Caspase-1: an important player and possible target for repair of the blood-brain barrier underlying neurodegeneration
2021-04-29DanielRandItzikCooper
Daniel Rand, Itzik Cooper
Our ageing population is bearing an increasing burden on society with an unprecedented rise in brain disease,especially neurodegeneration. Treatment for neurodegeneration is practically nonexistent, one major reason being the bloodbrain barrier (BBB). The BBB is a crucial component in maintaining a healthy brain as it protects the brain from the entrance of toxins and removes them from the brain’s vicinity, providing proper clearance.Therefore, BBB malfunction is the basis for numerous brain diseases making it a major target for potential new treatments. A major common underlying mechanism for BBB damage is excessive central nervous system (CNS) inflammation. In turn, BBB disruption has been linked to the initiation of neuroinflammation, forming a vicious cycle.The release of cytokines and destruction of cells are associated with BBB breakdown through multiple molecular mechanisms leading to the chemical and hormonal dysregulation of the brain, increased immune cell infiltration, decreased removal of waste, and degeneration of CNS cells that depending on the affected brain region—ultimately lead to cognitive decline and other CNS disorders (Van Dyken and Lacoste, 2018;Figure 1).
This perspective will briefly discuss BBB function in brain pathologies and the therapeutic potential of targeting caspase-1–the core component of inflammasomes–at the site of brain vasculature.
BBB:The BBB is a conserved structure composed of a continuous endothelial membrane within the brain capillaries which accounts for approximately 85% of cerebral vessels, reaching a total length of ~640 km(Begley and Brightman, 2003). It prevents the passage of most circulating molecules into the CNS, unless they have specialized transport proteins in the brain endothelium that facilitate their passage (Sweeney et al.,2018). Brain endothelial cells (BEC) express unique receptors, transporters and efflux pumps and are firmly connected via tight junctions. Brain capillaries are sheathed by perivascular astrocyte end feet and pericytes embedded in a joint basement membrane that maintain, support and regulate BBB function. Neurons and microglia also communicate with these cells to create a dynamic neurovascular unit (NVU;Figure 1).Maintaining BBB integrity is crucial for tightly controlling the chemical composition of the brain interstitial fluid, which in turn is critical for proper synaptic functioning, information processing and neuronal connectivity.Loss of BBB integrity results in increased vascular permeability and is associated with reduced cerebral blood flow and impaired hemodynamic responses. Furthermore,breakdown of the BBB enables toxic, bloodderived molecules, cells, microbial and viral agents to enter the brain and is associated with inflammatory and immune responses,which can initiate multiple pathways of neuropathologies (Sweeney et al., 2018).
Brain vasculature function is influenced by a variety of risk factors including age, lifestyle,environmental exposure to multiple toxins such as pollution, as well as classic vascularassociated metabolic factors including obesity and type 2 diabetes—all of which are often accelerated by genetic risk factors such as carrying the E4 allele of APOE in Alzheimer’s disease (AD). The BBB transport mechanisms can also be compromised,paradoxically delivering permeable drugs less effectively, such as L-DOPA that is transported across the healthy BBB via a carrier-mediated transport system (Sweeney et al., 2018).
A healthy BBB is not an impermeable wall; rather, it is a “communication center,” responding to and passing signals between the CNS and the blood (Keaney and Campbell, 2015). Under healthy physiological conditions, very few immune cells penetrate the BBB for the purpose of immune surveillance. However, in various pathological conditions, chronic or acute inflammation occurs in different brain regions. This may lead to the disruption of the BBB and can occur prior to the appearance of any pathological symptoms.The extravasation of most immune cells takes place via a paracellular route (between BEC), through interactions with adhesion molecules such as PECAM-1, ICAM-1 and VCAM-1 (Ransohoff and Engelhardt, 2012).It can also occur transcellularly (through the cell). This phenomenon involves clustering of ICAM-1 and can be inhibited by blocking PECAM-1. Caveolin-1 plays an important role in this process, especially in the transmigration of Th1 cells (Lutz et al.,2017). We recently found that caveolin-1 is upregulated in brain blood vessels in mice exposed to the organophosphate paraoxon,and that inhibition of caspase-1 restores its levels back to normal. PECAM-1, ICAM-1 and the chemoattractant MCP-1 were also involved, and their activation was blocked by a caspase-1 inhibitor (Israelov et al., 2020).
Caspase-1:Following stimulation by a myriad of microbial and endogenous signals,an assembly of the multiprotein platform named “inflammasome” occurs via the activation of several Nod-like receptors(Franchi et al., 2009). Caspase-1 is the core component of all inflammasome complexes that regulate the activation, production, and secretion of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 by cleaving their non-active precursors. In addition,caspase-1 cleaves other cellular protein substrates, including pro-caspase-7, and mediates unconventional protein secretion as well as membrane repair and cell survival in response to bacterial pore-forming toxins and other stimuli, some as-yet-to be defined(Lamkanfi et al., 2008). The importance of caspase-1 has been shown recently in many CNS disorders and specifically in AD,in which BBB dysfunction plays a major role.In mouse models, knocking out caspase-1 resulted in protection from memory deficits and improved Aβ clearance (Heneka et al.,2013; Flores et al., 2018). Treating an ADlike mouse model with VX- 765—a caspase-1 selective inhibitor—led to better cognition and reduced AD-like pathology (Flores et al.,2018). In humans, substantially increased amounts of cleaved (active) caspase-1 fragments were observed in brains from AD patients compared to controls, consistent with chronic inflammasome activation.Caspase-1 plays a major role in other brain pathologies; high protein levels of caspase-1 were recorded in the CSF of traumatic brain injury (TBI) patients with high intracranial pressure and caspase-1 expression correlated with poor outcome during the first 96 hours post TBI (Pérez-Bárcena et al.,2020). In a murine model of stroke, elevated expression of inflammasomes and caspase-1 was present on days one and three postinjury. This expression increase was inhibited in mice treated with VX-765 resulting in a better motor recovery as assessed by serial behavior tests and better neuronal survival(Li et al., 2020). In multiple sclerosis (MS)levels of inflammasome- and pyroptosisassociated genes and proteins, including caspase-1 were found to be up-regulated in the CNS of patients as well as in the MS animal model, experimental autoimmune encephalomyelitis. Inhibition of caspase-1 with VX-765 treatment in experimental autoimmune encephalomyelitis reduced the expression of inflammasome- and pyroptosis-associated proteins in the CNS,prevented axonal injury, and improved neurobehavioral performance (McKenzie et al., 2018).
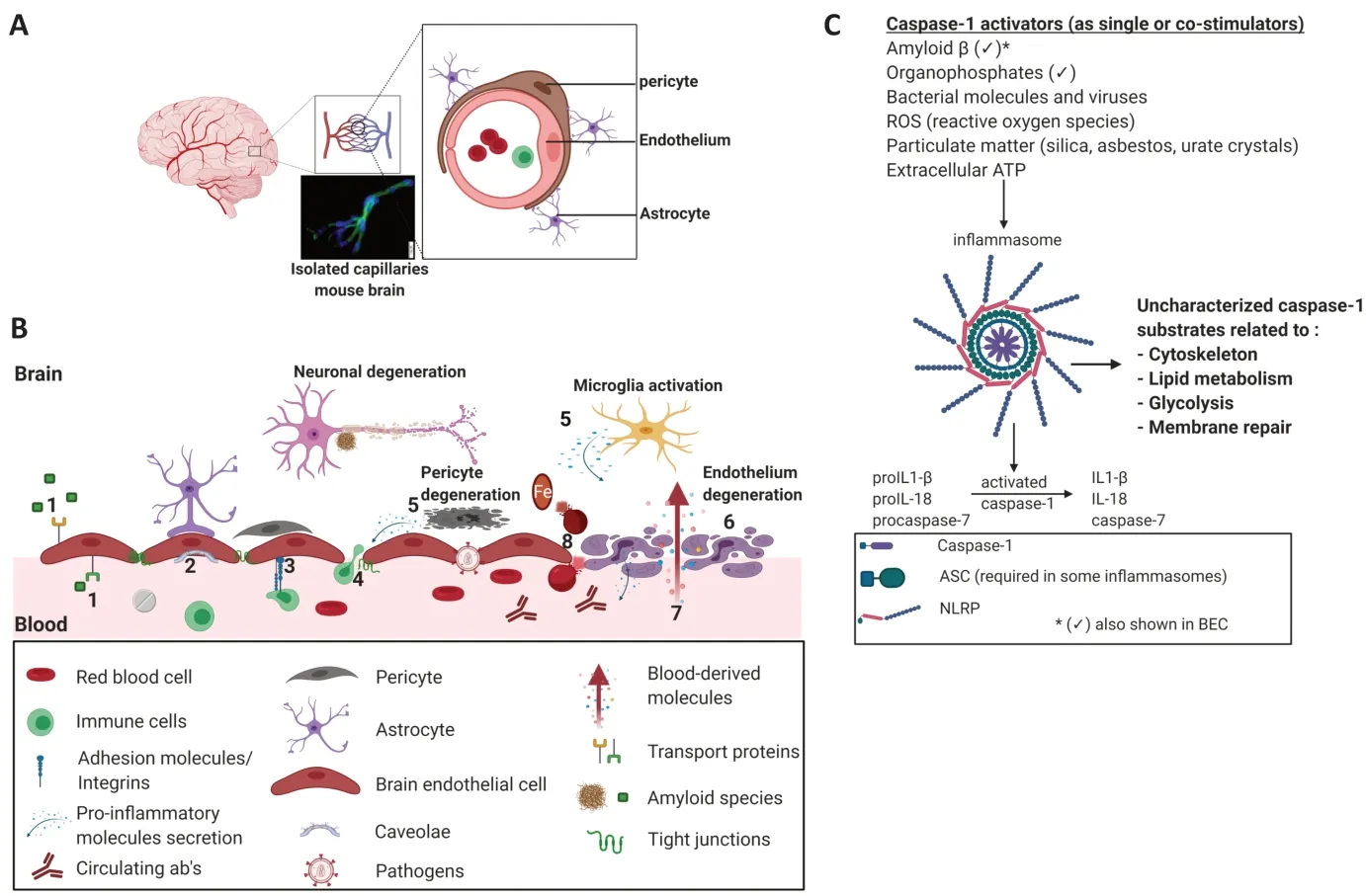
Figure 1|BBB breakdown and caspase-1 activation.
A link between inflammasome activation and brain exposure to chemicals has also been reported. This is important since chronic exposure to various chemicals has been associated with cognitive decline (Liu and Lewis, 2014), though the mechanism remains unknown. Exposure to the chemical herbicide paraquat caused elevated brain inflammation in mice, demonstrated by increased levels of activated caspase-1 and mature IL-1β in the hippocampus (Chen et al., 2015). Organophosphates have been shown to elevate caspase-1 levels in non-cerebral cells (Jang et al., 2015).These findings promoted our interest in examining the role of the inflammasome,and particularly of caspase-1, in mediating endothelium activation and hindering BBB integrity following exposure to toxic compounds.
BBB as a potential therapeutic target:Although the BBB is the interface between the brain and circulation, it has not been sufficiently researched as a potential target for the treatment of CNS diseases.This is despite growing evidence of its important role in various neurodegenerative diseases—not just as a consequence but also as a cause and/or at least as one of the key, early physiological defects leading to these disorders. Moreover, in various neurodegenerative diseases, including AD, Huntington’s disease (HD), Parkinson’s disease (PD) and MS, a disrupted BBB aggravates the disease. Sometimes this occurs in a vicious cycle in which other molecular hallmarks of these pathologies damage the BBB, which in turn fails to clear these toxic elements out of the brain(e.g., amyloid in AD, aggregated Huntingtin protein in HD, α-synuclein in PD, and infiltrating T cells and macrophages in MS).Thus, repairing the BBB holds premise for halting the course of disease and leading to better outcomes. Although the BBB is a major obstacle for CNS therapeutics and the main reason for the failure of CNS drug development, it is in itself readily exposed to drugs circulating in our blood stream. In fact,treating the injured BBB itself may be easier than treating the organ it protects.
Caspase-1 as a potential therapeutic target for repairing BBB damage:Caspase-1 activation functionally links vascular and neurological diseases and hence represents a promising therapeutic target (Lénárt et al.,2016). Humans who possess genetic variants of caspase-1 that display low enzymatic activity do not suffer from immunodeficiency(Luksch et al., 2013) and caspase-1 KO mice are fertile and healthy, indicating that the role of caspase-1 during development is either not critical or that it is replaceable by parallel compensating processes. Caspase-1 inhibition has been shown to protect multiple brain disorders in animal models,including TBI, stroke, MS, HD, cerebral malaria, depression, ALS, PD and AD. What is the connection between caspase-1 and neuronal function? One possibility may be via the regulation of the BBB, which is critical for the maintenance of a proper neuronal microenvironment. A direct effect on neuronal functioning or an indirect effect via other CNS cells, except the BEC, cannot be excluded as well.
他的乳牙我一直带在身上,在上面钻出一个小洞,穿着一根红绳吊在脖子上,可是后来,我跟人打架时被弄丢了,我挂着满脸的血趴在地上找了一个晚上,它就是不见了。
In recent years, numerous studies have focused on the development of multi-target drugs, due to the realization that targeting a single problem or pathway will usually not suffice in complex pathologies, especially in the field of neurodegeneration. Another attractive possibility in this regard will be to find a single molecule that, when targeted or blocked, will rescue multiple damaged phenotypes. We believe that caspase-1 may be such a molecule.
The fundamental molecular commonality of all types of inflammasomes is to activate caspase-1, which is the center of this proteins assembly and which in turn activates several downstream molecules. Some of them are well characterized and some are yet to be discovered or fully defined in terms of specificity and affinity (Figure 1).This means that caspase-1 is the central command of all inflammasomes, and thus when its function is decontrolled, multiple physiological irregularities might be initiated,resulting in pathology in many types of cells and organs. In recent years, non-canonical caspase-1 signaling cascades that are not dependent on its enzymatic activity were revealed, which are independent from the most characterized-NLRP3 inflammasomeand IL-1 cytokines, including tumor necrosis factor α mediated inflammation (Reinke et al.,2020). This further strengthens its important role in broad molecular pathological mechanisms. Targeting caspase-1 therefore has the potential to impose a wide range of therapeutic effects.
Indeed, we recently showed that blocking caspase-1 activation in an injured BBB results in multifaceted repair, including cell death, apoptosis, inflammation, and barrier permeability. A critical point is that rescuing cell death per se does not necessarily rescue the functionality of the organ composed by this cell (i.e., BEC and BBB respectively). For example, when we rescue BEC from toxininduced cell death, one might expect that the barrier function would improve, but this is not always the case. We demonstrated this using apoptosis blockers which completely rescued cell death but at the same time did not improve or even aggravated barrier functions such as the permeability of molecules and immune cells (Ravid et al.,2018; Israelov et al., 2020).
At least in our experience, targeting molecules which are downstream (IL-1β, IL-18, IL-8) or upstream (NLRP3) to caspase-1 may be insufficient to profoundly fix the damaged BBB. This suggests that the location of caspase-1, either physically or temporally,in the sequence of inflammasome activation (and maybe other less known or unknown pathways) makes it a robust molecule influencing multiple BBB assets and functions. Recently, we have examined the potential rescue effects of the selective caspase-1 inhibitor, VX-765, in a BBB injury modelin vitroandin vivo, and found that it can prevent multifaceted effects, including restoration of paracellular damage by averting the downregulation of Ve-cadherin and protecting important transport-related proteins such as caveolin-1 and LDL-R that line the mouse brain vasculature (Israelov et al., 2020). It also prevented inflammatory conditions in the cellular BBB, halting enhanced adhesion and transmigration of immune cells across the barrier via attenuation of important adhesion molecules and chemokines such as ICAM-1, PECAM-1 and MCP-1. The beneficial effects observed in BBB functionality when blocking caspase-1 might be as a direct result of activated inflammasome inhibition in endothelial cells as well as protecting surrounding NVU cells that dynamically communicate to influence barrier functions.
Unlike classic apoptotic caspases, caspase-1 supports cell death but can also support survival, which we believe is essential for rescuing BBB function because of the fine balance that exists between driving cells to death or to survival in order to rescue the organ’s functionality. This may be why,when we rescued BEC from apoptosis using caspase-8 and -9 blockers as well as with the pan-caspase inhibitor ZVAD, the barrier function was not improved and even deteriorated (Ravid et al., 2018; Israelov et al., 2020).
Conclusions:Ideally, novel treatments would focus on developing therapeutic approaches that selectively interfere with those aspects of the inflammatory response that promote damage, while protecting or promoting aspects of repair processes. We suggest the utilization of molecules that can inhibit caspase-1—and other molecular targets that benefit multifaceted pathways—as potential therapeutic candidates that may target the injured BBB and lead to brain repair.
We apologize to our colleagues whose work was not cited here because of space limitations. Figure 1 was created with BioRender.com.
This work was supported by the Defense Threat Reduction Agency-Joint Science &Technology Office for Chemical & Biological Defense (No. 11816372), and the Nehemia Rubin Excellence in Biomedical Research-The TELEM Program supported by the Aaron Gutwirth Fund and by Ministry of Science and Technology (No. 3-13576).
Daniel Rand, Itzik Cooper*
The Joseph Sagol Neuroscience Center, Sheba Medical Center, Ramat-Gan, Israel (Rand D,Cooper I)
Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel (Rand D)
School of Psychology, Interdisciplinary Center (IDC),Herzliya, Israel (Cooper I)
The Nehemia Rubin Excellence in Biomedical Research – The TELEM Program, Sheba Medical Center, Tel-Hashomer, Israel (Cooper I)
*Correspondence to:Itzik Cooper, PhD,itzik.cooper@sheba.health.gov.il.https://orcid.org/0000-0003-0723-5542(Itzik Cooper)
Date of submission:October 10, 2020
Date of decision:December 4, 2020
Date of acceptance:February 28, 2021
Date of web publication:April 23, 2021
https://doi.org/10.4103/1673-5374.313031 How to cite this article:Rand D, Cooper I (2021)Caspase-1: an important player and possible target for repair of the blood-brain barrier underlying neurodegeneration. Neural Regen Res 16(12):2390-2392.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Inhibition of extracellular vesicle pathway using neutral sphingomyelinase inhibitors as a neuroprotective treatment for brain injury
- Neuroglobin and neuroprotection: the role of natural and synthetic compounds in neuroglobin pharmacological induction
- Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders
- Epidural electrical stimulation for spinal cord injury
- Localization of the hydrogen sulfide and oxytocin systems at the depth of the sulci in a porcine model of acute subdural hematoma
- Lithium beyond psychiatric indications: the reincarnation of a new old drug
