X-ray absorption investigation of the site occupancies of the copper element in nominal Cu3Zn(OH)6FBr∗
2021-05-06RuitangWang王瑞塘XiaotingLi李效亭XinHan韩鑫JiaqiLin林佳琪YongWang王勇TianQian钱天HongDing丁洪YouguoShi石友国andXuerongLiu柳学榕
Ruitang Wang(王瑞塘), Xiaoting Li(李效亭), Xin Han(韩鑫), Jiaqi Lin(林佳琪), Yong Wang(王勇),Tian Qian(钱天), Hong Ding(丁洪), Youguo Shi(石友国), and Xuerong Liu(柳学榕),†
1Beijing National Laboratory for Condensed Matter Physics and Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2University of Chinese Academy of Sciences,Beijing 100049,China
3School of Physical Science and Technology,ShanghaiTech University,Shanghai 201210,China
4Shanghai Synchrotron Radiation Facility,Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
5Songshan Lake Materials Laboratory,Dongguan 250100,China
Keywords: x-ray absorption spectrum,barlowite spin liquid candidate,chemical occupations
1. Introduction
Quantum spin liquid (QSL) is a quantum state where the spins are long-range entangled but host no symmetry breaking.[2]With the spins being quantum coherent and arranged in a superposition state,QSL is predicted to have exotic properties.[3,4]Efforts to realize QSL state have been much focused on low dimensional geometrically spin frustrated systems,[5–8]such as two-dimensional kagom´e lattice,[9]but so far the results are still elusive. The main challenge is that,since the QSL results from the delicate balance of the microscopic interactions with quantum fluctuations, such state is highly susceptible to perturbations, including non-intrinsic chemical imperfectness.
For example, herbertsmithite ZnCu3(OH)6Cl2has been experimentally suggested to show many QSL properties.[10–15]However, it is known that keeping the fine balancing for herbertsmithite ZnCu3(OH)6Cl2is tricky because of the imperfect alignment of kagom´e planes[17]and possible lattice distortion due to the antisite disorder.[18,20]Nominally, the Cu is expected to occupy the kagom´e planes while the Zn is expected to stay in the interlayer sites. In ZnCu3(OH)6Cl2samples which showed no magnetic order down to 20 mK,[11,12,22]inelastic neutron scattering (INS) results suggested spinonlike dispersionless magnetic excitations.[23,24]But later it was found that the residual Cu2+on the interlayer site contributes mostly to these low energy excitations.[11,24–27]With x-ray anomalous scattering,Freedman et al. suggested that intersite Cu2+impurity concentration is about 15% in their nominal ZnCu3(OH)6Cl2sample.[20]
Recently, a new kagom´e layered system, barlowite Cu4(OH)6FBr and the end member of Zn-doped compound Cu3Zn(OH)6FBr were synthesized and investigated, which will be referred as Cu4and Cu3respectively in the following text. They are of a hexagonal crystal structure(P63/mmc)at room temperature,[1,28–30]as shown in Fig.1. This family is also built from kagom´e planes and interlayer planes, with their kagom´e planes proposed to be perfectly arranged.[17]And with a different coordination environment(trigonal prismatic) around the interlayer Cu2+site compared to herbertsmithite (octahedral), a lower amount of Cu2+defects were predicted.[18,19]
Experimental results showed that, while Cu4(OH)6FBr undergoes an antiferromagnetic (AF) transition at about 15 K,[26,28,31]no magnetic order is observed in Cu3Zn(OH)6FBr down to 50 mK.[28]Further, susceptibility and specific heat studies as well as theoretical calculations[29]suggested that a robust QSL is realized in partially Zn-doped compounds, consistent with former results which indicated that compounds with larger than 30% Zn replacement of the Cu in the interlayers may have intrinsic spin liquid kagom´e planes.[28]All these studies rely on the assumption that the kagom´e layer is perfect with full Cu occupation. Obviously,from the lessons we learned on the norminal ZnCu3(OH)6Cl2,a precise determination of the site occupations is critical in identifying QSL in real materials.
The inductively coupled plasma optical emission spectrometry (ICP-OES)[28,30]and the energy dispersive x-ray spectroscopy (EDS) are often used to determine the chemical ratio of a compound. But the former technique is siteinsensitive,thus can not disentangle the interlayer from intraplane impurities,[32]and the latter one often bears poor energy resolution for insulating materials and requires standards with similar composition, which limit the accuracy of this quantization.[33]Anomalous x-ray scattering has been used to site-selectively estimate the Cu and Zn occupations in herbertsmithite.[20,21]But the analysis depends on comparison to tabulated anomalous scattering factor calculated from the Hatree–Fock wave functions of atoms. These values may not be accurate enough since they depend on the particular chemical environments of the ions.[34]
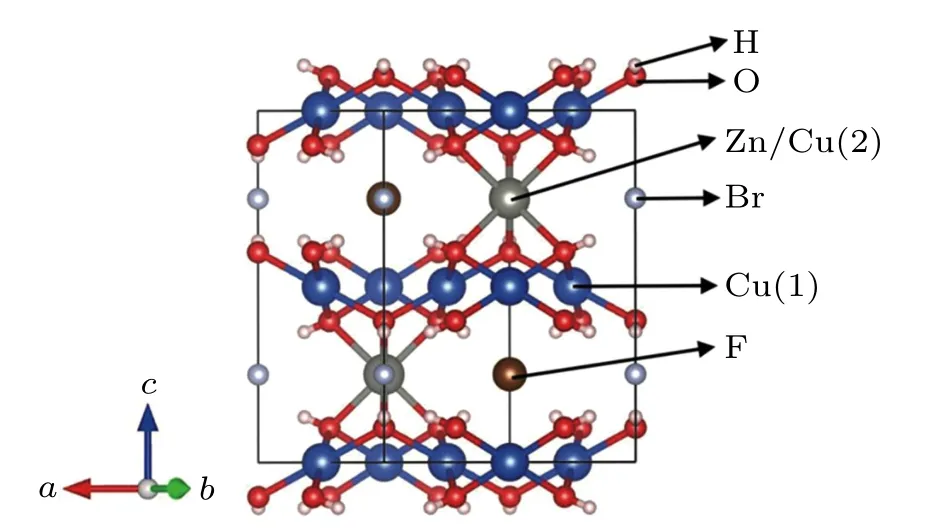
Fig.1. Crystal structure of Cu4(OH)6FBr (Cu4) and Cu3Zn(OH)6FBr(Cu3). Both materials crystalize in P63/mmc space group at 300 K.In Cu4(OH)6FBr, Cu2+ ions lie on intra-kagom´e plane site (Cu(1)) and inter-kagom´e plane site (Cu(2)), respectively. Cu(1) has a octahedral ligand field while Cu(2)has a trigonal prismatic ligand field.
Here we use Cu L-edge x-ray absorption spectroscopy,[35]combined with the MultiX multiplet calculations,[36]to evaluate the contents of inter-layer and intra-plane Cu2+in the nominal Cu3Zn(OH)6FBr. Our results suggest that the metal sites in the kagom´e planes are ∼82%occupied by Cu,while the interlayer metal sites are ∼34%occupied by Cu. Thus there is a strong antisite disorder,and likely the Zn substitution intrudes the kagom´e planes.By assuming that the rest of the metal sites are all occupied by Zn without voids, we estimate the atomic ratio between Cu and Zn to be 1:0.43, close to the values we reported earlier from the EDS measurements.[1]
2. Experimental methods
Nominal Cu4(OH)6FBr and Cu3Zn(OH)6FBr powders were synthesized by the hydrothermal method.[28]The powders were pressed with 8 GPa pressure into dense tablets. After fine polishing, 50 nm platinum electric contact was deposited on the tablet surfaces by pulsed laser deposition(PLD)method, leaving the tablet center an open area for x-ray absorption spectroscopy (XAS) measurements. Both samples were prepared with the same processes under identical conditions. XAS measurements in total-electron-yield(TEY)mode near Cu L3(2p3/2→3d)and L2(2p1/2→3d)edges were performed at beamline BL08U1-A,Shanghai Synchrotron Radiation Facility(SSRF).Incoming x-ray beam was perpendicular to the sample surface. All measurements were carried out at 300 K.
3. Results
The main results are shown in Fig.2. XAS spectra were normalized to the incident beam intensity. The two spectra from Cu4and Cu3are vertically stacked for clarity. In these measurements, the signal is sensitive to the unoccupied 3d states of the Cu element. The photoelectron absorption cross section can be written as[41]
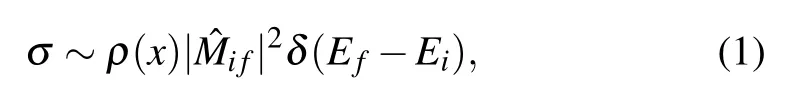
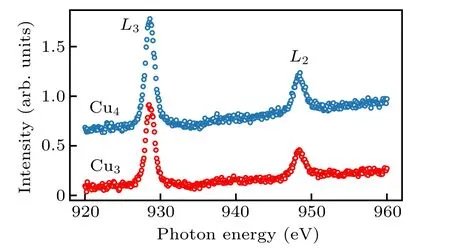
Fig.2. X-ray absorption spectra of Cu4 and Cu3. The measurements were carried out with total electron yield(TEY)mode. The spectra are vertically shifted for clarity.
As less Cu density is expected for the Cu3sample, the reduced absorption strength is also expected as suggested by Eq.(1).The data shown in Fig.2 agrees with such expectation.We will use this sensitivity to deduce the Cu concentration in our sample. It is interesting to notice that, although there are two non-equivalent Cu sites in the Cu4sample, the spectral peaks are quite similar to those of the Cu3sample. This observation indicates that the electronic configurations of the Cu(1)and Cu(2) (Fig.1) sites are quite close in energy, consistent with the DFT calculations.[17]

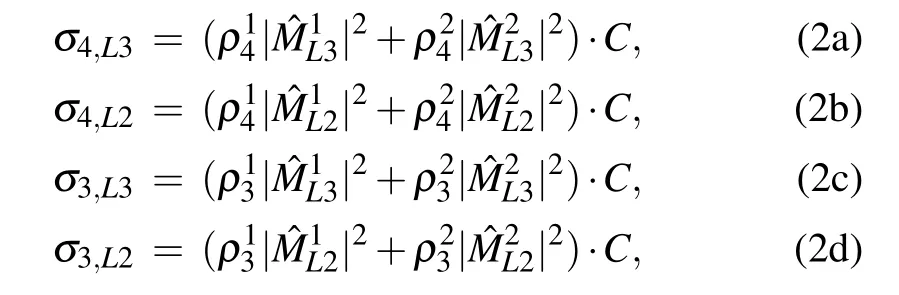
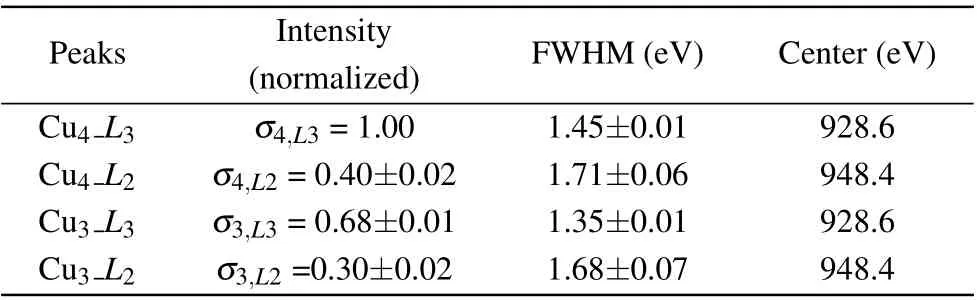
Table 1. Fitting results of L3 and L2 peaks. σm,Ln are the integrated intensities of the Ln peak for Cum samples. FWHM is the full width at half maximum of the peaks.
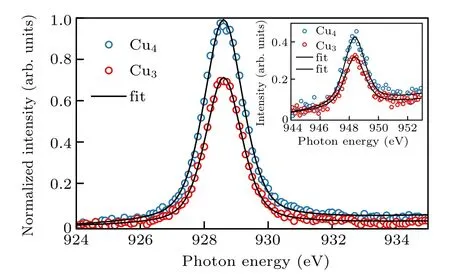

Table 2. The calculated photon absorption matrix elements. All the values are normalized to.
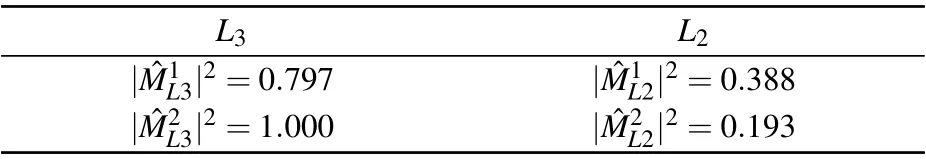
Table 2. The calculated photon absorption matrix elements. All the values are normalized to.
L3 L2| ˆM1L3|2=0.797 | ˆM1L2|2=0.388| ˆM2L3|2=1.000 | ˆM2L2|2=0.193
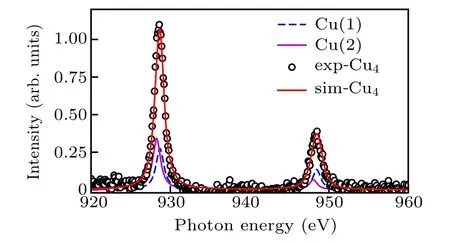
Fig.4.Simulation of the whole spectra of Cu4(OH)6FBr.The TEY data of Cu4(OH)6FBr is overlapped with the simulated result from MultiX.The red line is the weighted sum of the simulated results of Cu(1)and Cu(2)sites(see text).
Clearly, the absorption matrix elements are drastically different between the Cu(1)and Cu(2)sites at the two L-edges.From the crystal structure(shown in Fig.1),the Cu2+ions lie in two highly different local environments.Cu(1)is in kagom´e plane, surrounded by four oxygen atoms and two bromine atoms,forming an octahedral crystal field environment. While Cu(2)is in inter-kagom´e plane,whose nearest neighboring six oxygen atoms form a triangular prism. Thus strong contrast is expected in the transition matrix element ˆMiffor these two sites due to different crystal field effects.[36,42]
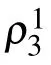
4. Discussion
Our results demonstrate the presence of significant antisite disorder in our measured nominal Cu3Zn(OH)6FBr sample. Assuming no vacancies, our analysis suggests that the chemical formula of our sample is(Cu0.823Zn0.177)3(Cu0.335Zn0.665)(OH)6FBr. It is helpful to compare these results with earlier measurements with other techniques. With ICP-OES measurements,[1,28,43]both Zn rich and Zn insufficient results were reported, and the degree of deviation from the ideal composition was suggested to be about 10%. However, ICP-OES only provides the total concentration of the elements rather than the site-specific content.Thus, the antisite disorder information is lost in ICP-OES results.
Another x-ray technique, namely, x-ray anomalous scattering, has been employed to determine the chemical disorder at different sites in herbertsmithite Cu3Zn(OH)6Cl2.[20]Their results suggested a nearly perfect Cu occupation in the kagom´e layer while the inter-layer site was mixed with Zn:Cu=0.85:0.15. In their analysis, the experimental results were compared to the calculated standard scattering factors for isolated Zn and Cu ions to extract the degree of Zn–Cu mixing on each site. These standard values from Hatree–Fock modeling with free atom approximation[34]may not be accurate for real materials since the anomalous scattering factors might vary in the specific chemical environments.[41]
The L-edge x-ray core-hole spectroscopy for Cu2+has the well-defined 2p →3d transition channel with only one unoccupied valence state. It can disentangle different local sites since the XAS feature depends on the local environment of the absorbing atoms.[44]Potentially it could be a good tool to determine the Cu concentration in Cu3Zn(OH)6FBr. We explored such possibility. As discussed in the main text, our analysis heavily depends on the output of the MultiX package.Although MultiX takes real crystal structure,it is a simplified multiplet calculation with ionic model. Thus certain error is expected.
5. Conclusion
Combining the Cu L-edge XAS measurements and the multiplet caculation with MultiX package,[36]we investigated the antisite mixing in the suggested spin-liquid system Cu3Zn(OH)6FBr. Our results suggest that, in our measured nominal Cu3Zn(OH)6FBr sample, the inter-kagom´e metal element site is 33.5% occupied by residual Cu2+, while about 17.7% of the in-plane Cu(1) site is either vacant or occupied by Zn. In a related compound, the herbertsmithite, it has been shown that since Zn2+and Cu2+are similar in size,Zn2+may occupies Cu2+site in the kagome plane,leading to imperfect kagome plane.[45,46]Our results suggest that similar Zn intrusion into the kagome plane might also happen in Cu3Zn(OH)6FBr.
The accurate determination of element concentration in materials with site sensitivities is generally difficult.Cu3Zn(OH)6FBr and Cu4(OH)6FBr serve as special cases where both Cu(1)and Cu(2)sites are of the same valence,and Zn and Cu are of similar ionic sizes. Our approach and the xray anomalous scattering analysis[20]could be complementary to each other.
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Speeding up generation of photon Fock state in a superconducting circuit via counterdiabatic driving∗
- Micro-scale photon source in a hybrid cQED system∗
- Quantum plasmon enhanced nonlinear wave mixing in graphene nanoflakes∗
- Restricted Boltzmann machine: Recent advances and mean-field theory*
- Nodal superconducting gap in LiFeP revealed by NMR:Contrast with LiFeAs*
- Origin of itinerant ferromagnetism in two-dimensional Fe3GeTe2∗
