Functional lymphaticovenular anastomosis for peripheral lymphedema: incision selection methods with muscle pumping
2021-05-07YukioSekiAkiyoshiKajikawaRintaroAsaiMeriTakadaTakumiYamamotoTakahiroTerashimaNorimitsuKurogi
Yukio Seki, Akiyoshi Kajikawa, Rintaro Asai, Meri Takada, Takumi Yamamoto, Takahiro Terashima,Norimitsu Kurogi
1Department of Plastic and Reconstructive Surgery, St. Marianna University School of Medicine, Kawasaki, Kanagawa 216-8511,Japan.
2Department of General Surgery, Shonan Atsugi Hospital, Atugi, Kanagawa 243-8551, Japan.
Abstract Lymphaticovenular anastomosis (LVA) is a highly effective, minimally invasive surgical treatment for lymphedema.The clinical effect of LVA begins immediately after the creation of the lymph-to-venous pathway. However, the long-term effect of LVA is not always promised when the lymph-to-venous bypass has any potential risk of occlusion, especially when the disorder has reached the late stage. The reasons of postoperative LVA occlusion are considered both a technical matter in performing LVA and a strategic matter in planning LVA. This article focuses on the effective preoperative LVA planning methodology of “functional LVA” for peripheral lymphedema, in which continuous and strong lymph flow at the anastomosis is created by the muscle pumping power of patients’ natural motions at the selected incision point. The current functional LVAs which we have developed are the dynamic LVA method for upper extremity lymphedema and the superior-edge-of-the-knee incision method for lower extremity lymphedema. Because these methods reduce the risk of postoperative LVA occlusion by continuous lymph-tovenous flow at the LVA, functional LVAs keep long-term clinical effect in reduction of lymphedema.
Keywords: Lymphedema, lymphaticovenular anastomosis, supermicrosurgery, functional LVA, dynamic LVA method, superior-edge-of-the-knee incision method, surgery
INTRODUCTION
Lymphaticovenular anastomosis (LVA) is widely recognized as a highly effective, minimally invasive surgical treatment for lymphedema[1-12], in which a new lymph-to-venous pathway is created at the affected limb by means of anastomosing the peripheral lymphatic vessel directly into the subcutaneous vein.Changes of lymph dynamics at the affected limb sometimes reveal an immediate effect, even intraoperatively.
In a variety of lymphatic surgeries, LVA has the advantages of minimal invasiveness without donor site morbidity. The therapeutic effect of LVA is obtained relatively early after the operation compared to other lymphatic surgeries including vascularized lymph node transfer, vascularized lymphatic vessel transfer, and lymphatic pathway reconstruction by local and free flap transfer[12-15]. LVA can be the first-line surgical treatment for peripheral lymphedema because of the high therapeutic efficacy with less invasiveness.
The clinical effect of LVA continues for a long time when the lymph-to-venous bypass maintains continuous lymphatic flow. However, LVA has the risk of occlusion in early-, mid-, or late-postoperative course[16,17]. That is why the long-term outcome of LVA is not always promised. There are many potential reasons of postoperative LVA occlusion, which include technical matter in surgery, surgical site infection,patients’ compliance, lymphatic vessels’ degenerations, postoperative management, changes of postoperative lymphatic dynamics, lymphedema severity, and selection of LVA location[16-20]. Although the reasons of postoperative LVA occlusion seem to vary in fundamentals, all occlusion risks are attributed to the absence of continuous lymph-to-venous flow at the anastomosis.
To keep continuous lymphatic flow at LVA, we developed a new LVA concept of “functional LVA” for peripheral lymphedema, in which continuous and strong lymph flow at the anastomosis is created constantly by the muscle pumping power of patients’ natural motions in daily life.
Functional LVA concept
Generally, an important power source to propel lymphatic fluid in the lymphatic system is the smooth muscle’s power of the lymphatic vessels. In addition, this smooth muscle’s power has an important role in propelling lymphedematous fluid into the lymph-to-venous bypass of LVA.
However, lymphedema patients reveal sclerosis of the lymphatic vessels with progression of edema[12,18-20].Whether lymphatic vessels are sclerosed or not is basically assessed by pathological study. However,Yamamotoet al.[12]reported an easy way to assess lymphatic sclerosis intraoperatively by the operating microscopic features of lymphatic vessel’s wall thickness, appearance, wall expandability, and status of lumen. These lymphatic vessels with degenerated smooth muscle are an inadequate power source; they are too weak to propel lymphedematous fluid to the LVA. This limits the therapeutic effect of LVA in patients with progressive lymphedema. Furthermore, LVAs using these sclerotic vessels are easily occluded postoperatively.
To overcome the difficulty to treat lymphedema by means of LVA, we developed and reported new LVA methods by which LVA incisions are placed at the points where patient’s muscle pumping power is enlisted as an alternative power source to propel lymph to the anastomosed vein[21,22]. This new muscle pumping power creates continuous lymphatic flow at the site of LVA. In other words, this new LVA itself acts as a perpetual motion, preventing its own obstruction and efficiently draining lymph into the anastomosed veins; therefore, we describe this new LVA concept as “functional LVA”.
In the functional LVA concept, the method of maximizing muscle pumping is different between the upper limb and lower limbs, because the anatomical and physiological characteristics of lymphatic and venous dynamics differ between them. The lymphatic function in the arm works more longitudinal with low gravity effect on the veins. In contrast, the lymphatic function in the leg works more transversal with high gravity effect on the veins[9]. These differences in lymphatic function and gravity effect on the vein between upper and lower extremities are important for making LVA strategy.
The current functional LVA methods we have developed are the dynamic LVA method for upper extremity lymphedema (UEL) and the superior-edge-of-the-knee incision method for lower extremity lymphedema(LEL).
The dynamic LVA method for upper extremity lymphedema
To keep continuous lymphatic flow at the lymph-to-venous anastomosis in upper extremity, the muscle pumping power by patient’s natural hand motions can be utilized as functional LVA.
The dynamic LVA method is a reliable LVA technique at the forearm for UEL, which the senior author developed in November 2016, and we reported a case-control study in January 2019[21]. After the detection of the lymphatic vessels at the arm by other modalities, preoperative dynamic ultrasonography (US) is performed to determine the best incision point on each lymphatic vessel on each patient forearm where muscle pumping by hand movements works maximally.
Because the patient’s dominant hand, lifestyle, work, and other activities affect muscle development and tissue structure in the arm, the anatomical and physiological function of lymphatic and venous systems are not uniform in UEL patients. That is why the best incision sites for LVA in patients with UEL must be determined individually.
The clinical effect of the dynamic LVA is obtained at the whole upper extremity because LVA at the arm can be seen at a longitudinally far distal or far proximal region[9,23]. Preoperative determination of incision points using traditional US technology is the essence of this method. An important point in the dynamic LVA method is that US is utilized not to detect lymphatic vessels but to evaluate muscle pumping and subcutaneous veins over 1.0 mm in diameter. Thus, no special US techniques are needed to perform dynamic LVA.
As preparation of the method, the lymphatic vessels’ pathways are identified first. Although our preferred way to detect lymphatic vessels for dynamic LVA is utilizing ICG lymphography findings at affected and non-affected arms[9,23,24], any modalities to visualize the lymphatic vessels can be used to identify lymphatic pathways in this method. Several modalities, including ICG lymphography, MRI, high-frequency US, and photoacoustic imaging, can be combined to identify lymphatic pathways for dynamic LVA[3-14,25].
Once a lymphatic vessel is detected at the patient’s arm, dynamic US is performed along the detected vessel to determine the incision point with maximum muscle pumping function. Throughout dynamic US examination, patients are instructed to move the lymphedematous hand continuously to evaluate muscle and venous pumping in the subcutaneous tissue at the forearm. If the large subcutaneous vein (> 1.0 mm) is pumped with the muscle movements in dynamic US, the point is defined as the incision. The pumping subcutaneous vein has negative pressure in the lumen when this vein is opening in cooperation with muscle pumping[21,23]. Because LVA is created using the small subcutaneous vein that is the first to third branch of the large pumping vein (> 1.0 mm) at the incision, lymphatic fluid is drawn from the lymphatic vessel to the anastomosed vein of LVA by the power of negative pressure created by the large pumping subcutaneous vein (> 1.0 mm) [Figure 1]. The dynamic US findings of the pumping vein with movements of the blood at the lymphedematous arm are depicted in Supplementary Video 1. The other details of ultrasonographic findings of the dynamic LVA method are described in our previous paper with the video of dynamic US[21].
The clinical significance of the dynamic LVA method is very early improvement of lymphedema, even often seen intraoperatively as decreased stiffness and volume reduction around the site of LVAs. In addition,intraoperative hand movements can enhance flow of lymphatic fluid to the anastomosed vein, and the confirmation of the patency of the anastomosis can become more precise. The effect of intraoperative hand movement in the dynamic LVA method is depicted in Supplementary Video 2.
The superior-edge-of-the-knee incision method for lower extremity lymphedema
The strategy of creating functional LVA differs between patients with UEL and those with LEL[23]. In UEL,selecting the muscle pumping point as an incision especially where the pumping vein exists is important,because the hand movements even created by small muscle have enough power to propel lymphatic fluid into the anastomosed vein of LVA.
However, in LVA at the lower limb, the existence of the pumping vein with small muscle pumping is not enough to propel lymphatic fluid against gravity. The difficulty to treat LEL is the gravity effect, which is considered stronger in the lower limb than that in the upper limb. To propel lymphatic fluid against gravity,evaluation of the subcutaneous veins for LVA is important[25]. Because the venous valves in the lower limb originally work against gravity, utilizing a vein with good valvar function is theoretically effective for LVA.In fact, selecting the subcutaneous vein with good valvar function in LVA is reported as beneficial to prevent venous reflux at the anastomosis[26,27].
The other difficulty in treating progressive LEL is degeneration of the lymphatic vessels. Sclerosis of lymphatic vessels already occurs even in early LEL patients[19]. Performing precise intima-to-intima LVA using these degenerated lymphatic vessels is a difficult procedure for the microsurgeon because the lumen of the vessel becomes narrow and fragile. In addition, these degenerated lymphatic vessels are often not sufficient as a power source to propel lymphatic fluid to the site of LVA. Therefore, alternative power to propel lymphatic fluid is required in treating severe LEL. Because only utilizing small muscle pumping seems to be not enough against gravity, strong muscle pumping by joint movements is essential.
For these reasons, ideal functional LVA in treating LEL is created with a combination of the subcutaneous vein with good valvar function and strong muscle pumping power of the joint movements at the incision point[25]. The superior-edge-of-the-knee incision method is particularly useful for functional LVA in lower extremity[21,22], because this method is characterized in utilizing both the strong muscle pumping power of the knee joint movement and the subcutaneous vein with good valvar function at the specific incision point in the medial thigh [Figure 2]. Furthermore, lymphatic vessels detected at the incision of the method are relatively large in diameter with less degeneration.
As preparation of the method, the incision site is identified as the intersection of a transverse line drawn at the superior edge of the patella and a longitudinal line drawn along the medial axis of the distal thigh with the patient in the supine position. From the point of intersection, a 2.5 cm transverse incision is made posteriorly [Figure 3]. The incision site can be set without any imaging study because the lymphatic vessel for functional LVA is always detected at this point in all patients.
At the incision point, many lymphatic vessels can be identified over and under the superficial fascia in subcutaneous tissue[21,22]. Lymphatic vessels under the superficial fascia should be selected for LVA in this method because strong upward propulsion of lymphatic fluid to the anastomosed vein is created by the gracilis muscle pumping, which compress soft tissues between the deep fascia and the superficial fascia that cooperate in knee joint movement. Before dissection under the superficial fascia, the subcutaneous vein with good valvar function is detected and dissected for LVA over the subcutaneous fascia at this point.
To obtain a lymphatic vessel residing in rich fatty tissue under the superficial fascia, 3-0 nylon monofilament is used to catch the lymphatic vessel. The lymphatic vessel is then dissected from rich fatty tissue up to 1.5 cm proximally, supported by careful traction of the vessel with the 3-0 nylon monofilament.The lymphatic vessel is carefully cut with microscissors at the most proximal point of dissection. The vessel is pulled out over the superficial fascia to be anastomosed with the subcutaneous vein with good valvar function there.
Because relatively large and less degenerated lymphatic vessels can be detected at the incision, the lymphatic vessel can be anastomosed to the subcutaneous vein with 11-0 nylon suture without using 12-0 nylon suture. The procedure of the superior-edge-of-the-knee incision method is depicted in Supplementary Video 3.
OUTCOMES
The functional LVA for UEL, i.e., the dynamic LVA method, was reported in a case-control study of 30 patients[20]. All patients were under sleeve compression. The 30 International Society of Lymphology (ISL)stage 2 UEL patients were divided into two groups: 15 patients were treated by means of three traditional LVAs via three incisions without preoperative dynamic US, while 15 were treated by means of three dynamic LVAs via three incisions. The characteristics of the patients per group are shown in Table 1, and there was no significant difference between the two groups. Postoperative volume reduction in UEL index at 1 month, 6 months, and one year were compared between the two groups [Table 2]. The dynamic LVA group patients revealed very early improvement of decreased stiffness and volume reduction before restarting compression therapy. Although the conventional LVA group patients had some recurrence of edema after the LVA, the dynamic LVA group patients revealed no recurrence of edema after the surgery.The rates of finishing compression therapy at two years postoperative were significantly greater in the dynamic LVA group than in the conventional LVA group [12 of 15 follow-up patients (80.0%)vs. 5 of 13 follow-up patients (38.5%);P =0.023][28][Figure 4].
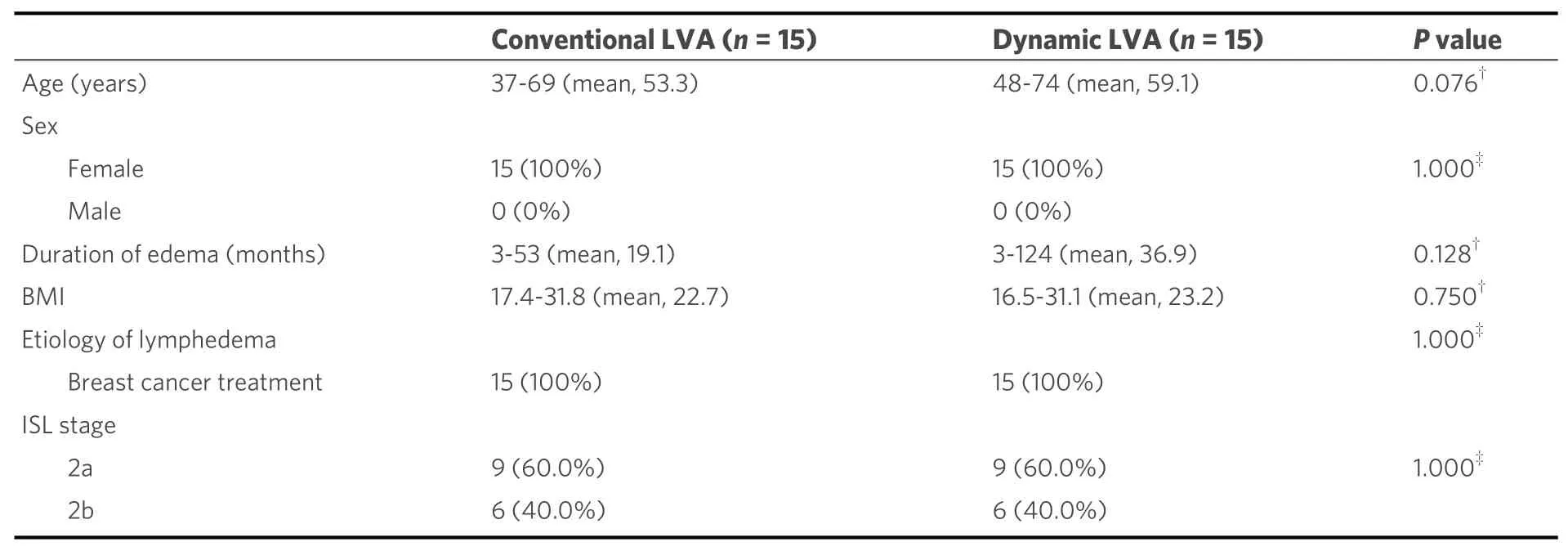
Table 1. Characteristics of patients in the conventional LVA group and dynamic LVA group[20]
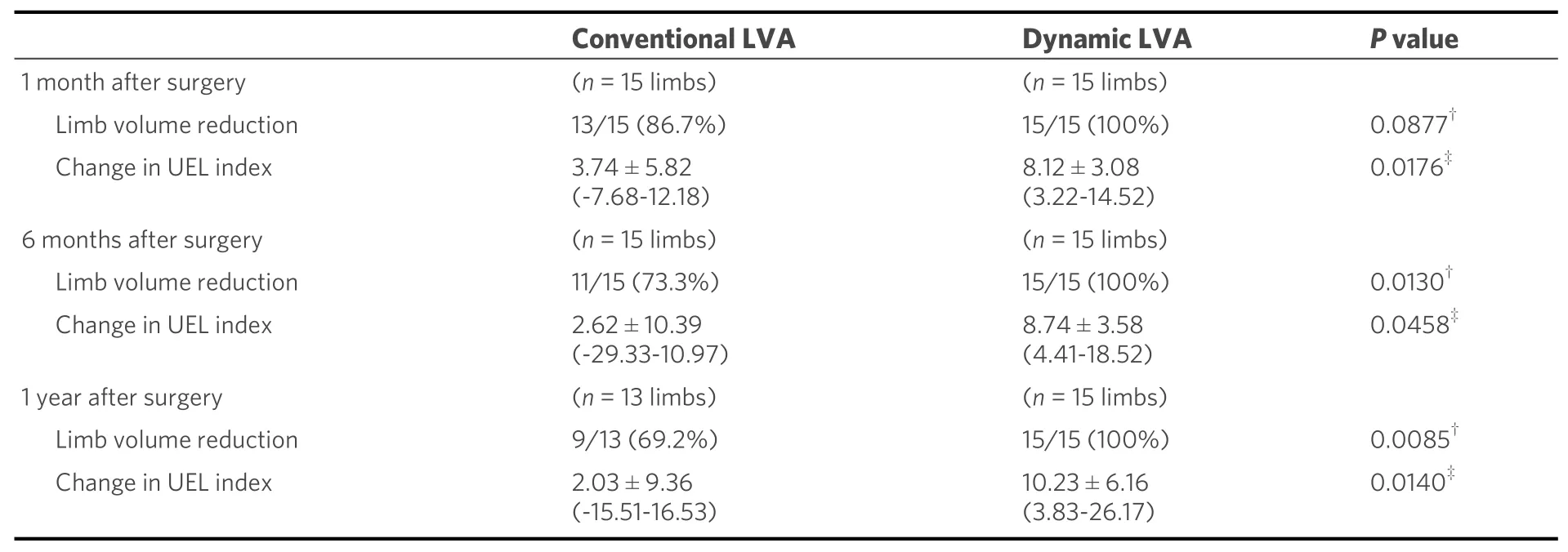
Table 2. Postoperative improvement in the conventional LVA group and dynamic LVA group[20]
The functional LVA for LEL, i.e., the superior-edge-of-the-knee incision LVA, was first reported in a casecontrol study of 30 patients[21]. All patients were under compression stockings. The 30 ISL stage 2 LEL patients were divided in two groups. Fifteen patients were treated by traditional multiple LVAs without the superior-edge-of-the-knee incision method, while the other 15 were treated by multiple LVAs including the superior-edge-of-the-knee incision method. Large lymphatic vessels (≥ 0.65 mm) were detected significantlymore frequently in the superior-edge-of-the-knee incision group than in the non-superior-edge-of-the-knee incision group (60.0%vs. 18.8%,P= 0.002). The postoperative volume reduction in LEL index at one year was significantly greater in the superior-edge-of-the-knee incision group than in the non-superior-edge-ofthe-knee incision group (24.427 ± 12.400vs.0.032 ± 20.535;P< 0.001) [Figure 5].
Another study of the superior-edge-of-the-knee incision method, a case series study of 10 early ISL stage 2 LEL patients, was performed[22]. All 10 patients were treated by only a single LVA using the superior-edgeof-the-knee incision method. The mean follow-up time was 7.70 ± 3.30 months, and the volume changes in LEL index was significantly reduced in all 10 patients postoperatively (postoperative LEL index 222.532 ±19.390vs. preoperative LEL index 242.692 ± 19.026;P= 0.031).
CONCLUSIONS
The functional LVA utilizing muscle pumping in daily movements as an alternative power source to propel lymphatic fluid at the point of LVA is beneficial for treating upper and lower extremity lymphedema.
Further studies are required to confirm the long-term effects of functional LVA, and the authors intend to provide long-term follow-up data to confirm the efficacy of functional LVAs and patency of the anastomoses in a later study. We also intend to develop other functional points for LVA.
DECLARATIONS
Acknowledgments
The authors thanks Isao Koshima for sharing his knowledge, surgical techniques, passion, and zeal for lymphedema treatment. This article is reported with all our respect of the father of supermicrosurgery, Isao Koshima. And the senior author thanks Yoshiharu, Miyuki, Hanano, Mayoko, and all members of our department for their kind support during preparation of this manuscript.
Authors’ contributions
Preparation of the article, and study design: Seki Y
Data analysis: Seki Y, Asai R
Performed data acquisition, as well as provided administrative, technical, and material support: Kajikawa A,
Asai R, Takada T, Takumi Y, Terashima T, Kurogi N
Availability of data and materials
Not applicable.
Financial support and sponsorship
JSPS KAKENHI Grant Number JP17K17038 (to Seki Y).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study was conducted under approval from the St. Marianna University School of Medicine ethics committee (Approval number: 4768).
Consent for publication
Figures 1 and 2 and partial of figures 4 and 5 are from our articles in PRS and JPRAS. I have copyright permissions from to journals to reuse them in this PAR issue including how to use them in this article.
Copyright
© The Author(s) 2021.
