Microstructure and corrosion resistance of a duplex structured Mg-7.5Li-3Al-1Zn
2021-05-21AnnDobkowskBogusAmzykCieslkJiKubsekDliborVojtehDriuszKuEugeniuszHsikJrosMizer
Ann Dobkowsk, Bogusłw Amzyk - Cie´slk, Jiˇrí Kubásek, Dlibor Vojtˇeh,Driusz Ku, Eugeniusz Hsik, Jrosłw Mizer
a Faculty of Materials Science and Engineering, Warsaw University of Technology, 141 Wołoska St, 02-507 Warsaw, Poland
b Chemistry Department, Western University of Ontario, 1151 Richmond St, London, ON N6A 5B7, Canada
c Department of Metals and Corrosion Engineering, University of Chemistry and Technology, Technicka 5, 166 28 Prague 6, Czechia
d Institute of Materials Engineering, Silesian University of Technology, Krasinskiego 8St, 40-019 Katowice, Poland
Received 5 April 2020; received in revised form 23 June 2020; accepted 31 July 2020
Available online 7 October 2020
Abstract
Keywords: Magnesium-Lithium corrosion; Extrusion; Annealing.
1. Introduction
With the rapid expansion of the automotive and aircraft industries, there are new and ever-increasing requirements for lightweight structural Mg-Li alloys. Due to their low density, and relatively good formability, Mg-Li alloys have a variety of applications [1] and some of them are already in commercial use [2,3]. A group of Mg-Li alloys of particular interest are those that contain between 5 and 11wt% of Li and possess a dual-phase structure composed of a hexagonal close-packed α(Mg) phase (hcp) and body centered-cubic β(Li) phase (bcc) [4-7]. Dual-phase alloys are an excellent compromise between the strength of the α(Mg) and the excellent plasticity of the β(Li) phase [3,8,9]. A mixture of bcc and hcp phases is expected to change the deformation mechanisms and enhance the formability and strength of the hcp structured α(Mg) [10-15].
Although the effect of alloying elements, grain size distribution and other microstructural features on the mechanical properties of dual-phase Mg-Li alloys are described in literature data [16-22], there is very little information about their corrosion resistance [23]. This underrepresentation can be attributed to the fact that corrosion of dual-phase Mg-Li is a complex process [24-26], as Mg can readily form either a galvanic corrosion system with other participationsor, a microgalvanic corrosion system with some secondary phases [27-29]. It is worth noting, that the effects of Clconcentration and pH value on corrosion behavior of dualphase Mg-Li in aqueous solutions have been preliminarily studied by analyzing the corrosion rate, potentiodynamic polarization,and impedance spectroscopy[30-32].However,the detailed description of a corrosion behavior of duplex Mg-Li alloys includes many other factors[33],i.e.the type and number of alloying elements. It was shown by Song et al. [24],that the corrosion rate of Mg-8Li was more rapid than pure Mg, however, there are strong indications that some phases can improve the corrosion resistance of Mg-Li alloys. As described by Zheng et al. [34], the presence of Mg2Ca in Mg-Li-Ca supports the corrosion product formation,and thus prevents further substrate corrosion.Liu et al.[33]proved that the Al4Sr phases distributed along the grain boundaries behave as a barrier for spreading corrosion. Investigations of ascast Mg-6Li and Mg-6Li-6Zn-1.2Y show that the Mg3Zn6Y formed in Mg-6Li-6Zn-1.2Y improved its corrosion resistance [35].
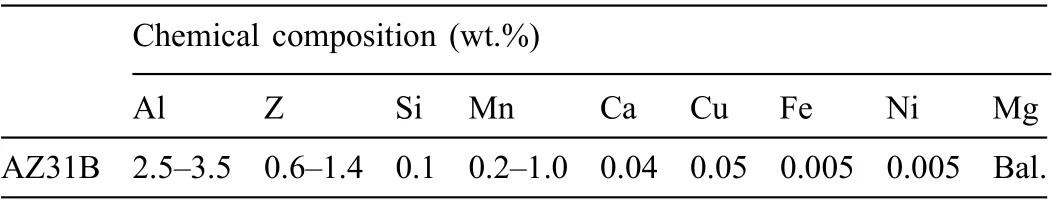
Table 1 Chemical composition of AZ31B [40].
The electrochemical stability of the dual-phase Mg-Li is also dependent on the microstructure refinemen caused by subsequent plastic or thermal deformation [36]. Considering,that microgalvanic corrosion may occur between α(Mg) and β(Li) phases [37], a volume ratio of α(Mg) to β(Li) is of a critical importance for the ongoing research on the corrosive properties of the dual-phase Mg-Li alloys.
The aim of this study is to investigate the role of the microstructural changes induced by post-processing annealing on the corrosion behavior of AZ31 (Mg-3Al-1Zn) where 7.5wt% Li was added.
2. Materials
For the purpose of this work, the dual-phase Mg-7.5Li-3Al-1Zn alloy was designed and prepared utilizing traditional casting and conventional extrusion. The AZ31B (chemical composition is shown in Table 1) was smelted with the addition of 7.5wt% Li (high purity) in a vacuum induction furnace VSG 02 manufactured by Balzers Inc., and ingots about 40mm in diameter were obtained (Fig. 1). Next, the ingots were machined, and extruded to 20mm by conventional hydrostatic extrusion at 350 °C with an extrusion speed of 0.5 s-1. One group of the extruded rods was also subsequently annealed at 350 °C for 1 h in Ar atmosphere and cooled in the air. The conditions of the extrusion and subsequent annealing, were chosen to prevent the unfavorable growth of grains in fabricated alloys [38,39].

Fig. 1. A vacuum induction furnace VSG 02 (Balzers Inc.), and fabricated rod Mg-7.5Li-3Al-1Zn.
3. Methodology
3.1. Microstructure characterization
3.1.1. Chemical and phase analyses
The relative concentration of Li in the microstructure of the alloys was determined by atomic absorption spectroscopy(AAS) with the use of a GBC 932 Plus with an air-acetylene oxidizing flam and a wavelength 670.8nm. The chemical composition of the analyzed alloys was performed using the high-performance wavelength dispersive X-ray fluorescenc(WDXRF) Bruker S4 Explorer spectrometer. To characterize phase composition the X-ray diffraction (XRD) and scanning electron microscopic SEM observations of non-etched samples were used. The XRD patterns were obtained using a Bruker D8 Advance diffractometer. The measurements were done at 40kV and 40mA with Cu Kα radiation using step scanning 2θ from 20° to 100° with a step size of 0.02°, and a count time of 10s per step was used. The semi-quantitative determination of the relative composition of the microstructure components was calculated based on obtained XRD patterns,by comparing the integrated intensities of the diffraction peaks from each of known phases.
3.1.2. Optical, scanning and transmission observations
Optical observations of the alloys after extrusion, and after extrusion with post-processing annealing, were also performed. The samples were molded in epoxy resin, and polished up to 1μm using monocrystalline water-free diamond suspension (with glycol based lubricant). To obtain optical images of the microstructure, the samples were etched with picric etching reagent and optical images were made using an Axio Scope Zeiss A1 microscope.
The phases formed in the materials were observed using a transmission electron microscope (TEM) JEOL JEM-1200EX with an acceleration voltage of 120kV. Thin foils for TEM investigation were prepared using a Gatan Model 656 Dimple Grinder and Gatan Model 691 Precision Ion Polishing System(PIPS).
Automatic EBSD scans were performed using a Hitachi SU70 SEM equipped with HKL data acquisition software.Prior to EBSD scanning, samples were mechanically ground and polished to a mirror-like finish and cleaning in Ar+ion beam using a Hitachi IM4000 Ion Milling System was subsequently employed. All EBSD measurements were conducted with a step size of 1μm at 20kV and at a working distance of 18mm and a sample tilt angle of 70°
The microstructure of the alloys was also described based on the scanning electron microscopy observations (Hitachi SU70 high resolution SEM).The microstructural images were taken prior to EBSD measurements. To describe the phases formed in the material energy dispersive X-ray spectroscopy was used (EDX).
3.2. Corrosion testing and post-corrosion characterization
The electrochemical behavior of samples was investigated in aerated 3.5wt% sodium chloride solution (3.5wt% NaCl)at ambient temperature. The traditional 3 components setup was used, where the Pt was a counter electrode, Ag/AgCl was a reference electrode, and a sample was a working electrode.The samples were immersed for 5 h in open circuit,and the corrosion potential (Ecorr) was recorded. Next, the electrochemical impedance spectroscopy (EIS) was registered, and at the end of the loop, the potentiodynamic polarization tests were done. The electrochemical data was analyzed with Nova 2.1.2 software. Samples in the shape of rod with the dimensions 1cm×0.5cm were threaded and ground with SiC papers (#600, #1200, #2500 and $4000). Prior to measurements samples were ultrasonically cleaned in the ethanol and dried with high purity Ar gas.
To compare the corrosion rate of the alloys, the traditional mass loss measurements were performed. To approach this,the samples with the dimensions of 1cm×1cm×0.5cm were polished and immersed for 5 h in naturally aerated 3.5wt%NaCl, and the 150ml of the solution was used per 1 cm2of the sample surface. The coupons were weighted prior to, and after the immersion. The corrosion product was removed during immersion in a water solution of 200g chromium trioxide(CrO3), 10g silver nitrate (AgNO3) and 20g barium nitrate(Ba(NO3)2)for 1 l distilled water.The corrosion rate(Vc)was calculated using the following formula [41]:

where: Δm is a mass loss (g), t is a time of exposure (day),s is a surface area (m2).
The post-corrosion observations were performed using scanning electron microscopy (Tescan Vega, SEM), and the phase composition of the corrosion product was analyzed.
4. Results
4.1. Microstructure characterization
The designed materials were expected to contain 7.5wt%Li, however, as Li easily oxidizes, after solidificatio and extrusion its concentration was found to be 7.3wt%. Despite the annealing was carried out in Ar atmosphere, the subsequent annealing still resulted in higher Li evaporation, and the amount of Li in the annealed alloy was around 6.9wt%(Table 2).
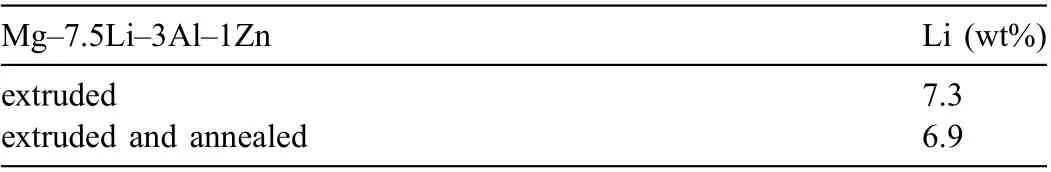
Table 2 Li concentration measured by AAS (wt%).
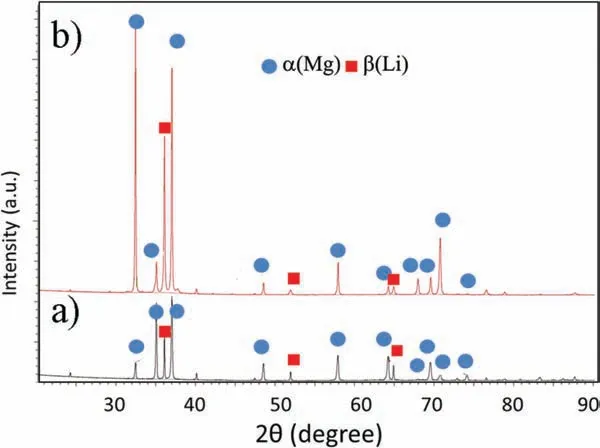
Fig. 2. XRD patterns obtained for a) extruded Mg-7.5Li-3Al-1Zn, b) extruded and annealed Mg-7.5Li-3Al-1Zn.
The XRD patterns presented in Fig. 2 shows that both alloys are mainly composed of α(Mg) and β(Li). The microstructures of the alloys are shown in Figs. 3 and 4. A typical dendritic microstructure is observed for the extruded alloy(Fig.3a).The over-etching occurs in the shoulders of the dendrites (Fig. 3a) suggesting that some elements were segregated at the dendrite’s boundaries, and the EDX mapping shown in Fig. 5a confirm presence of Al and Zn at these locations (Li is not detectable). As observed in SEM images(Fig. 3b and c), this dual-phase microstructure consists of light irregular regions of hcp structured α(Mg) surrounded by dark regions of bcc structured β(Li). The amount of α(Mg)to β(Li) was found to be 71 and 29%, respectively (Table 4).
To initiate the material recrystallization, the extruded rod was annealed at 350°C for 1 h. The microstructure observations show that the thermal processing did not fully trigger the transformation of the dendritic microstructure into a granular one (Fig. 4), however, some grains were created, and they are observed in Fig. 4a. As a consequence of the β(Li) to α(Mg) transformation, the proportions of α(Mg) to β(Li) in the annealed alloy changed and were found to be 65-35%(Table 4). After annealing the dark β(Li) areas became larger than those observed in the extruded alloy (Figs. 3c and 4c).The higher volume of β(Li) formed during annealing may be explained by Li diffusion, which at high temperature is rapid,and occurs faster than Mg diffusion [42]. As XRF analysis shows the chemical composition of the alloy (excluding Li)is in agreement with the normative one (Tables 1 and 3). The distribution of Al, Zn, Mn in the annealed alloy also changed,the color intensity in some points on EDX maps suggest they most likely formed precipitates (Fig. 5b). The gray dots located within α(Mg) in Fig. 4c indicate that secondary phases precipitated within the α(Mg) during post-processing annealing.
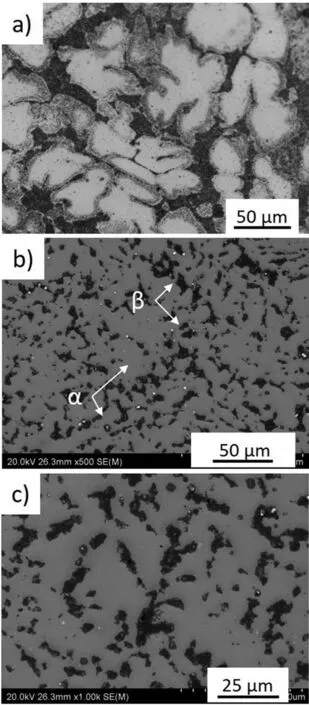
Fig. 3. Microstructure of the extruded Mg-7.5Li-3Al-1Zn: a) optical image after chemical etching, b) and c) SEM images taken from the sample performed for EBSD mapping.
XRD is a reliable method to determine the phase composition of the alloys, however, due to its detection limit, we were not able to identify the rest of the phases formed in the investigated materials. Hence, to confir the presence of the secondary phases,selected area diffraction(SAD)patterns were performed.
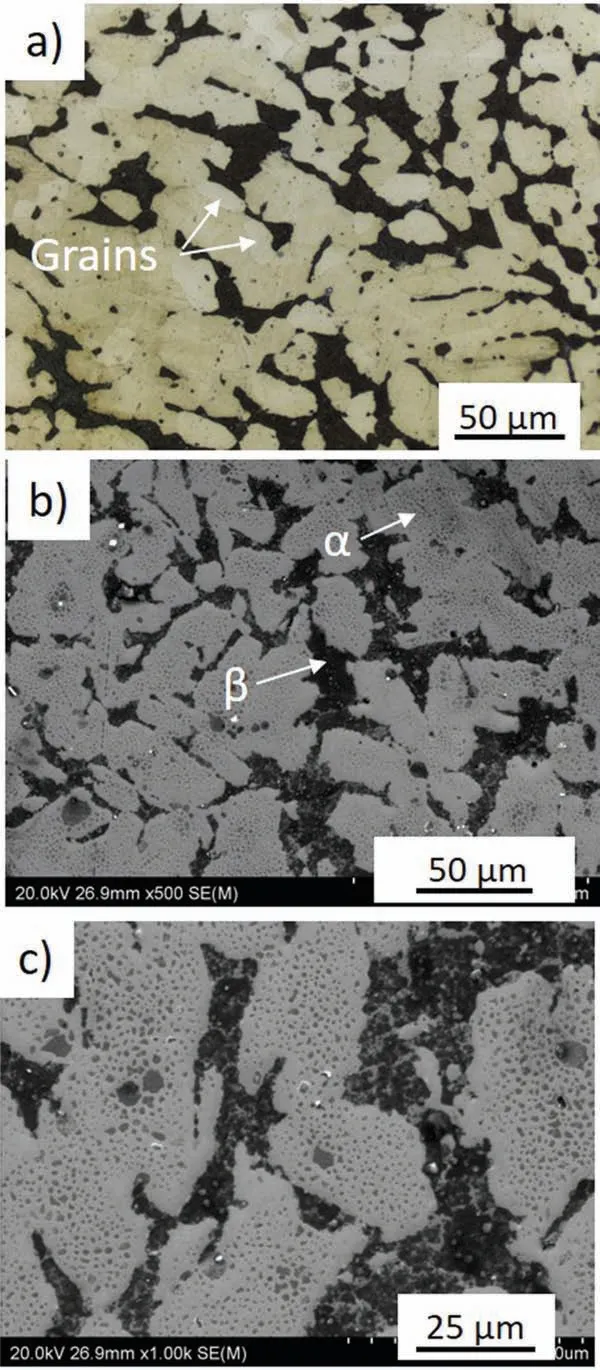
Fig. 4. Microstructure of the extruded and annealed Mg-7.5Li-3Al-1Zn: a)optical image after chemical etching, b) and c) SEM images taken from the sample performed for EBSD mapping.
The electron diffraction patterns verify that randomly distributed AlLi was formed in the extruded alloy (Fig. 6a),and small lamellar Mg17Al12precipitated within the matrix(Fig. 6b). The presence of Θ-MgLi2Al was also identifie(Fig. 6c). After subsequent annealing, the rounded particles of AlLi were also detected by TEM (shown in Fig. 7b and c). Analysis of the diffraction pattern presented in Fig. 7c indicates that the Mg17Al12were still in the alloy. The selected TEM image obtained for α(Mg) and β(Li) in the alloy after subsequent annealing is shown in Fig. 7a.
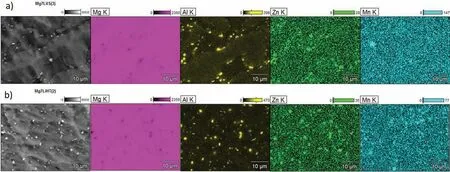
Fig. 5. EDX analyses performed for Mg-7.5Li-3Al-1Zn a) extruded, and b) extruded with subsequent annealing.
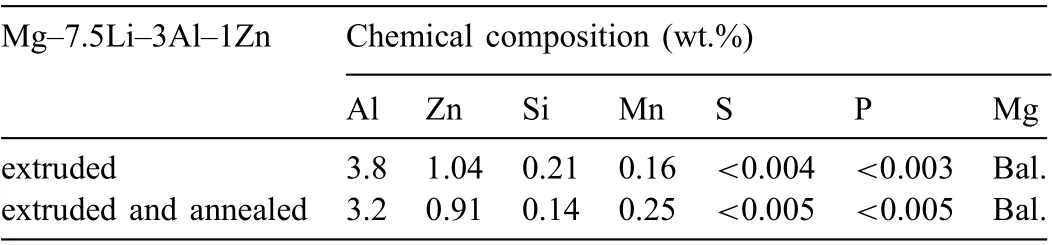
Table 3 Chemical composition of the extruded, and extruded with subsequent annealing Mg-7.5Li-3Al-1Zn alloy obtained using XRF.
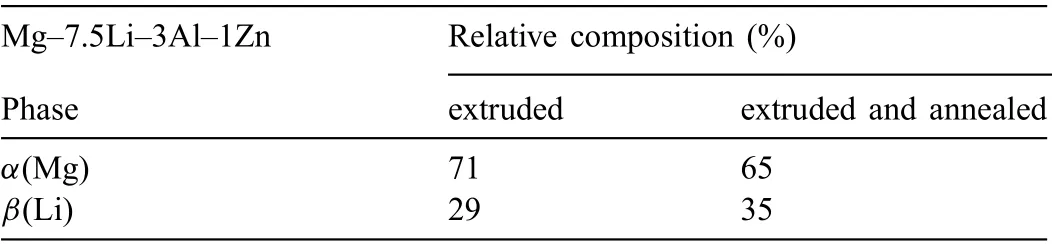
Table 4 Semi-quantitative analysis obtained by XRD for the extruded, and extruded with subsequent annealing Mg-7.5Li-3Al-1Zn.
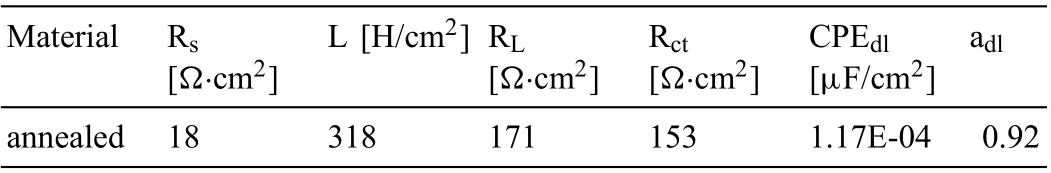
Table 5 The fitte EIS data.
It should be pointed out that the diffraction spots of AlLi,Θ-MgLi2Al and Mg17Al12were seldom detected in the TEM observations, and there is a possibility that further phases can exist in these alloys, however, they could not be easily found during TEM observations.
The IPF maps with corresponding distribution of β(Li)and α(Mg) in the analyzed alloys are shown in Fig. 8. Both microstructures were randomly oriented, and a high amount of grains oriented to {01-10} (blue color) were detected(Fig. 8a and c). The non-uniform distribution of the grains with an orientation of {-12-10} (green color) and {0001}(red color) were also observed. As expected, after annealing the crystallographic orientations of the grains changed, and when comparing Fig. 8a and c, a higher overall area of grains oriented to {0001} and a smaller number of grains oriented to {-12-10} were created during post-processing annealing.The distribution of β(Li) and α(Mg) in the extruded, and extruded with subsequent annealing alloys, are shown in Fig. 8b and d, respectively. The increased amount of β(Li)is observed after subsequent thermal processing, and this is in agreement with the XRD data.
4.2. Corrosion description
The Ecorrthat was measured for 5 h for both analyzed alloys is shown in Fig. 9a. A stable trend of Ecorrdue to the timing of the immersion was recorded for the extruded sample. The Ecorrfor this material was oscillating around-1.60V/REF. A slight shift in the Ecorrtowards more positive values was observed for the annealed sample. In this case, a rapid decline in the Ecorrwas observed during firs 30min of the measurement, which could be related to fast propagation of the anodic reactions at the beginning of the measurement [43]. After that time, the Ecorrwas consistent and oscillated around -1.55V/REF.
To more thoroughly characterize the impact of the annealing on the corrosion behavior of the analyzed materials, EIS measurements were carried out. The impedance data were plotted as Nyquist plots and are shown in Fig.9b and the electrochemical parameters obtained from the fittin of equivalent circuits are presented in Table 5. The Nyquist plot obtained for the annealed alloy consisted of a single capacitive loop at a high/middle frequency and one inductive loop at a low frequency and was described using the equivalent circuit (EEC)given in Fig. 10. The high/medium capacitive loops represented by Rctand CPEdl, which correspond to a double layer at the interface of the corroded material and solution, and an inductive loop was described using specifi L and RLcharacteristics [44]. In this case, the inductive loop represented precipitation of the corrosion products on the substrate materials. Instead of a classical capacitance due to the depressed semicircles, the constant phase elements (CPE) were selected.

Fig. 6. TEM images in bright fiel and selected area diffraction (SAD) patterns obtained from the extruded Mg-7.5Li-3Al-1Zn.
There was a wide variance in the radius of the Nyquist plots recorded for both the extruded alloy, and the alloy that was extruded with subsequent annealing. The Nyquist plot for the extruded Mg-7.5Li-3Al-1Zn was not fitte with EEC because the alloy was very active in the analyzed solution;fast dissolution of the extruded Mg-7.5Li-3Al-1Zn was observed, and the precipitated alloy was visible at the bottom of the electrochemical cell. The Nyquist plot for this sample was registered over a very narrow range of high frequencies,which confirme the poor resistance of the extruded alloy in naturally aerated 3.5wt% NaCl. It can be assumed that the anodic reactions of the substrate were very intense and were not compensated for by the reactions that led to corrosion product formation. After annealing, the capacitive loop became significantl wider and Rctincreased to 153 Ω·cm2. As the extruded alloy was very active, this result was expected.
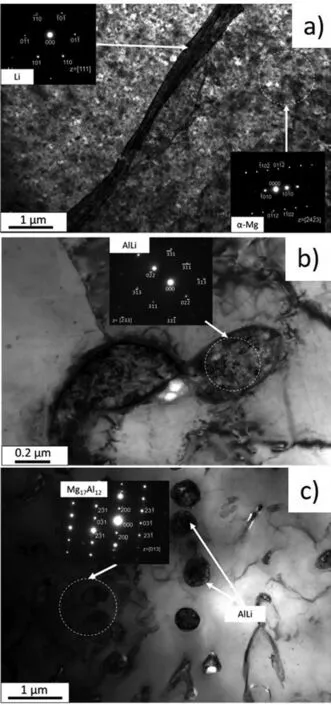
Fig. 7. TEM images in bright fiel and selected area diffraction (SAD) patterns obtained from the extruded and annealed Mg-7.5Li-3Al-1Zn.
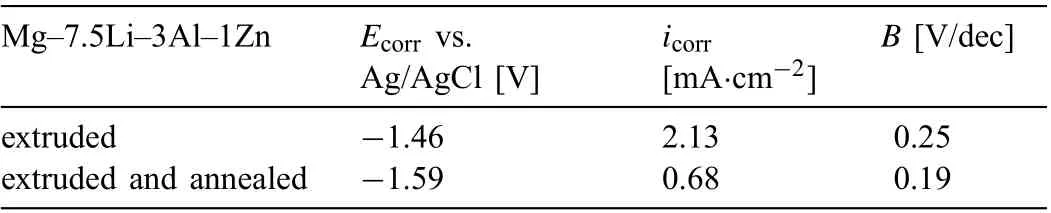
Table 6 The electrochemical parameters obtained from Tafel fittin fitte results of the polarization curves.
The potentiodynamic polarization curves obtained for both analyzed materials presented in Fig.9c are typical for actively corroded alloys. The recorded current densities are slightly lower for the annealed sample and the potentials shift to more positive values. As the data from Table 6 shows, higher icorrwas observed for extruded alloy (icorr=2.13 mA·cm-2) when compared to the annealed sample (icorr=0.68 mA·cm-2).
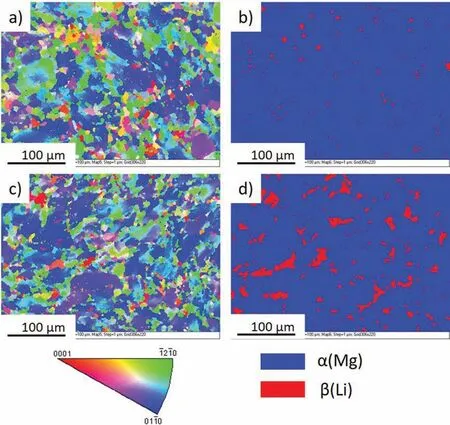
Fig. 8. EBSD data obtained from the surface perpendicular to the extrusion direction: a) IPF map for the as-extruded Mg-7.5Li-3Al-1Zn, b) β(Li) distribution in the as-extruded Mg-7.5Li-3Al-1Zn, c) IPF map for the as-extruded and annealed Mg-7.5Li-3Al-1Zn, d) β(Li) distribution in the as-extruded and annealed Mg-7.5Li-3Al-1Zn.
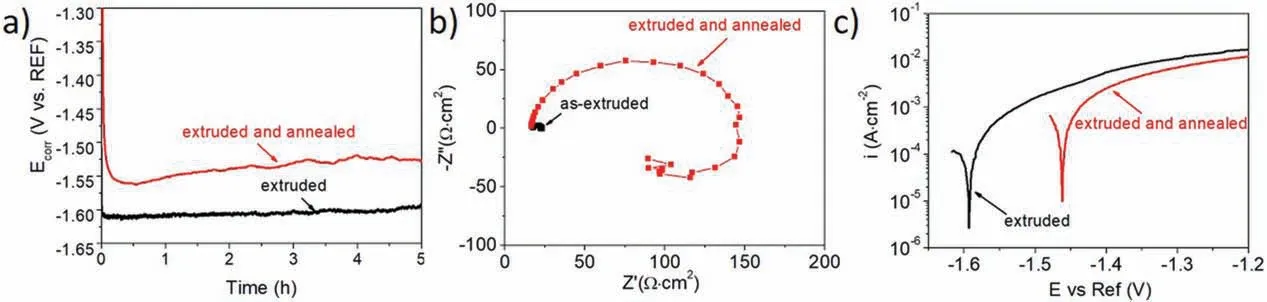
Fig. 9. Electrochemical results: a) Ecorr with respect to immersion time, b) Nyquist plot, c) potentiodynamic polarization curves.
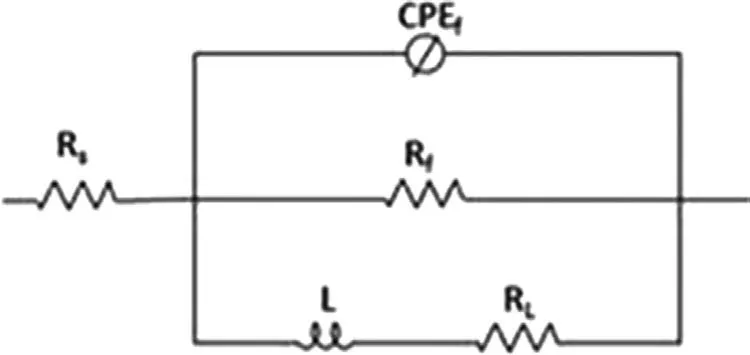
Fig. 10. The equivalent circuit used for EIS data fitting
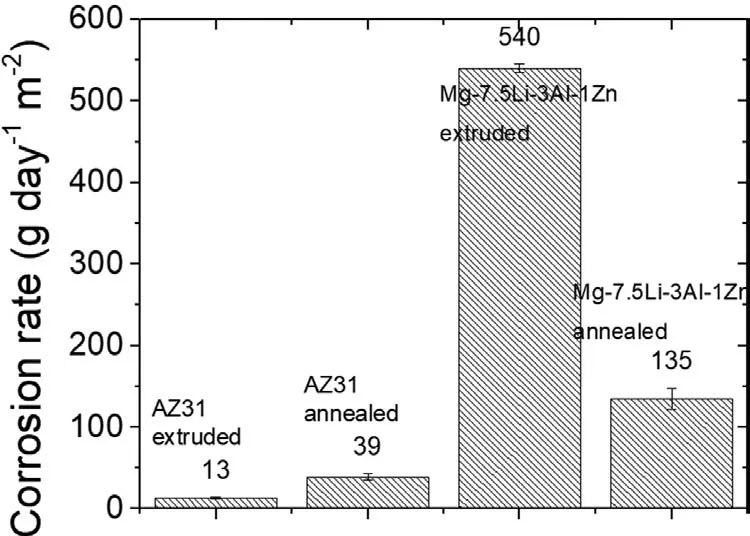
Fig. 11. Corrosion rate obtained from mass loss measurements for the extruded, and extruded with subsequent annealing AZ31 and Mg-7.5Li-3Al-1Zn.
The rapid dissolution of the extruded alloy was also observed during immersion tests, the results of which are shown in Fig. 11. The calculated corrosion rate for the extruded sample was almost 4 times higher than for the annealed one, and was found to be around 540 and 135 g·day-1·m-2for the extruded, and extruded with post-processing annealing, respectively. To compare the corrosion rate of the fabricated alloys with traditional AZ31, the immersion tests were also performed for the AZ31 alloys produced in the same manner. As observed, the corrosion rate of the alloys with Li addition is 10 times higher than the corrosion rate of AZ31. The corrosion rate of the extruded alloys was 13 and 540 g·day-1·m-2for AZ31 and Mg-7.5Li-3Al-1Zn, respectively. After annealing the corrosion rate calculated for AZ31 was 39 g·day-1·m-2and was135 g·day-1·m-2for the sample with Li addition.
4.3. Surface morphology
The surface morphologies and cross-sections of the corroded samples were investigated by SEM after immersion in 3.5wt% NaCl under open circuit conditions. The observations revealed that the entire surface of the extruded sample dissolved swiftly(Fig.12a).Corrosion proceeded quickly into the depth of the material, causing fast dissolution and exfoliation of the corroded products.Over time,the corrosion moved through the surface and into the material, and the strong dissolution of the substrate was observed. Cross-sectional observations revealed that micro-galvanic processes were dominant in this case, and as β(Li) is more active than α(Mg), corrosion reactions propagated mainly along β(Li), causing further degradation of the substrate (Fig. 12b). These processes were supported by interdendritic segregation. We suppose that corrosion also procceded between second phases formed in the material and α(Mg) and/or β(Li).
The SEM image taken from the surface of the annealed sample after immersion in 3.5wt%NaCl with the corresponding cross-sectional image are shown in Fig. 13. Fewer corrosion products that were randomly distributed were visible on the surface (Fig. 13a). The corrosion proceeded slower than on the extruded sample, and was accompanied by adsorption reactions, which resulted in corrosion product formation(Fig. 13b). However, there is also localized corrosion which was propagated into the depth of the material. The corrosion product formed was not stable and easily dropped off from the surface. The XRD measurements presented in Fig. 14a and b indicate that the observed corrosion products formed on the extruded, and extruded with subsequent annealing Mg-7.3Li-3Al-1Zn were mainly composed of Mg(OH)2and Al2O3.
5. Discussion
The Mg-7.5Li-3Al-1Zn was successfully manufactured using conventional casting, and extrusion processes. The processing parameters were not sufficien to provide full microstructure recrystallization, even post-processing annealing(350°C for 1h) did not trigger the full microstructure transformation from dendritic to the granular structure. This study demonstrates that the microstructure of the extruded Mg-7.5Li-3Al-1Zn was mainly composed of α(Mg), β(Li), and secondary phases such as: AlLi, Mg17Al12and θ-MgLi2Al.Moreover, EDX performed on the surface indicates that the second phases composed of Al and Zn (and Mn in the annealed material)with an unknown stoichiometry,were formed in the alloys.
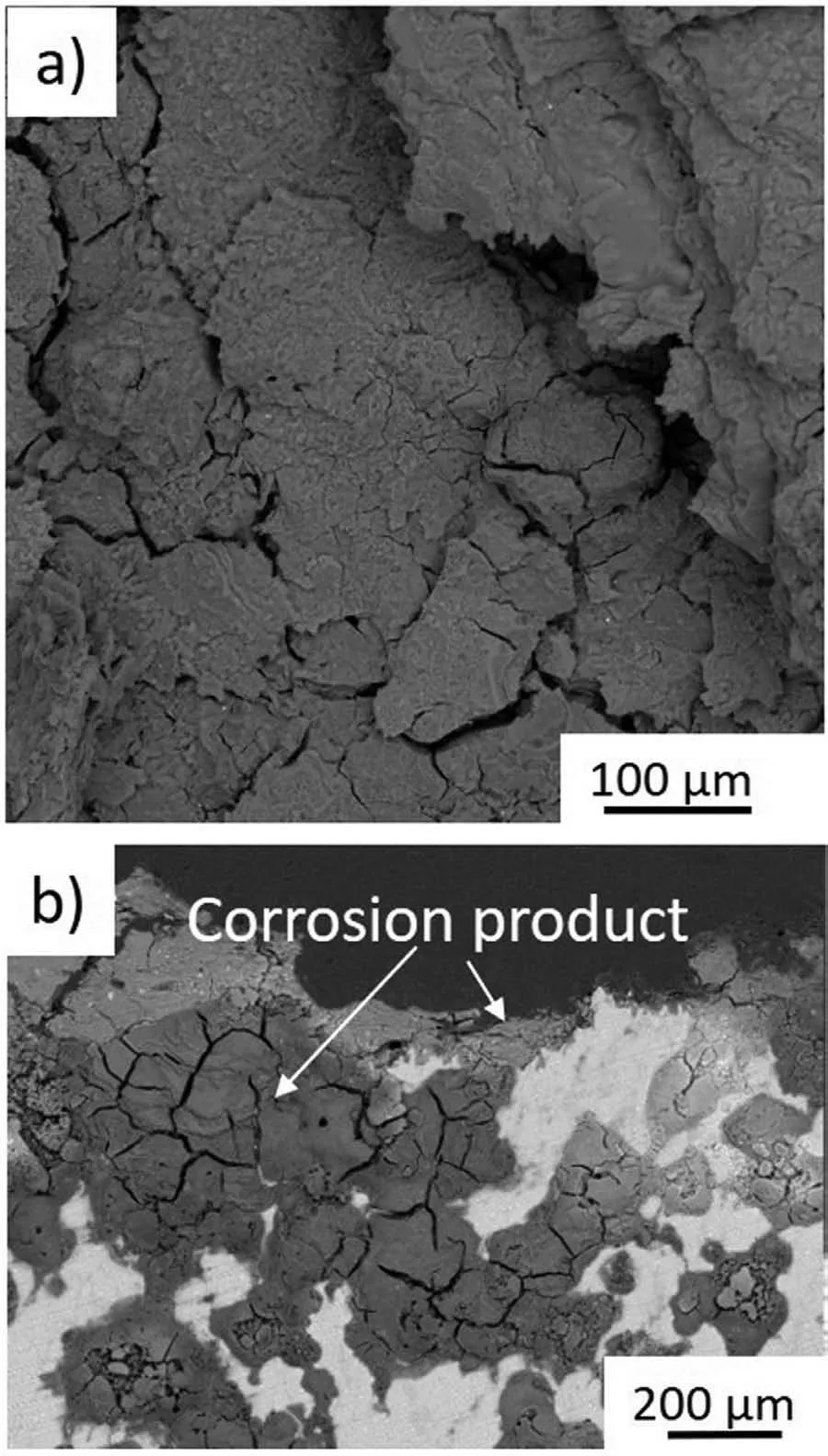
Fig. 12. Post-corrosion observations performed on the extruded Mg-7.5Li-3Al-1Zn after immersion in 3.5wt% NaCl: a) SEM image taken from the surface, b) SEM image taken from the cross-section.
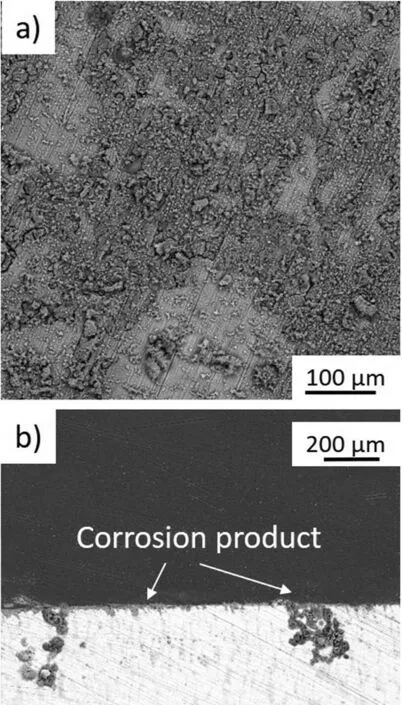
Fig. 13. Post-corrosion observations performed on the extruded and annealed Mg-7.5Li-3Al-1Zn after immersion in 3.5wt% NaCl: a) SEM image taken from the surface, b) SEM image taken from the cross-section.
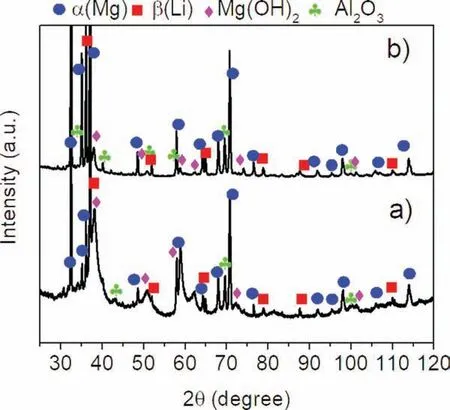
Fig. 14. XRD patterns obtained from the surface of the a) extruded and b)extruded an annealed samples after immersion in 3.5wt% NaCl.
As observed, the extruded Mg-7.5Li-3Al-1Zn was not resistive in 3.5wt% NaCl. The strong microgalvanic corrosion supported by interdendritic segregation made this alloy very active in the analyzed system. Different corrosion behavior was observed for the annealed alloy with a dual-phase, partially granular microstructure. The post-processing annealing improved the corrosion resistance of the alloy. Although after testing corrosion products that formed were not compact and could not fully protect the substrate from the propagated corrosion mechanisms, the formation of the corrosion products retarded further corrosion of the substrate. A similar situation was also shown for the AZ31 alloy tested in 3.5wt% NaCl by Lin et al. [45], where the occurred adsorption processes were insufficien to compensate for the dissolution reaction of the substrate. Additionally, results of our work showed that in the case of the annealed Mg-7.5Li-3Al-1Zn the corrosion mechanisms did not propagate as easily as in the case of the extruded alloy and also moved beyond the surface into the substrate,however,its propagation was unquestionably slower.The spreading, local corrosion was mainly the result of microgalvanic reactions between the α(Mg) and β(Li), where the interface of these two phases was the preferable path for the spreading microgalvanic reactions [46]. Interestingly, the change in the size and distribution of the different phases in the material increased its corrosion behavior. It was expected that a higher amount of β(Li) in the annealed alloy can support corrosion reactions, and the alloy with a higher amount of β(Li) should corrode faster. However, the predominant role in this system played not the higher activity of the β phase, but the area ratio of β(Li) to α(Mg) and the anodic/cathodic nature of the two adjacent phases. Knowing that in a heterogenous corrosive system the corrosion reactions are strongly dependent on the spatial distribution of the potential on the surface, and that the β(Li) is more preferential to corrode than α(Mg), it can be concluded that the larger area of β(Li) compared to α(Mg) influence the degree of dissolution of the alloy. This phenomenon is related to the half-reactions of the corrosive system. When the cathodic area is large, this facilitates a reduction reaction, the anodic dissolution current must increase to adjust the local electric neutrality. In other words, the resulting from post-processing annealing the increased amount of β(Li) reduces the large area ratio of cathodic to anodic sites of corrosion.
In duplex structured Mg-Li alloys the main corrosion processes can spread in two ways: between α(Mg) and β(Li),which is the most intensive reaction, or between matrix and intermetallic compounds, which can also affect the corrosion resistance of the alloy. Therefore, the role of discontinuously distributed secondary phases in the microstructure of the annealed alloy must also be noted.
This study proved, that the corrosion mechanism of the annealed alloy was complex, and that the dissolution reactions were partially compensated for by passivation reactions.For Mg alloys, the main reaction in the corrosion products formation is [45,47-49]:
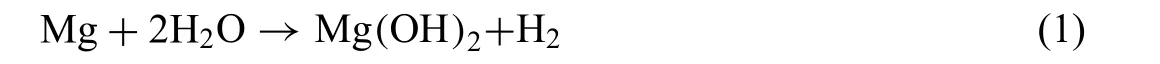
According to this reaction, Mg(OH)2was identifie as the main compound of the adsorbed species on the surface. Interestingly, corrosion products created on the surface of the annealed alloy were also composed of Al2O3. It is worth noting, that Al addition has a significan influenc on the mechanical properties, and as outlined by Bhagat Singh et al.[50], Al increases the strength of the Mg-Li alloys. This work indicates, that Al dissolution enriched the interface of substrate/electrolyte with Al3+and improved the passivation ability of the alloy, according to the reaction [48]:
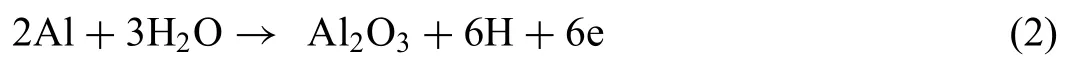
This observation is related to the presence of the secondary phases enriched with Al such as Mg17Al12,AlLi and θ-MgLi2Al. However, since θ-MgLi2Al is a metastable phase and undergoes reformation to AlLi during thermal processes,its role in the corrosion processes should be investigated more thoroughly in a less aggressive corrosive medium. It is possible that higher amount of AlLi in the extruded alloy drastically enhanced the dissolution reactions. It is also worth noting, that the Li most likely underwent dynamic local anodic reactions [51]. Also, local corrosion reactions occurred between secondary phases enriched in Al, Zn and Mn, however, due to their unknown stoichiometry, their role in the microgalvanic corrosion in the investigated material cannot be analyzed.
In our opinion, the dissolution rate of the materials investigated in this research was also dependent on their texture. Both materials (extruded and annealed) displayed a random crystallographic orientation,however,subsequent annealing resulted in an increase in the overall area of the grains oriented to {0001}, and the amount of grains near {11-20}decreased. The surface energy of Mg {0001}, {10-10} and{11-20} is different, and the lowest surface energy had the grain oriented near {0001}. That suggested that the grains oriented to {0001} should dissolve slower than those grains oriented to {10-10} and {11-20} [52,53]. It is reasonable assumption that higher amount of grains with an orientation near {0001} might also affect the corrosion resistance of the annealed alloy, and facilitate its higher corrosion resistance in the 3.5wt% NaCl. Our observations are supported by results obtained by Liu et al.[54]., where the authors stated that the grains of pure Mg oriented near {0001} were the most corrosion resistant.Similar observations can be found in[55]where the corrosion rate of AZ31 dramatically increased when the(0001) texture intensity decreased and the (10-10)/(11-20)increased.
The overall results derived from the presented experiments revealed the poor corrosion resistance of Mg-7.5Li-3Al-1Zn in both, the as-extruded and annealed alloys, when compare to pure magnesium [56] and other magnesium alloys [57,58].This is the result of Li, the addition of which to the AZ31 enhanced the cathodic reaction and caused faster corrosion propagation [51].
6. Conclusions
A novel dual-phase Mg-7.5Li-3Al-1Zn alloy was extruded, and subsequently thermal processed. The following conclusions are drawn:
1. The post-processing annealing of the Mg-7.5Li-3Al-1Zn at 350 °C was not sufficien to provide full microstructure recrystallization. Both alloys, as-extruded, and as-extruded with post-processing annealing, were mainly composed of α(Mg) and β(Li). The existence of AlLi, Mg17Al12and MgLi2Al was detected in the extruded alloy, AlLi and Mg17Al12were determined in the annealed alloy. The EDX results suggest that secondary phases composed of Al, Mn and Zn may be also present in the investigated alloys.
2. The as-extruded alloy was not corrosion resistive in 3.5wt% NaCl. Subsequent annealing at 350 °C for 1 h improved the corrosion resistance of the alloy. Mixed corrosion processes were observed, and a loose and inhomogeneous fil of corrosion products formed on the surface.
3. The corrosion resistance of the dual-structured Mg-7.5Li-
3Al-1Zn is mostly dependent on the area ratio of β(Li)α(Mg), however, the minor role of the secondary phases in the micro-galvanic processes cannot be dismissed.
Declaration of Competing Interest
The authors declare that they have no known competing financia or personal interests that could have appeared to influenc the work reported in this paper.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Mechanism of Mn on inhibiting Fe-caused magnesium corrosion
- An efficien and comparative adsorption of Congo red and Trypan blue dyes on MgO nanoparticles: Kinetics, thermodynamics and isotherm studies
- Twin recrystallization mechanisms in a high strain rate compressed Mg-Zn alloy
- Corrosion behaviour and cytocompatibility of selected binary magnesium-rare earth alloys
- Correlation between test temperature, applied load and wear transition of Mg97Zn1Y2 alloy
- Residual stress and precipitation of Mg-5Zn-3.5Sn-1Mn-0.5Ca-0.5Cu alloy with different quenching rates
