Anterior skull base reconstruction: a contemporary review
2021-05-07AkashNaikPeterLancioneAnuraagParikhChenLinDustinSilvermanRicardoCarrauKyleVanKoeveringNolanSeimMatthewOldStephenKang
Akash N. Naik, Peter J. Lancione, Anuraag S. Parikh, Chen Lin, Dustin A. Silverman, Ricardo L. Carrau,Kyle K. VanKoevering, Nolan B. Seim, Matthew O. Old, Stephen Y. Kang
Department of Otolaryngology-Head and Neck Surgery, James Cancer Hospital and Solove Research Institute, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA.
Abstract
Keywords: Anterior skull base, orbitocranial defects, locoregional flaps, free tissue transfer, head and neck reconstruction, skull base surgery
INTRODUCTION
Anterior craniofacial defects present significant challenges in head and neck reconstruction[1]. Conceptually,the goal of anterior cranial base reconstruction is to separate the cranial cavity from the aerodigestive tract,which demands a watertight closure to prevent cerebrospinal fluid (CSF) leak, pneumocephalus, and meningitis. Soft tissue may also be required to obliterate dead space, provide coverage for critical structures such as the internal carotid artery, and restore optimal function and cosmesis[2,3]. Traditional skull base surgery involved open transfacial or transcranial approaches, allowing wide exposure for both tumor excision and reconstruction, albeit with significant associated morbidity. With the advent and evolution of endoscopic skull base surgery, there have been advances in the ablation of complex skull base pathology with minimal access approaches[4]. The adoption of endoscopic endonasal skull base surgery has created new challenges for reconstructive surgeons as defects may need to be reconstructed through the narrow sinonasal corridor[5].
Whether an open or endoscopic approach is planned, the method of reconstruction depends on the extent and volume of the defect, presence of CSF leak, need for adjuvant therapy, patient co-morbidities, desired functional/aesthetic outcomes, and available options that may be limited by prior surgery, trauma, or radiation therapy[1,6-8]. Among patients with large volume defects, history of radiation treatment, or previously failed reconstruction, local and regional flaps may offer inadequate tissue bulk or vascularity,necessitating microvascular free tissue transfer. Despite the challenging and varied nature of these defects,many reconstructive techniques have been reported[9-12]. In particular, open and endoscopic defects often differ greatly in size, location, subsites involved, and accessibility, thus creating a separate set of reconstructive options for these two defect classes. In this article, we comprehensively review a variety of methods and innovative techniques used to reconstruct anterior cranial base and craniofacial defects encountered with both open and endoscopic approaches.
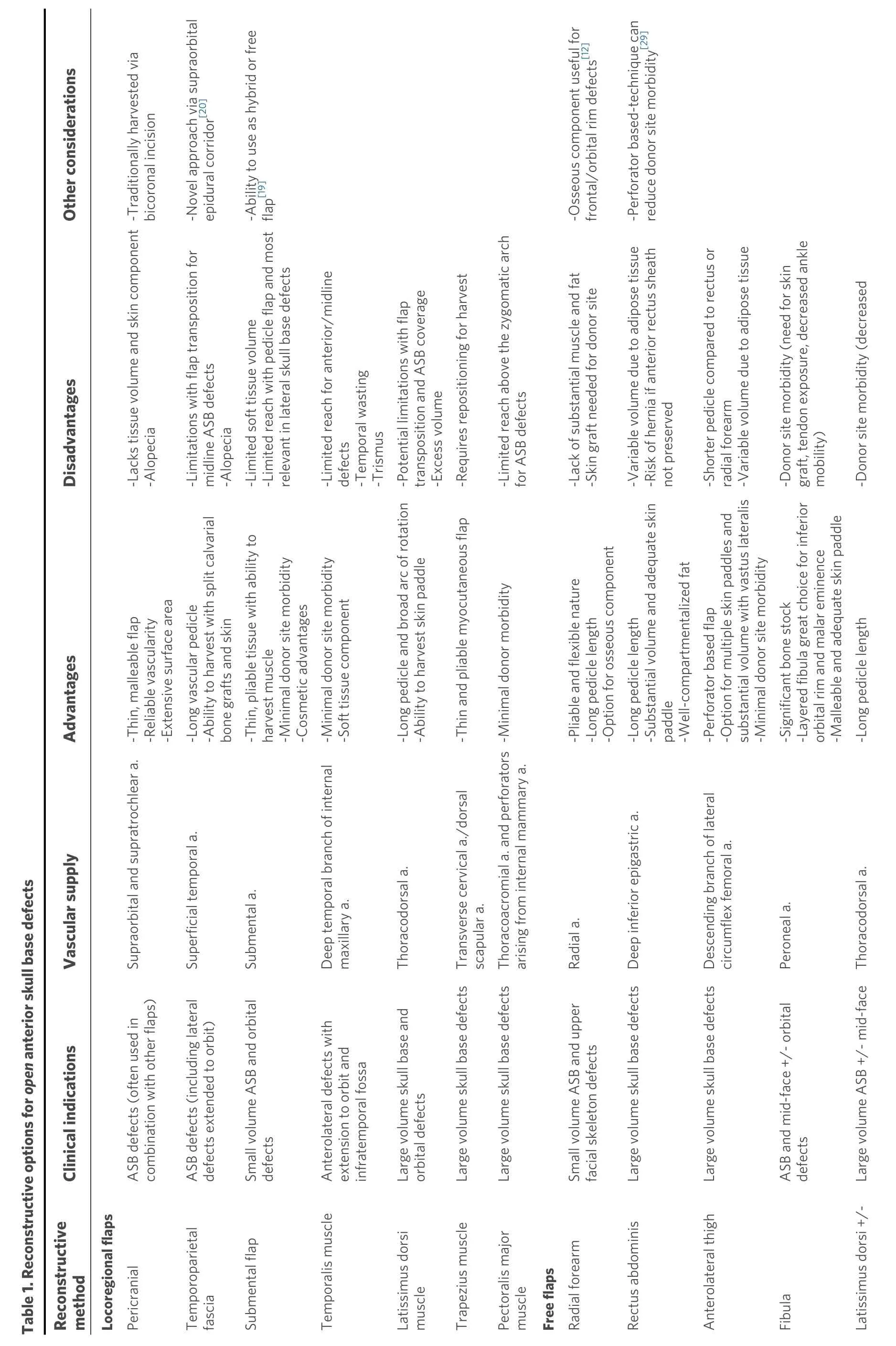
OPEN ANTERIOR SKULL BASE DEFECTS
Despite a significant shift towards endoscopic skull base surgery, open skull base approaches and reconstruction are still indicated for large skull base defects, extensive craniofacial trauma,osteoradionecrosis, and salvage surgery[6][Table 1].
Locoregional
The pericranial or galeopericranial flap has historically been the mainstay option for open anterior skull base (ASB) and craniofacial reconstruction. It is a well vascularized axial flap supplied by the supraorbital and supratrochlear arteries that offers a wide surface area and covers ventral defects extending from the frontal sinus to the planum sphenoidale and sella[4,6,13,14]. It has traditionally been harvested via a coronal incision and is comprised of pericranium and subaponeurotic connective tissue[6,14]. Others have included the galea to increase the sturdiness and blood supply of the flap. Re-establishment of the dura and skull base integrity are paramount to a successful skull base reconstruction[15]. Dural defects are reconstructed with a fascia graft, acellular dermis, pericardium, or cadaveric dura in order to create a watertight closure. The malleable and thin nature of the pericranium is an optimal choice for subsequent skull base reconstruction especially when it is used in conjunction with other soft tissue and bony reconstructions[6,16,17,18]. However, it lacks a cutaneous component and the mass needed to reconstruct extensive defects with significant volume loss[19].
?

?
The temporoparietal fascia flap, based off the superficial temporal artery, is another excellent reconstructive option but may be better suited for more lateral aspects of the anterior cranial skull base involving the orbit.It is a pliable fascial graft with a long vascular pedicle that can cover a substantial surface area obliterate complex pyramid-shaped defects in addition to an adequate skin paddle for sinonasal lining and external defects[3,6,9,12]. In a retrospective review by Chiuet al.[28], the rectus abdominis was used in 68 patients who underwent anterior and middle cranial skull base resection and reconstruction. CSF leak was avoided in 93% of cases in which the flap was used for reconstruction. Variable subcutaneous tissue volume that is dependent on body habitus and the risk of a ventral hernia are notable disadvantages of this flap[4,9,31]. A recent modification of the flap harvest uses a perforator-based technique with three main reconstructive advantages: exceptional pedicle length (> 13 cm), highly moldable adipose tissue for flap customization, and the ability to reconstruct closed-space defects due to absence of flap atrophy (insignificant muscular volume)[29]. Compared to older methods of harvest that include a large portion of the rectus abdominis muscle, this approach also limits donor site morbidity by preserving the anterior rectus sheath in its entirety. The rectus abdominis continues to be an excellent option but a variety of other free flaps are now being utilized for ASB reconstruction.
The ALT is a great option for ASB and orbital reconstruction due to its versatility and reliability[6,31,38,39]. The vascular anatomy, based off the descending branch of the lateral circumflex femoral artery, can be variable but typically includes more than one perforating vessel, which is a particularly useful benefit of perforator flaps when distinct skin paddles are needed[9,10,30,31]. A portion of the vastus lateralis can also be harvested as a chimeric flap for extensive orbitomaxillary defects requiring extra coverage for critical neurovascular structures and exposed dura[38,40]. Additional advantages include minimal donor site morbidity and feasibility of the simultaneous two-team approach to help decrease operative time[9,31]. Aminet al.[41]described their use of the ALT to reconstruct ASB defects in five cases and midface defects in seven cases.The major complication rate in this series, which also included lateral skull base and scalp reconstruction,was 22%[41]. A potential disadvantage of the ALT is a shorter pedicle length in comparison to the rectus abdominis or radial forearm[4,42].
The fasciocutaneous or osteocutaneous radial forearm free flap (RFFF) is another reliable flap for ASB defects[2,4,7,17]. The main advantages for ASB reconstruction are its pliable nature and ability to allow flap folding and contouring of complex defects[9]. It also has substantial pedicle length (up to 15 cm) based off the radial artery that can reach recipient vessels and reduce the need for interposition vein grafts[17]. In a case series of 20 patients, Chepehaet al.[7]demonstrated the role of the RFFF in reinforcing low-volume skull base defects among patients who had prior skull base surgery or radiation. The RFFF was utilized in 14 patients (70%) not only to provide a layer of vascularized tissue between the sinonasal cavity and intracranial space but also for local reconstruction such as obliteration of orbital defects. An osseous component was harvested in six of these 14 patients to help reconstruct the frontal orbital bar and upper facial skeleton[7,12]. The lack of substantial muscle and fat can be a disadvantage when large tissue volume is needed. Potential donor site complications include poor wound healing, tendon exposure, and decreased wrist range of motion[15].
Extensive orbitomaxillary pathology may result in large palatal or midface defects requiring bony reconstruction in addition to coverage of dura and significant soft tissue replacement to obliterate dead space. Osseous reconstruction is often required to provide structural competence for anterior maxillary projection and along the inferior orbital rim and/or orbital floor. The scapula has often been considered the technique of choice for reconstructing total maxillectomy defects[35,43]. Recent popularization of the thoracodorsal artery scapular tip flap, initially described by Chepehaet al.[44], permits the harvest of the versatile scapular tip with a separate, flexible soft tissue component to recontour the complex craniofacial skeleton. This technique omits the need for two separate free flaps and provides a long vascular pedicle reducing the role for interposition vein grafts[44]. The ability to harvest a variety of flaps off the subscapular system is particularly advantageous and allows for both watertight closure of dura and reinforcement of the bony facial structure with orbitomaxillary defects[9,34]. The simultaneous two-team approach is also very feasible for this donor site, particularly when it is harvested from the anterior approach[34]. Shipchandleret al.[35]described the use of a layered osteocutaneous fibula free flap to provide excellent long-term outcomes in total maxillectomy defects including those with OE. The layered fibula is particularly adept at reconstructing the inferior orbital rim and the malar eminence. The authors concluded that utilizing the bone depth along with a malleable skin paddle results in a donor flap that is flexible enough to manage contiguous orbitomaxillary defects[35].
Free flap selection
Despite previously reported algorithms for skull base reconstruction management[2,15,45-48], there is no clear consensus regarding free flap selection due to the heterogeneity and complexity of ASB defects. The ability to create an effective watertight closure and separate the intracranial contents from the sinonasal cavity,orbit, and external environment is the most critical determinant for flap selection. There are a variety of other factors including defect size, location, vascular pedicle length, patient co-morbidities, surgeon experience, and institutional preference that influence flap choice. The thin, pliable nature of the RFFF is often ideal for smaller, low volume skull base defects and especially for recalcitrant CSF leaks or in the salvage setting[2,7]. Based on a retrospective review of 45 free flaps for skull base reconstruction, Weberet al.[2]specifically defined small defects as < 40 mL in volume and primarily recommended the RFFF for such defects. For larger ASB defects with substantial volume requirements, the myocutaneous or myofascial rectus abdominis, ALT, and latissimus are all viable options. The need for adequate skin coverage or multiple skin paddles as well as associated donor site morbidities can also influence free flap selection.Osseous free tissue transfer should be considered for defects extending to the orbit, nasal bone, or other craniofacial buttresses[6,9]. The scapula, fibula, and osteocutaneous RFFF have all been utilized to reconstruct mid-face and associated skull base defects with various advantages and disadvantages[7,34,35,44]. Vargoet al.[12]recommended the osteocutaneous RFFF for high and middle anterior cranial defects due to similar thickness of the radial and frontal bones. Middle defects in this series included the supraorbital bar, frontal sinus, and overlying glabellar soft tissue, while high defects included the squamous portion of the frontal bone and upper forehead soft tissue[12]. Of note, free flaps are often used in conjunction with regional flaps such as the pericranial flap, if available, to create a multilayered closure.
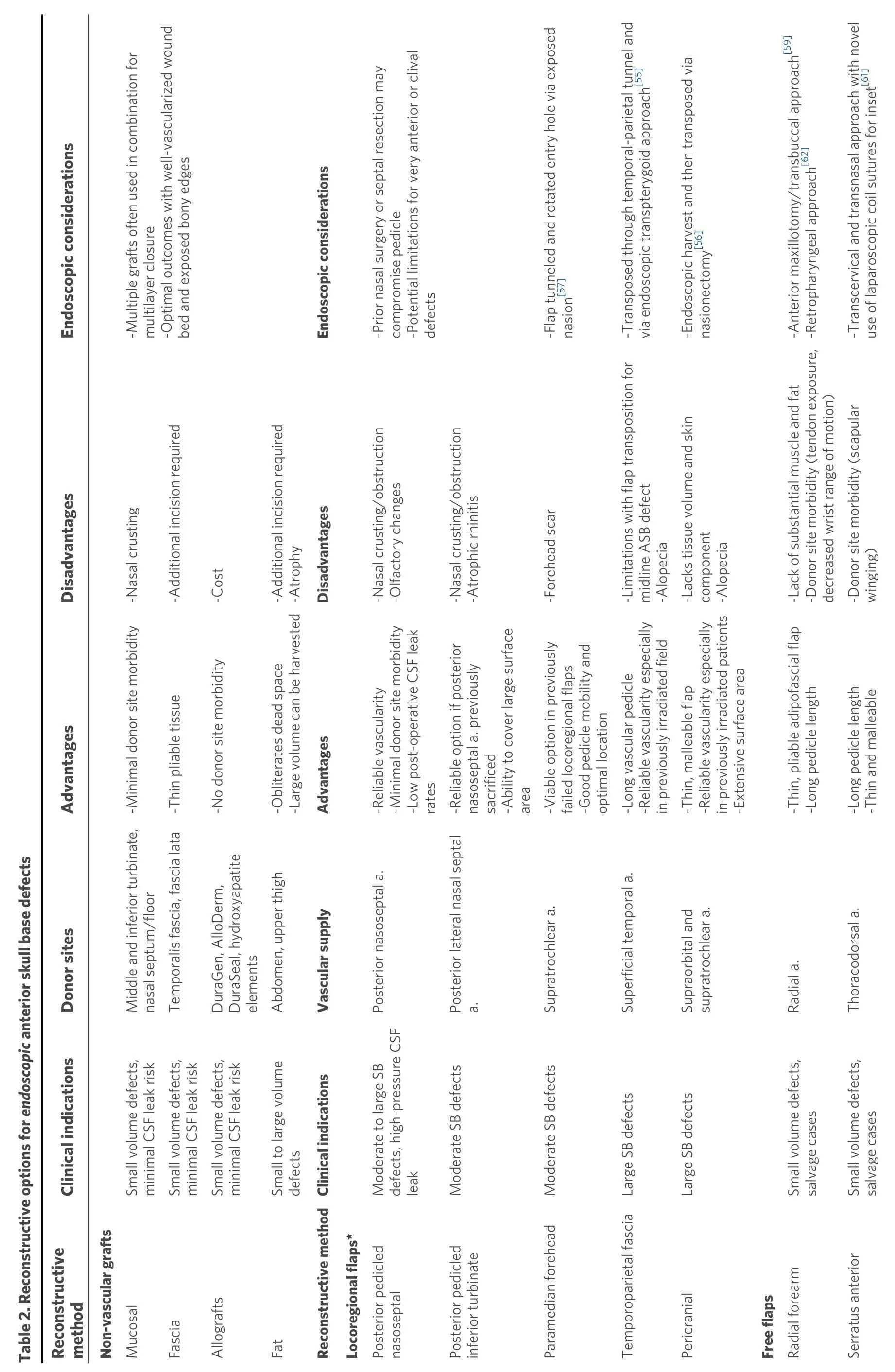
ENDOSCOPIC ENDONASAL DEFECTS
As endoscopic endonasal techniques for ASB pathologies advance, the variety of reconstructive options including free grafts, local/regional vascularized pedicle flaps, and free tissue transfers also continues to evolve [Table 2].
Non-vascular grafts
Free grafts used in a multilayer fashion can be a simple but a highly effective method to reconstruct small ASB defects[8]. These are comprised of both autologous tissue grafts and non-autologous manufactured materials. Common autologous grafts include middle turbinate mucosal grafts, abdominal fat, fascia lata,and temporalis fascia[4,8,14]. Synthetic, non-cellular grafts (i.e., alloplastic and allogenic implants) include collagen matrix, acellular dermis, biological glues, and hydroxyapatite cements[4,6,8]. These non-vascular grafts are most successful among small defects with low-flow CSF leaks, exposed bony edges, and a wellvascularized tissue bed[8,14,49,50].
Locoregional grafts
?

?
The majority of small endoscopic skull base defects can be reconstructed successfully with free tissue grafts,locoregional flaps, or a combination[14]. The posterior pedicled nasoseptal, or the Hadad-Bassagaisteguy flap,used to reconstruct open ASB defects due to their versatility, long vascular pedicles, and adequate skin paddles[5,8,14]. However, there are a multitude of challenges to consider with free tissue transfer via an endoscopic approach. These include access to the defect, narrow constraints of the sinonasal cavity,recipient vessels for microvascular anastomosis, and inset[57]. Thus, a disadvantage of some traditional flaps is excessive bulk resulting in difficult tunneling and/or flap inset.
The first description of free microvascular transfer for endoscopic skull base reconstruction used the vastus lateralis donor site for cribiform and planum sphenoidale defects in a salvage setting[5]. This technique utilizes an endoscopic inset by placing the flap into the sinonasal cavity via a sublabial approach through an anterior maxillotomy to access an ASB defect in order to prevent a craniotomy. Endoscopic medial maxillectomy is also required to place the flap against the skull base. The narrow and flexible strip of vastus lateralis is an ideal free flap as it can be packed and conformed within the sinonasal cavity. The substantial length that can be harvested with the vastus lateralis flap (up to 26 cm) in addition to a long pedicle length(8 cm) is a significant advantage of this flap. No vein grafts were needed in this case series. The long pedicle based off the descending branch of the lateral femoral circumflex artery also helps to ensure excellent pedicle geometry and a tension-free anastomosis after it is tunneled through the buccal space, deep to the buccinator, to reach the recipient facial vessels. The authors highlight the importance of meticulous flap inset and placement only after all the sinonasal mucosa along the skull base and medial orbital walls has been debrided. This helps the muscle flap adhere to the skull base surfaces and completely obliterate the space between the sinonasal and intracranial cavities. The flap is then bolstered in place with a combination of absorbable and non-absorbable nasal packing subsequently followed by nasal trumpets. The exposed muscle bulk will gradually atrophy and rapidly remucosalize within the sinonasal cavity. The main disadvantage of this technique is near total nasal obstruction in the immediate postoperative period[5].
In a recent case series, Pipkornet al.[58]described the use of a RFFF via a similar transbuccal-anterior antrostomy approach for skull base reconstruction after endoscopic endonasal surgery. The authors advocate for the use of an adipofascial flap without a skin paddle, wide transmaxillary exposure for safe delivery of the flap, and precise defect measurement prior to harvest due to frequent overestimation with endoscopic views[58]. Rodriguez-Lorenzoet al.[59]used a similar transmaxillary approach for endoscopic inset of both muscle (i.e., vastus) and adipofascial flaps (i.e., ALT and RFFF) for complex ASB reconstructions in a small case series.
Kraneet al.[60]described a novel surgical technique utilizing a laparoscopic fixation device to assist with free tissue transfer inset for a complex skull base defect in the setting of chronic clival and cervical spine osteomyelitis. Using a combined transcervical and transnasal endoscopic approach, the pedicle of an anterior serratus free tissue transfer was tunneled from the posterior nasopharynx to the neck. Due to concerns for free flap prolapse and ineffective endoscopic suturing in the setting of osteomyelitis,laparoscopic coil sutures were used to circumferentially secure the muscular free flap to the surrounding mucosa under endoscopic visualization. The soft palate was also sutured to the lateral and posterior pharyngeal walls to help suspend the inferior aspect of the flap[60].
A case report by Londonet al.[61]described a retropharyngeal approach for tunneling a RFFF to repair a clival defect in the setting of osteoradionecrosis. Vieiraet al.[62]also reported a case in which a vastus lateralis free flap was utilized for clival reconstruction due to osteoradionecrosis secondary to proton beam therapy. Another approach using the transmaxillary corridor to tunnel the pedicle across the face in the maxillary subcutaneous tissue for contralateral facial vessel anastomosis has also been described[63].
OTHER CONSIDERATIONS
Complications
Post-operative complications can be a significant challenge among this patient cohort due to the complex anatomy, prior surgery, irradiated tissue, and other medical co-morbidities[5,7,9]. CSF leak is the most common complication after reconstructive skull base surgery but other complications, such as meningitis,pneumocephalus, wound dehiscence, and stroke, can also occur[7,31]. In a large retrospective comparison study, Neliganet al.[22]demonstrated that the overall complication rate was 33.5% in free flaps, 38.8% in local flaps, and 75% in pedicled flaps. The authors also reported compromised wound healing in 36.3% of pedicled flaps compared to only 10% of free flaps[22]. The rotational limitations and gravitation pull associated with regional flaps are hypothesized to contribute to increased rates of wound dehiscence[9].Additionally, the distal portion of regional pedicled flaps is often the most vital aspect for effective reconstruction but can also be the most at risk with poor flap circulation[30]. In a different retrospective series of cranial base reconstructions, Changet al.[21]reported an overall complication rate of 29% with the temporalis muscle flap and 31% with free tissue transfer. Llorenteet al.[15]reported an overall complication rate of 17% and a free flap success rate of 94% among 62 flaps used for skull base defects. The majority of these defects involved the ASB (81%) and reconstruction was primarily performed with an ALT (37%).Chepehaet al.[7]also demonstrated that a RFFF in conjunction with the pericranial flap can result in a low rate of major post-operative complications (15%) in salvage skull base surgery. It is important to recognize limitations associated with highlighted retrospective studies including the inherent selection bias and heterogeneity regarding methods of reconstruction, complications reported, and various patient comorbidities.
Recipient vessels
The choice of recipient vessels is an integral aspect of successful free flap outcomes for both open and endoscopic reconstruction[15]. The ipsilateral facial vessels are used most frequently if they are available because they can be traced to level of the mandible and have an accompanying vein. In a retrospective review of 11 free flaps, Vargoet al.[12]exclusively used the superficial temporal vessels for microvascular anastomosis. While the artery is typically acceptable for anastomosis, the size of the vein can be variable.Superior thyroid, lingual, and occipital arteries can also be utilized[45]. Typical recipient vessels may not be viable options in salvage cases, patients with recent infection, or in a vessel-depleted neck, in which use of the transverse cervical artery is often required. The dorsal scapular artery has also been described as a viable recipient vessel during free flap reconstruction in a vessel-depleted neck[64]. Interposition vein grafts may be required to lengthen the venous and/or arterial pedicle despite appropriate pre-operative planning and intra-operative efforts with overall success rates around 85%[65]. Different vein grafts have been described in the literature, such as the external jugular, facial, and cephalic, but the saphenous vein is most commonly used.
Based on a recent retrospective cohort study of 31 patients, Hanicket al.[66]reported the use of the angular vessels as a viable option for microvascular anastomosis in head and neck reconstruction. Despite smallercaliber vessels, the angular artery and vein can be advantageous for ASB defects due to their proximity to the sinonasal cavity and shorter pedicle requirements. This vascular supply can also be beneficial in the setting of previously failed reconstructions or irradiation to the neck[66]. Other potential recipient vessels that have been described based on cadaveric studies include the superior trochlear system[23]. and the third segment of the maxillary artery[67]. Reyeset al.[23]reported an average diameter of 2.5 mm2for the superior trochlear artery in six cadavers, which was then anastomosed to a fascia lata free flap for ASB reconstruction. The superior trochlear vessels were isolated via a Lynch incision and the fascia lata pedicle was then tunneled through a radix osteotomy[23]. This novel technique could be a feasible option for complex skull base defects especially in salvage cases but furtherin vivostudies are warranted.
CONCLUSION
Craniofacial and ASB reconstruction can be challenging due to complex anatomy and associated postoperative complications. A variety of reconstructive techniques have been described for both open and endoscopic approaches, and it is important to be familiar with these options to optimize surgical outcomes.Free tissue transfer is a viable option for extensive ASB defects especially in the salvage setting. The primary goal is to create a watertight closure and the optimal choice depends on location of the defect, size, prior surgery/radiation, medical comorbidities, and surgeon experience. As open and endoscopic skull base surgeries continue to advance, innovative techniques to reconstruct these challenging defects will also continue to evolve including free tissue transfer.
DECLARATIONS
Authors’ contributions
Conceived and designed the article's objective: Naik AN, Parikh AS, Kang SY Performed the literature review and drafted of the article: Naik AN, Lancione PJ All authors were involved in interpretation of the data, provided critical revisions, and approved this version to be published.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
