO2conversion increased to 24.8%, the CO selectivity decreased to 90.2%and CH4selectivity increased to 9.8%.When Co/Zr-MOF-M was added to the plasma reactor, the CO2conversion increased to 58.9%,which was 2.9 times and 2.3 times greater than with Zr-MOF and Co/Zr-MOF-N, respectively.CH4was the main reaction product,and its selectivity increased to 94.7%.The CO selectivity was only 5.3%.The carbon balance value of CO2methanation under plasma-assisted Co/Zr-MOF-M, Co/Zr-MOF-N and Zr-MOF was in the range of 98%–102%.The above findings indicate that the Co/Zr-MOF-N and atmospheric-pressure plasma had a certain synergistic effect, which improved the CO2conversion and the CH4selectivity.Meanwhile, the synergistic effect of Co/Zr-MOF-M and the atmospheric-pressure plasma greatly improved the CO2conversion rate and CH4selectivity,which may have been due to the differences in the structures and compositions of Co/Zr-MOF-M and Co/Zr-MOF-N.We also investigated the influence of active components and Co loading on CO2methanation.The results are shown in figures S3 and 2(b).It can be seen from figure S3 that Co/Zr-MOFM gave the highest CO2conversion and CH4selectivity than that of Ni/Zr-MOF-M, Cu/Zr-MOF-M and Fe/Zr-MOF-M,and from figure 2(b)that CO2conversion and CH4selectivity increase gradually with increased Co loading.
Figure 3 shows the XRD patterns of Zr-MOF, Co/Zr-MOF-M and Co/Zr-MOF-N.The three samples showed the characteristic diffraction peaks of Zr-MOF at 7.4°, 8.5° and 25.7°, corresponding to the simulated Zr-MOF standard characteristic peaks.In the XRD patterns of Co/Zr-MOF-M and Co/Zr-MOF-N,no diffraction peaks of Co and its oxides were found.This indicated that the basic framework structure of Zr-MOF was not destroyed in the process of preparing Co/Zr-MOF-M and Co/Zr-MOF-N, and that calcination at 350°C for 2 h had a minor effect on the framework structure of the Zr-MOF.At the same time,compared with other supports,Zr-MOF had a larger specific surface area, and cobalt or its oxide could be highly dispersed with a relatively high loading in the Zr-MOF support.Therefore, when the cobalt loading was greater than 10%, no diffraction peaks of cobalt and its oxides were found in the XRD pattern.

Figure 2.Plasma-assisted CO2 conversion,CO and CH4 selectivity and C balance over(a)Zr-MOF,Co/Zr-MOF-M and Co/Zr-MOF-N and(b) Co/Zr-MOF-M with different Co loadings.
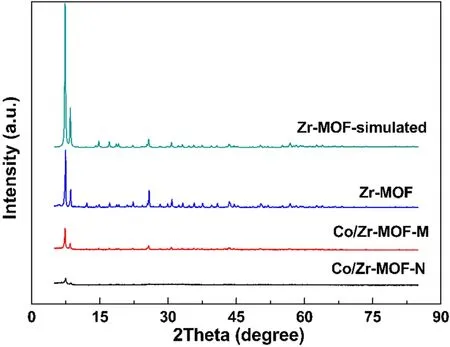
Figure 3.XRD patterns of Zr-MOF, Co/Zr-MOF-M and Co/Zr-MOF-N.
Figure 4 shows SEM images of Zr-MOF,Co-MOF,Co/Zr-MOF-M and Co/Zr-MOF-N.Figure 4(a) shows the complete Zr-MOF structure, which is consistent with literature results [22].Figure 4(b) shows that the Co-MOF had a regular octahedral morphology with a high degree of crystallinity, suggesting that the Co-MOF prepared by liquidphase precipitation had a good crystalline state.Figures 4(c)and (d) show SEM images of Co/Zr-MOF-M and Co/Zr-MOF-N, respectively.Their morphologies are essentially consistent with that of Zr-MOF shown in figure 4(a),demonstrating that the morphology of Zr-MOF is generally maintained in the precursor of the catalytic material after calcination at 350 °C for 2 h.
Table 1 lists the specific surface area, pore size and pore volume data obtained from the nitrogen adsorption–desorption isotherms and pore size distribution diagrams of the Zr-MOF, Co/Zr-MOF-M and Co/Zr-MOF-N samples.Compared with the specific surface area of Zr-MOF (1085.8 m2·g−1),the specific surface areas of Co/Zr-MOF-M and Co/Zr-MOF-N were greatly reduced (438.2 and 255.2 m2·g−1,respectively),indicating that covering of the surface of the Zr-MOF by cobalt and its oxides reduced the specific surface area of the Zr-MOF.Comparison of the pore volume and the average pore diameter of the three samples showed that the pore volume and average pore diameter of Co/Zr-MOF-N were equivalent to those of the Zr-MOF,but the pore volume and average pore diameter of Co/Zr-MOF-M were slightly larger than those of the Zr-MOF.This may have been related to the cobalt precursor of Co/Zr-MOF-M being the Co-MOF and the porous structure formed during the calcination process.This was also confirmed by the fact that the specific surface area of Co/Zr-MOF-M was significantly larger than that of Co/Zr-MOF-N.
Figure 5 shows the XPS spectra of Co/Zr-MOF-M and Co/Zr-MOF-N, including the full spectrum and Co 2p highresolution spectra.The full spectrum in figure 5(a)shows that the surfaces of the Co/Zr-MOF-M and Co/Zr-MOF-N samples all contained Zr, Co, O and C, which is consistent with the elemental compositions of the precursor after calcination.The peaks with binding energies of about 780.8 and 796.5 eV in figure 5(b) are associated with Co 2p3/2and Co 2p1/2,respectively, which are consistent with values reported previously [23].The strong peaks at 785.8 and 803.1 eV are the Co 2p satellite peak signals, indicating that the two catalysts contained a relatively high amount of Co2+.To quantitatively compare the Co content in different valence states on the surface of the catalytic material,the Co 2p in figure 5(b)was subjected to a peak fitting process.The deconvoluted peak of Co 2p showed that only Co2+and Co3+existed on the surfaces of the two samples.Furthermore, the binding energies of Co2+and Co3+in the Co/Zr-MOF-M sample were slightly lower than those in the Co/Zr-MOF-N sample,indicating that the chemical environments of the Co in the two catalysts are slightly different.The surface Co2+/Cototaland Co/Zr ratios of the two samples obtained from the Co 2p peak fitting results and the surface element atomic ratios are listed in table 1.The Co2+/Cototalratio (0.85) and Co/Zr ratio (0.55)of Co/Zr-MOF-M were higher than those of Co/Zr-MOF-N(Co2+/Cototalof 0.78 and Co/Zr ratio of 0.27), which may have been the main reason why the activity of Co/Zr-MOFM was higher than that of Co/Zr-MOF-N.Liet al[24]reported Co/ZrO2catalysts prepared by an organic acidassisted incipient wetness impregnation method.The Co/ZrO2exhibited excellent performance for CO2methanation, the activity was more than twice that the catalyst prepared by a conventional method and Co2+was beneficial for CO2methanation.

Figure 4.SEM images of (a) Zr-MOF, (b) Co-MOF, (c) Co/Zr-MOF-M and (d) Co/Zr-MOF-N.
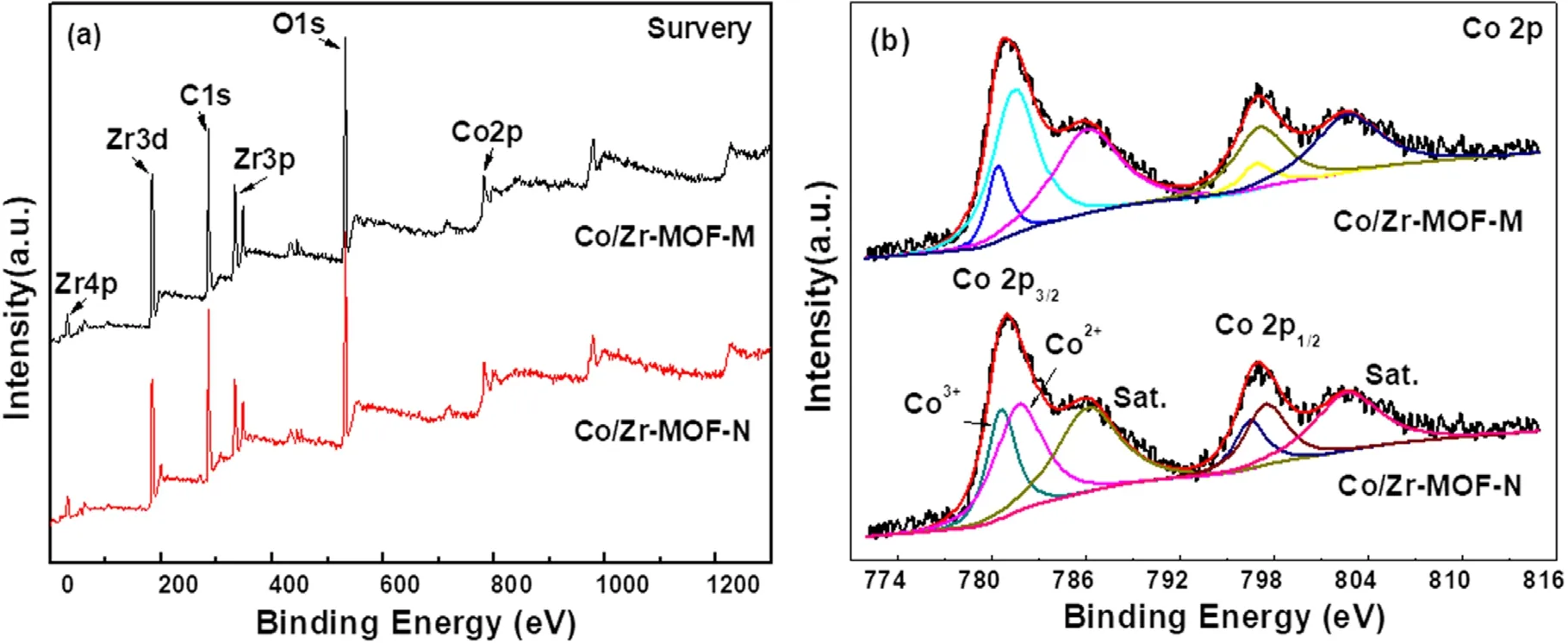
Figure 5.XPS spectra of Co/Zr-MOF-M and Co/Zr-MOF-N: (a) survey spectrum and (b) Co 2p spectra.
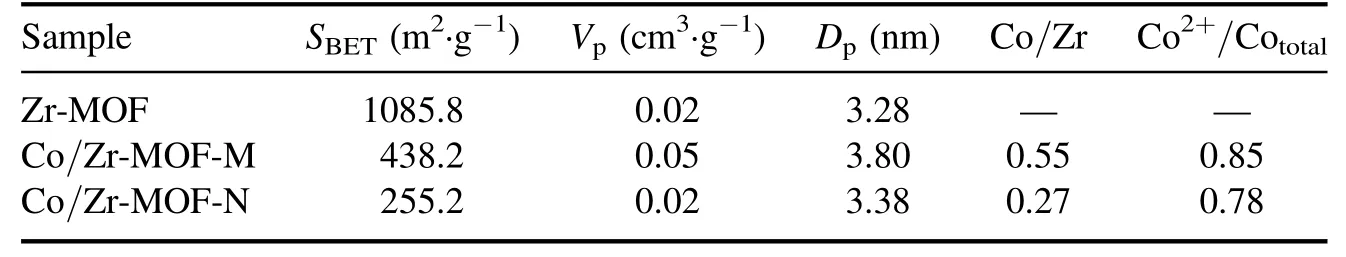
Table 1.Specific surface areas,pore diameters,pore volumes,Co/Zr ratios and Co2+/Cototal ratios of Zr-MOF,Co/Zr-MOF-M and Co/Zr-MOF-N.
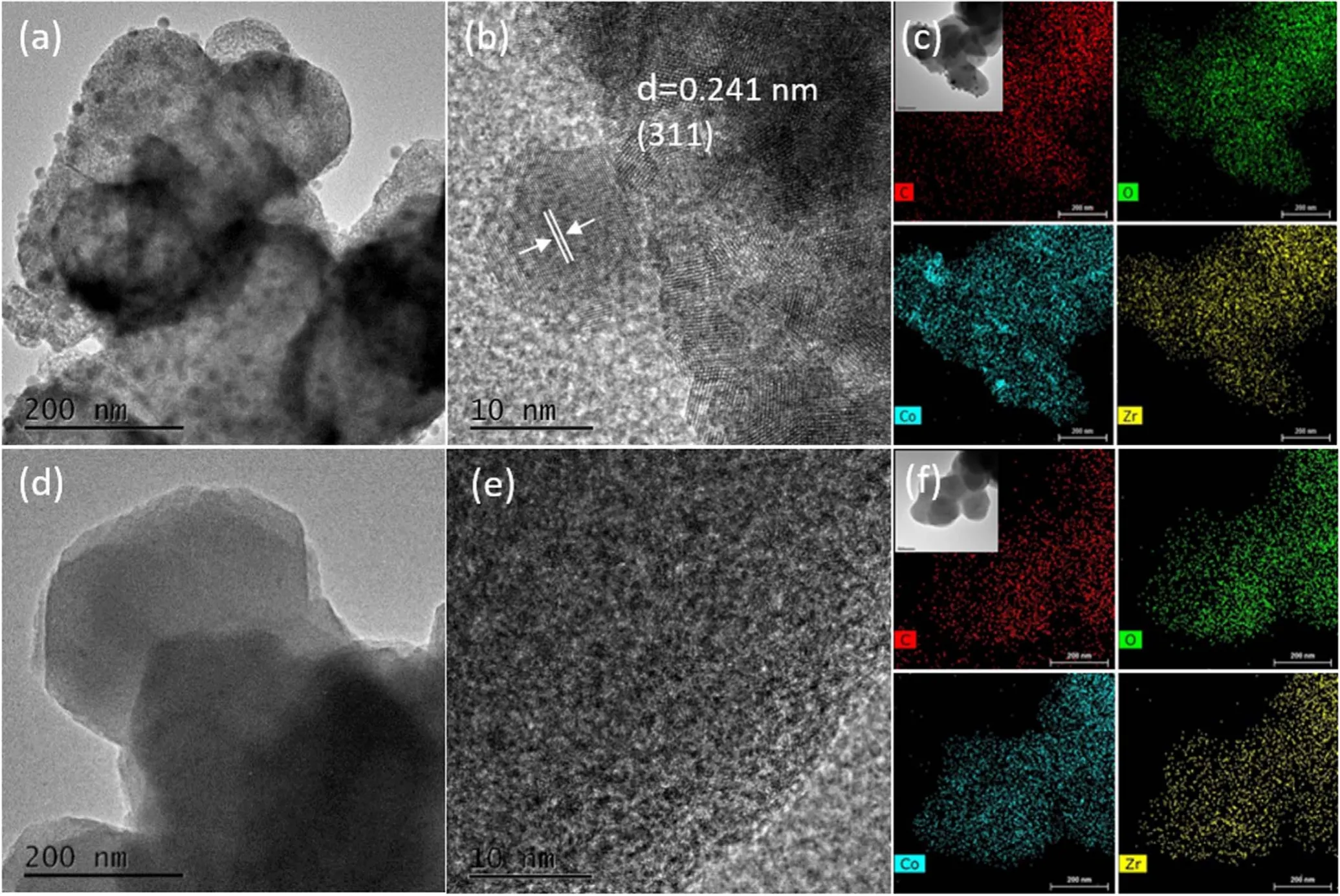
Figure 6.(a),(b),(d),(e)HRTEM images of Co/Zr-MOF-M and Co/Zr-MOF-N.(c),(f)Elemental mappings of C,O,Zr and Co on Co/Zr-MOF-M and Co/Zr-MOF-N.
Figure 6 shows the TEM measurement results of the two samples.Figures 6(a) and (b) show the high-resolution TEM(HRTEM) images and figure 6(c) shows the elemental mapping of Co/Zr-MOF-M.Figure 6(a) clearly shows that Co oxide was distributed on the Zr-MOF in the form of microparticles, with a particle size of about 16±0.26 nm.Lattice fringes are evident in figure 6(b).The lattice spacing was 0.241 nm, corresponding to the (311) crystal plane in the crystal structure of Co3O4cubic spinel [25].The elemental mapping in figure 6(c)clearly shows the distributions of C,O,Zr and Co in the Co/Zr-MOF-M sample.The distributions of C, O and Zr are uniform, the Co oxide is uniformly distributed in the Zr-MOF; some of the bright spots are caused by Co oxide particles.Figures 6(d)and(e)show the HRTEM images and figure 6(f) shows the elemental mapping of Co/Zr-MOF-N.Figure 6(d)shows that the Co oxide is uniformly distributed on the Zr-MOF.According to figure 6(d), the average particle size of Co oxide is 0.52±0.11 nm.The elemental mapping in figure 6(f) shows that Co, C, O and Zr are uniformly distributed in the Co/Zr-MOF-N sample.Comparison of the TEM analysis results of the two samples reveals that the cobalt oxide in the Co/Zr-MOF-M is distributed on the surface of the Zr-MOF in the form of porous particles, facilitating the increase in the specific surface area of the sample, consistent with the BET measurement results.However, the cobalt oxide in the Co/Zr-MOF-N is dispersed on the surface of the Zr-MOF.
4.Conclusions
Zr-MOF material,with a high specific surface area,was used as the carrier to support the Co-MOF and Co(NO3)2·6H2O precursors to obtain Co/Zr-MOF catalysts.The CO2methanation reaction under the combined action of the prepared material and a cold atmospheric-pressure plasma was investigated.Co/Zr-MOF-M with Co-MOF as the precursor had a synergistic effect with the atmospheric-pressure cold plasma,which could effectively increase the CO2conversion(58.9%)and methane selectivity(94.7%).With Co(NO3)2·6H2O as the precursor, Co/Zr-MOF-N was prepared, and the combined effect of Co/Zr-MOF-N and the atmospheric-pressure cold plasma increased the CO2conversion (from 20.2% to 24.8%compared with Zr-MOF),but the main gas phase product was CO.XRD and SEM analyses revealed that Co/Zr-MOF-M and Co/Zr-MOF-N both maintained a good Zr-MOF framework structure.N2adsorption–desorption (BET) analysis showed that Co/Zr-MOF-M had a larger specific surface area, pore volume and average pore diameter than Co/Zr-MOF-N.XPS analysis indicated that Co2+/Cototaland Co/Zr on the surface of the Co/Zr-MOF-M catalytic material were higher than on Co/Zr-MOF-N.TEM results further illustrated the difference in the surface active component distribution between Co/Zr-MOF-M and Co/Zr-MOF-N.The higher catalytic activity of the Co/Zr-MOF-M catalytic material could be attributed to the following:(1)the Co/Zr-MOF-M catalytic material had a higher specific surface area and higher Co2+/Cototaland Co/Zr ratios; (2) the cobalt active component in the Co/Zr-MOF-M catalytic material was distributed on the surface of the Zr-MOF as porous microparticles,while the cobalt active component in the Co/Zr-MOF-N catalytic material was dispersed on the Zr-MOF surface.
Acknowledgments
This work is supported by National Natural Science Foundation of China (Nos.21673026, 11605020), Innovative Training Program for College Student of Liaoning Province(No.S202011258068)
ORCID iDs
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- UV and soft x-ray emission from gaseous and solid targets employing SiC detectors
- The acceleration mechanism of shock wave induced by millisecond-nanosecond combined-pulse laser on silicon
- Investigation of non-thermal atmospheric plasma for the degradation of avermectin solution
- Laser-induced breakdown spectroscopy for the classification of wood materials using machine learning methods combined with feature selection
- Colorimetric quantification of aqueous hydrogen peroxide in the DC plasma-liquid system
- Quantitative analysis of uranium in electrorecovery salt of pyroprocessing using laserinduced breakdown spectroscopy
