One-step spraying method to construct superhydrophobic magnesium surface with extraordinary robustness and multi-functions
2021-05-21LingjieLiXiLiJinlongChenLeiLiuJingleiLeiNiningLiGoLiu
Lingjie Li, Xi Li, Jinlong Chen, Lei Liu, Jinglei Lei,*, Nining Li, Go Liu,
Fusheng Pand
aSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400044 PR China
b School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715 PR China
cEnergy Storage & Distributed Resources Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720 USA
d School of Materials Science and Engineering, Chongqing University, Chongqing, 400044 PR China
Received 1 December 2019; received in revised form 11 June 2020; accepted 22 June 2020
Available online 6 October 2020
Abstract
Keywords: Magnesium alloy; Spraying method; Superhydrophobicity; Robustness; Multi-functions.
1. Introduction
Magnesium alloys, due to their superior strength-to-weight ratio, low density, excellent castability and other unique characteristics, are expected to fin wide applications in many field such like aerospace, transportation and etc. [1-4].Nevertheless, the application of magnesium alloys has been severely limited by their active chemical property and highsusceptibility to be damaged in harsh environments [5,6].To protect magnesium alloys from various damages and extend their service life as well as expand their applications,various surface treatments have been attempted and applied[7-9]. Among them, the superhydrophobic coating, which has a static contact angle (CA) more than 150° and a roll-off angle lower than 10° [10,11], is considered as one of the most promising surface treatments due to the great potentials in corrosion protection[12],self-cleaning[13-15],oil/water separation[16,17],anti-icing[18,19],and so forth[20,21].However,at present the practical applicability of the superhydrophobic coatings still faces great challenges.For example,the poor robustness to various damages cannot satisfy the requirements for applications in harsh environments [22-24]. Meanwhile,the surface multi-functions have not yet been realized and applied,which block the further promotion of magnesium alloys[25-28]. Hence, the superhydrophobic coatings with extraordinary robustness and multi-functions on magnesium alloys are highly desirable for improving the service performance and expanding the application field of magnesium alloys.
As well known,rough structure and low surface energy are two indispensable factors for a superhydrophobic coating.The conventional methods to prepare a superhydrophobic coating include constructing a rough structure on the metallic substrates and modifying the rough structure with low surfacefree-energy compounds [28-30]. Generally, the above methods need two or more steps,and the complexity seriously limits the large-scale fabrication of the superhydrophobic coatings[31-33].So,the simple and scalable methods to fabricate the superhydrophobic coatings with extraordinary robustness and multi-functions are urgently indispensable.
Spraying method, with the advantages of simplicity, scalability, substrate-independence and etc., has been widely used in industry field to fabricate various coatings [12,34-37].However, the superhydrophobic coatings fabricated via spraying always suffer from the poor durability that caused by incompatibility of nanoparticles and weak adhesion to substrates. To resolve these problems, in this work, we develop a simple and scalable method based on spraying to construct the superhydrophobic coatings with extraordinary robustness and multi-functions on magnesium alloys.The homemade spraying suspension contains nanoparticles with low surface-free-energy, which can provide the rough structure and decrease the surface energy, endowing superhydrophobicity. Meanwhile, the polymer component in the spraying suspension can bind the nanoparticles tightly in the coating and enhance the adhesion to the substrate, promoting the coating robustness and functionality. The as-prepared coating is superhydrophobic with a high static contact angle and a low roll-off angle. The as-treated magnesium surface demonstrates extraordinary robustness to resist various physical and chemical damages,including sand impact,water impact,abrasion, peeling, high temperature, strong acidic/salty/basic media and long-time immersion in different organic-solvents.Moreover, the surface displays multi-functions of corrosion protection, anti-fouling and heat insulation. Such robust and multi-functional coatings will undoubtedly boost various practical applications of magnesium alloys.
2. Experimental
2.1. Materials and chemicals
AZ31 Mg alloy (Chongqing Magnesium Co., Ltd.,Chongqing, PR China; composition: 95.75wt% magnesium,3.0wt% aluminum, 1.0wt% zinc, 0.15wt% manganese, and 0.1wt% silicon) was used as the substrates. Chemical reagents, including acetone and ethanol, were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, P.R. China. Poly(methyl methacrylate) (PMMA) (Mw=120 000gmol-1) and ZnO nanoparticles (ca. 30nm) were purchased from Aladdin Reagent Co., Ltd., Shanghai, P. R.China. Stearic acid (SA) was obtained from Boyi Chemical Reagent Co., Ltd., Chongqing, P. R. China. All chemicals were of analytical grade and used without further purification
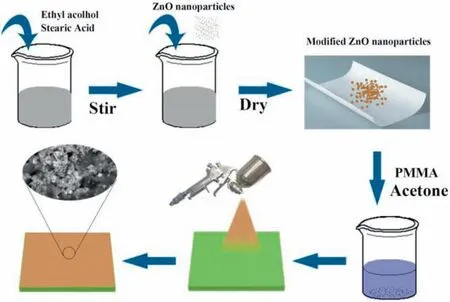
Fig. 1. Schematic illustration of the fabricating process.
2.2. Coating fabrication
First, the AZ31 Mg substrates were abraded with 180#,400# and 800# grit emery papers, and then ultrasonicated in ethanol for 5min and rinsed with pure water. Subsequently,the home-made spraying suspension (1.0mL) was uniformly sprayed on the clean AZ31 Mg substrate (with the surface area of 6.0cm2).The homemade spraying suspension was prepared as follows: 2.0g PMMA was dissolved completely in 20mL acetone, and then 2.0g SA-modifie ZnO nanoparticles were dispersed in the above solution and stirred for 1h.The SA-modifie ZnO nanoparticles (2.0g) were synthesized by dispersing SA (0.5g) and ZnO nanoparticles (0.9g) in the absolute ethanol (12mL) and stirring for 6h at room temperature, and then dried at 60°C overnight. The as-sprayed AZ31 Mg sample was finall dried at 60°C for 2h to obtain the as-sprayed coating. The above fabricating process is schematically illustrated in Fig. 1.
2.3. Characterization
The static and dynamic CAs of the samples were evaluated at room temperature by a contact angle meter (Dataphysics OCA20, Germany) with droplets of 2μL and 4μL, respectively. The surface and cross-section morphologies were characterized by a fiel emission scanning electron microscope(FESEM, JEOL JSM-7800F, Japan). The surface roughness was measured by an atomic force microscope (AFM, MFP-3D Origin, USA). The surface composition was analyzed by X-ray photoelectron spectroscopy (XPS, Thermo electron ESCALAB 250, USA) using Al Ka radiation and Fourier transform infrared spectroscopy (FTIR, Thermo Scientifi Nicolet iS10, USA).The dynamic impact processes of droplets on the sample surfaces were captured by using a high-speed camera(Phatom V7.3, USA) at 5000 frames per second.
2.4. Robustness tests
Mechanical stability tests: The sand impact test was conducted by releasing sand grains (with the diameter of 150-250μm) at a rate of 10gmin-1from a height of 20cm and recorded the variations of CAs every 2min. In the water impact test, a water droplet of 10μL was released from a height of 10cm and impacted on the inclined surface (with an angle of 45°) at a rate of 2 impacts s-1, and the variations of CAs were recorded every 30min. The abrasion test was performed by moving the sample on a sand paper (800#)under a pressure of 1.00kPa for 50cm, which was define as one cycle, and the variations of CAs were recorded every fi e cycles. The peeling test was conducted and the process is as follows: A tape (4.5cm width) was pressed tightly and completely on the sample surface (≥2.15kPa), and then was slowly peeled off from one end to the other end. The above process was repeated several times and the CAs after every time were recorded.
Chemical stability tests: The chemical stability of the assprayed sample in strong acidic,salty and basic media as well as in different organic solvents for long time was evaluated by measuring the static CAs of droplets with different pH values ranging from 1 to 13 on the sample surface or by recording the CAs variations during immersion of the sample in different solvents(including ethanol and n-hexane)for 24h.
Thermal stability test: The thermal stability test was performed by placing the as-sprayed sample in an oven of 200°C for 24h and recording the CAs of the sample surface every 3h.
2.5. Multi-function evaluation
Corrosion protection test: The corrosion resistance of the surface coating was characterized by the electrochemical impedance spectroscopy (EIS) technique, which was performed on an electrochemical workstation (AUTOLAB PGSTAT302N, Switzerland) at room temperature. A classical three-electrode configuratio was employed in the EIS measurements with the different AZ31 Mg samples (with a working area of 1.0cm2) as the working electrodes (WE), a saturated calomel electrode(SCE)as the reference electrode(RE),a platinum sheet (2cm×2cm) as the counter electrode (CE)and 3.5 wt% NaCl corrosive solution as the electrolyte. The EIS measurement was carried out in a frequency range of 0.01 Hz-100kHz at open circuit potential (OCP) with the AC amplitude of 50mV.
Anti-fouling test: The anti-fouling function of the assprayed magnesium surfaces against different contaminants was verifie by sprinkling the contaminants (carbon black powder and soil) on the inclined samples (with an angle of 10°) and recording the dynamic behavior of the rolling droplets to take away the contaminants.
Heat insulation test: First, the as-sprayed sample and control sample was placed individually into two foam boxes of the same size (7.0 dm3), where the samples were illustrated by infrared lamps (250W) at the distance of 20cm. Then the temperature variations of the interior air in the two boxes were probed by a thermocouple thermometer (putting in the center of the box). The heat insulation function of the surface coating was proved by the comparison results.
3. Results and discussion
3.1. Characterization results on morphology and composition
The surface morphology of the bare AZ31 Mg substrate is shown in Fig. 2a, which is smooth without any featured microstructure. As for the as-sprayed sample, the surface is rough with well dispersed clusters (Fig. 2b). Fig. 2c shows the cross-section image of the as-sprayed sample. The coating with a thickness of ~50μm is free from cracks, which adheres tightly to the substrate. Because the surface roughness plays a key role in construction of the as-sprayed surface, the AFM characterization was carried out to quantify the surface roughness of the as-sprayed sample. From the AFM image in Fig. 2d, the roughness value Rqcan be calculated, which is ca. 440.79nm.
To identify the chemical composition of the as-sprayed AZ31 Mg surface, XPS technique was employed. Fig. 3a illustrates the XPS survey spectrum of the as-sprayed surface, from which the elements of Zn, C and O are confirmed Fig. 3b shows the high-resolution spectrum of Zn 2p. The peaks at BE (binding energy) of 1021.3 and 1044.4eV correspond to Zn 2p1/2 and Zn 2p3/2 [38,39], respectively, which are the characteristic of Zn element in +2 valency. The C 1s high-resolution spectrum (Fig. 3c) has two peaks: the main peak at BE=284.6eV is attributed to C-C and C-H, and the other small peak at BE=288. 6eV is related to O-C=O[38-42]. As for the O 1s high-resolution spectrum (Fig. 3d),it can be fitte by two peaks, among which the peak at BE=531.9eV is assigned to -COO- from SA and PMMA while that at BE=530.8eV correlates with Zn-O [38-40].Furthermore, FTIR characterization was conducted for the assprayed sample and the result is shown in Fig. 3e. Combining with the FTIR spectra of the control samples of the pristine ZnO, SA, and SA-modifie ZnO, we can get a clear understanding on the origin of the featured groups of the as-sprayed surface.The absorption peaks centered at 1538 and 1463cm-1are attributed to the asymmetric and symmetric stretching vibrations of the carbonyl groups (-COO-) from SA while that at about 1725cm-1is caused by the stretching vibration of C=O in PMMA [42,43]. The adsorption peaks at about 2915 and 2846cm-1are assigned to the asymmetric and symmetric stretching vibrations of -CH2- bands [42]. From the above results, it can be inferred that SA-modifie ZnO nanoparticles were incorporated into the polymer PMMA to form the coating. The SA-modifie ZnO nanoparticles can provide the appropriate roughness and the long-chain alkyl groups of SA can decrease the surface energy while PMMA binds them tightly to the substrate, which are all crucial for the coating superhydrophobicity and robustness.
3.2. Surface superhydrophobicity and robustness
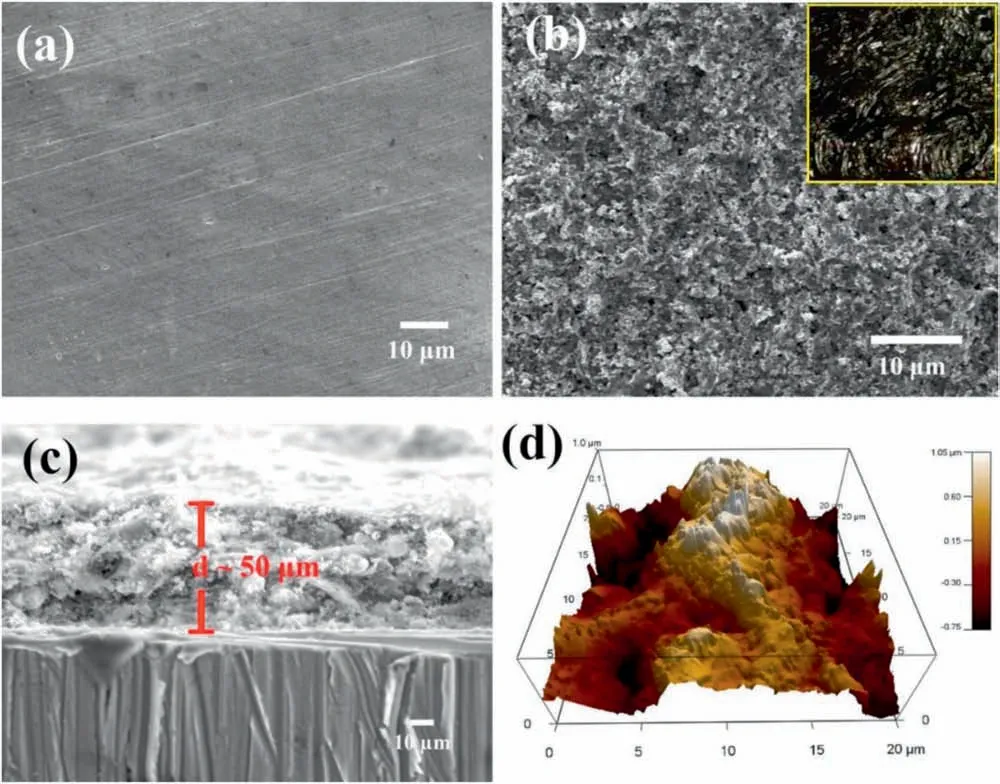
Fig. 2. FESEM images of (a) AZ31 Mg substrate surface, (b) as-sprayed AZ31 Mg surface, and (c) cross-section of as-sprayed AZ31 Mg sample. (d) AFM 3D image of as-sprayed AZ31 Mg surface.
The bare AZ31 Mg surface has a static CA of 67.3°(Fig. 4a) and a dyed water droplet (in green) spreads on it(Fig. 4b), indicating the hydrophilic nature of the untreated surface. The static CA of the as-sprayed sample is 157.0°(Fig. 4c) and a water droplet (dyed in green) remains the spherical shape on the surface (inset of Fig. 4c), suggesting the superhydrophobic character of the as-sprayed sample. The adhesive property of the as-sprayed sample was evaluated by measuring the rolling angle, which is 6.0° (Fig. 4d), implying the low adhesion of the sample surface. The above results demonstrate the successful construction of the superhydrophobic coating on the AZ31 Mg substrate via the facile one-step spaying method.
The practical applications of magnesium alloys in harsh environments highly depend on the durability of the surface coatings,which are required to durably resist various mechanical and chemical damages. To study the mechanical stability of the as-sprayed sample,the tests to resist sand impact,water impact, abrasion and peeling were carried out. As presented in Fig. 5a, the CAs of the as-sprayed sample surface changed little (from 156.4° to 152.0°) after sand impact for 20min.The result of water impact is shown in Fig. 5b, which indicates the unchangeable CAs of the as-sprayed sample surface even after continuous water impact for 3 h. Fig. 5c shows the CAs variation of the as-sprayed sample surface during the abrasion test, which presents no significan differences even after 25 cycles. Meanwhile, the surface morphology of the as-sprayed sample after the abrasion test shows little change(inset of Fig. 5c), further confirmin the strong resistance of the as-sprayed sample surface. Additionally, the tape-peeling test was performed to study the resistance of the as-sprayed sample surface to peeling as well as the adhesion between the surface coating and the underlying substrate. Fig. 5d shows the CAs variation of the as-sprayed sample surface during the peeling test, which indicates that the surface coating has strong adhesion to the substrate and the surface wettability keeps very well. The bouncing behavior of a water-droplet on the sample surface after the peeling test (inset of Fig. 5d)further confirm the high resistance of the as-sprayed sample to peeling damage.
Moreover, the chemical stability of the as-sprayed sample in strong acidic, salty and basic media as well as in organic solvents for long time was investigated. A series of liquid droplets with different pH values from 1 to 13 were dropped on the sample surface respectively, and the static CAs were measured. Fig. 6a shows the CAs of the liquid droplets with different pH values from 1 to 13 on the sample surface,which are all more than 150°, suggesting the brilliant stability of the sample surface to withstand strong acid, salt and alkali.In addition, the CAs variations of the sample surface during immersion in the organic solvents of ethyl alcohol and n-hexane for 24h are shown in Fig. 6b, which display stable wettability. The surface morphology of the sample after immersion in ethyl alcohol for 24h shows little change (inset of Fig. 6b), further confirmin the excellent chemical stability of the as-sprayed sample surface. Notably, the as-sprayed sample demonstrates superb thermal stability, which can keep the stable CAs upon exposure in 200°C atmosphere for at least 24h (Fig. 6c). Such extraordinary thermal stability is crucial for the applications of magnesium alloys in high-temperature environment.
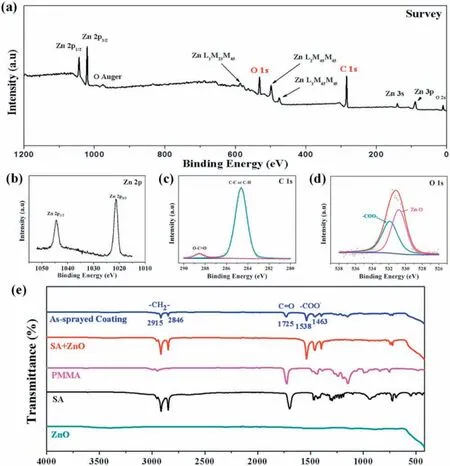
Fig. 3. (a) XPS survey spectrum. High-resolution spectra of (b) Zn 2p, (c) C 1s, and (d) O 1s. (e) FTIR spectra of different samples.
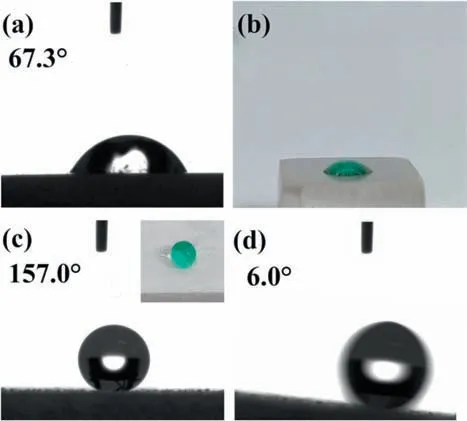
Fig. 4. Static CAs of 2 μL water droplets on different samples: (a) AZ31 Mg substrate and (c) as-sprayed AZ31 Mg. Digital photos of water droplets dyed in green on different samples: (b) AZ31 Mg substrate and inset of (c)as-sprayed AZ31 Mg. (d) Roll-off angle of 4μL water droplet on as-sprayed AZ31 Mg sample.
3.3. Multi-functions
The corrosion-protection function of a surface coating on a metallic substrate is essential for the practical application of the metallic materials. EIS technique is usually used to qualify the corrosion-protection ability of a coating. Fig. 7 shows the EIS results of the bare AZ31 Mg substrate and as-sprayed AZ31 Mg sample. The Nyquist plot of the bare substrate is characterized by a capacitive loop at high frequencies and an inductive loop at low frequencies. The capacitive loop is caused by the charge transfer process while the inductive loop is attributed to the adsorbed intermediate products at the electrode-electrolyte interface. But for the as-sprayed AZ31 Mg sample, the Nyquist plot is composed of a small loop at the high-frequency range correlating with the as-sprayed coating and a large loop representing the charge transfer process at the coating-electrolyte interface. The insets of Fig. 7a and b illustrate the corresponding equivalent circuits used to fi the EIS spectra, where Rs, Rtand CPEdlrepresent the solution resistance, charge transfer resistance and double layer capacitance, respectively, and additional inductance (L) as well as the resistance (RL) are added for describing the inductive loop for the bare substrate while CPEcand Rcare depicted as the capacitance and resistance of the as-sprayed coating.The fittin spectra are also plotted in Fig. 7, which are in good agreement with the experimental spectra. The electrochemical parameters obtained through EIS fittin are listed in Table 1. Obviously, the Rtvalue of the as-sprayed sample (3.05×106Ωcm2) is much larger than that of the untreated substrate (441.60Ωcm2), suggesting the as-sprayed coating effectively retards the charge transfer at the electrodeelectrolyte interface.The Rcvalue of the as-sprayed coating is 1.23×105Ωcm2, which is higher than that of other reported coatings [5,6], indicating that the coating has a high corrosion resistance and can play an important role in protection of magnesium alloys from corrosion.
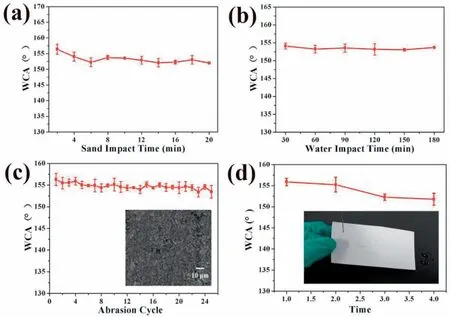
Fig. 5. Variations of CAs in (a) sand impact test, (b) water impact test, (c) abrasion test and (d) peeling test. Inset of (c): FESEM image of the sample surface morphology after the abrasion test, and inset of (d): snapshot of the water-droplet bouncing on the sample surface after the peeling test. (The error bars in Fig. 5(a)-(d) are the average +/- standard deviations of three measurements.).
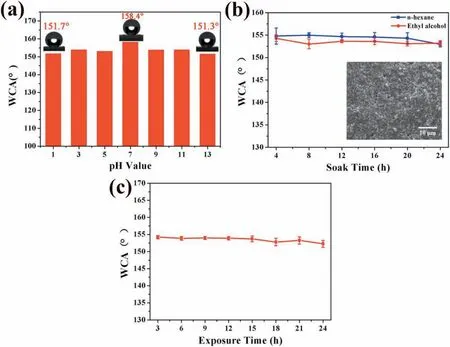
Fig. 6. (a) CAs of the liquid droplets with different pH values on the sample surface. Variations of CAs during (b) immersion in the organic solvents of ethyl alcohol and n-hexane for 24h, and (c) exposure in 200°C atmosphere for 24h. Inset of (b): FESEM image of the sample surface morphology after immersion in ethyl alcohol for 24h. (The error bars in Fig. 6(b) and (c) are the average +/- standard deviations of three measurements.).

Table 1 Electrochemical parameters obtained by fittin EIS spectra shown in Fig. 7.
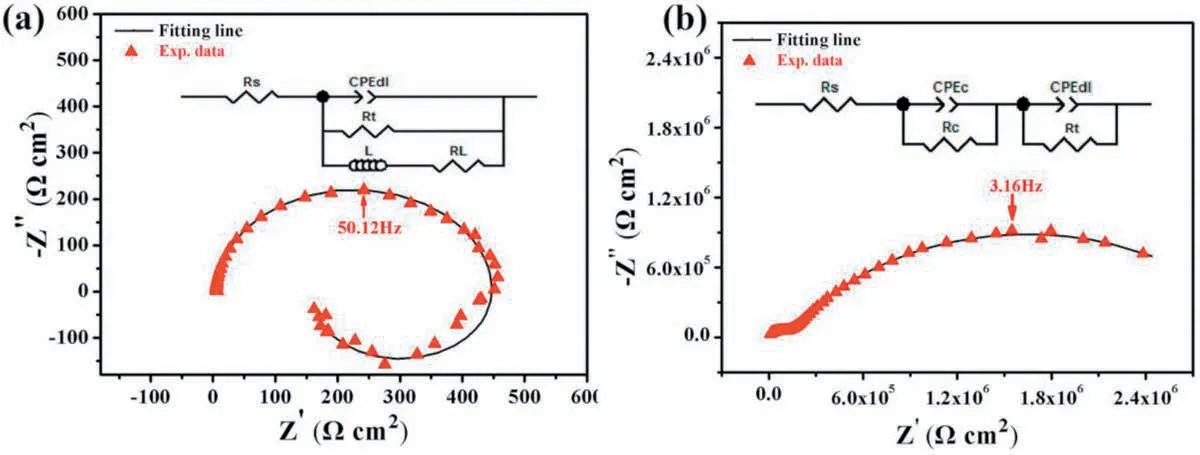
Fig. 7. Nyquist plots of (a) bare AZ31 Mg substrate and (b) as-sprayed AZ31 Mg sample. Insets are the corresponding equivalent circuits for fittin the experimental spectra.
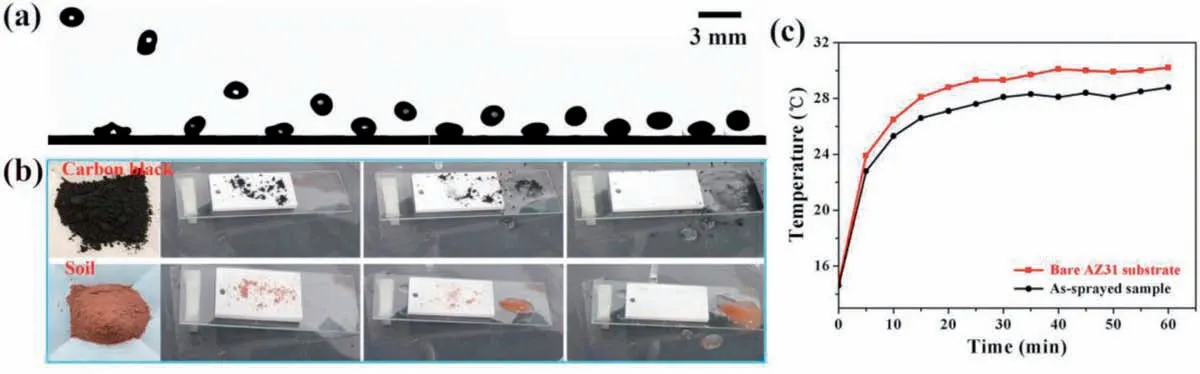
Fig. 8. (a) Snapshots of the water-repellency behavior and (b) Digital photographs of the anti-fouling performance of the as-sprayed sample surface. (c)Temperature variations of the interior air in the two boxes that respectively placed of the as-sprayed sample and bare AZ31 substrate during the IR irradiation.
Since most magnesium alloys are used outdoors and their surfaces are inevitably fouled by various contaminants, the anti-fouling function of magnesium surfaces is crucial for the practical applications. Fig. 8a displays the dynamic bouncing behavior of a water droplet (10μL) on the as-sprayed sample surface. The droplet rebounds back and forth for many times on the sample surface, indicating the as-sprayed surface has desirable water-repellency for further anti-fouling application.As shown in Fig. 8b, the target contaminants (carbon black and soil) were sprinkled on the as-sprayed sample surfaces(inclined with a tilt angle of about 10°). The water droplets were released from a distance of 1cm, and then slid off the surfaces and took away the contaminants. In a short time the contaminants were cleaned up and the sample surfaces recover tidiness, which demonstrate the brilliant anti-fouling ability of the as-sprayed samples. Interestingly, the as-sprayed sample has a certain heat insulation effect. Fig. 8c illustrates the temperature variations of the interior air in the two boxes that respectively placed of the as-sprayed sample and bare AZ31 substrate during the IR irradiation. The temperature in the box with the as-sprayed sample is lower than that in the box with the bare magnesium alloy, and the temperature difference remains stable at 1.5°C after irradiating for 30 min,suggesting a certain heat insulation effect of the as-sprayed sample.
4. Conclusion
In summary, the superhydrophobic coating with extraordinary robustness and multi-functions was facilely fabricated on magnesium alloys via a one-step spraying method. The as-treated magnesium surface displays excellent mechanical,chemical and thermal stabilities under harsh conditions including various physical damages (sand impact, water impact,abrasion, peeling, high temperature) and chemical damages(strong acidic/salty/basic media, long-time immersion in different organic solvents), which are highly desirable in practical applications. Remarkably, the surface demonstrates multi-functions of corrosion protection, anti-fouling and heat insulation. The present work provides an effective way for the surface treatment of magnesium alloys, which will undoubtedly promote their much wider applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China(21773019,21972012),the Graduate Research and Innovation Foundation of Chongqing (CYB18044)and the sharing fund of Chongqing University′s Large-scale Equipment.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- An efficien and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies
- Twin recrystallization mechanisms in a high strain rate compressed Mg-Zn alloy
- Corrosion behaviour and cytocompatibility of selected binary magnesium-rare earth alloys
- Correlation between test temperature, applied load and wear transition of Mg97Zn1Y2 alloy
- Residual stress and precipitation of Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy with different quenching rates
