Effect of Mechanical Forces on the Behavior of Dental Stem Cells:A Scoping Review of In-Vitro Studies
2021-04-25MaryamRezaiRadSadraMohagheghFarnazKouhestaniandSaeedRezaMotamedian
Maryam Rezai Rad, Sadra Mohaghegh,Farnaz Kouhestani and Saeed Reza Motamedian
1Dental Research Center,Research Institute of Dental Sciences,School of Dentistry,Shahid Beheshti University of Medical Sciences,Tehran, 1983963113, Iran
2Student Research Committee, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, 1983963113,Iran
3Department of Periodontics, School of Dentistry, Tehran University of Medical Sciences, Tehran, 1439955991, Iran
4Dentofacial Deformities Research Center,Research Institute of Dental Sciences,Department of Orthodontics,School of Dentistry,Shahid Beheshti University of Medical Sciences, Tehran, 1983963113, Iran
ABSTRACT This article is a scoping review of the studies that assessed the effect of mechanical forces on the behavior of dental stem cells (DSCs).PubMed and Scopus searches were done for in-vitro studies evaluating the effect of tension,hydrostatic pressure (i.e., the pressure applied through an incompressible fluid), compression, simulated microgravity,and vibration on DSCs.The following factors were analyzed:osteogenic/odontogenic differentiation,proliferation, adhesion and migration.Articles were reviewed according to the Preferred Reporting Items for Systematic Reviews extension for scoping reviews(PRISMA-ScR)guideline.Included studies were evaluated based on the modified Consolidated Standards of Reporting Trials (CONSORT).A total of 18 studies published from 2008-2019 were included.Nine studies were focusing on Periodontal ligament Stem Cells(PDLSCs),eight studies on Dental Pulp Stem Cells(DPSCs)and one study on Stem Cells from Apical Papilla(SCAP).Results showed that tension,three-dimensional stress and simulated microgravity promoted the proliferation and osteogenic differentiation of PDLSCs.DPSCs proliferation increased after microgravity and tension exertion.In addition, dynamic hydrostatic pressure and compression promoted odontogenic differentiation of DPSCs.Besides, mechanical stimuli increased the osteogenic differentiation of DPSCs.One study analyzed the effect of carrier features on the response of DSCs to 3D-stress and showed that cells cultivated on scaffolds with 30% bioactive glass (BAG) had the highest osteogenic differentiation compared to other ratios of BAG.It has been shown that increasing the duration of tension (i.e., from 3 h to 24 h force application) enhanced the positive effect of force application on the osteogenic differentiation of DSCs.In conclusion, all types of mechanical forces except uniaxial tension increased the osteogenic/odontogenic differentiation of DSCs.In addition, the effect of mechanical stimulation on the proliferation of DSCs differs based on the type of stem cells and mechanical force.
KEYWORDS Tension; compression;biophysics; stem cells; proliferation; differentiation
1 Introduction
Stem cells(SCs)can be isolated from adipose tissue,neural tissue,lung,peripheral blood,bone marrow and heart[1].Dental tissues are a more recent source of SCs,which can be widely used due to their ease of access,considering that teeth are commonly extracted and distracted as waste[2].Indeed,dental stem cells(DSCs)can be isolated from dental pulp(DPSCs),periodontal ligament(PDLSCs),apical papilla(SCAP),and gingival tissue (GMSCs) through minimally invasive procedures [3-7].The response of DSCs to physiological forces can affect the function and structure of the teeth and surrounding tissues [8-11].Besides, alternation of the biological behavior of DSCs through mechanical forces can be useful for therapeutic and regenerative purposes.
DSCs proliferation and differentiation can be affected by physiologic mechanical stimulations during jaw movements and occlusion.For instance, DPSCs differentiate towards the odontoblasts and produce dentin when they are subjected to biomechanical forces such as trauma and erosion [12-15].On the other aspect, PDLSCs can increase or decrease bone uptake based on the magnitude and direction of mastication force [16].Therefore, investigating the response of DSCs to mechanical forces can be beneficial for explaining the physiological phenomena.
Therapeutic procedures are the other field which can benefit from altering the behavior of DSCs through mechanical forces.For instance,PDLSCs can respond to the mechanical stimuli of stretching or compression during orthodontic movements and produce chemical signals.These signals induce bone remodeling by regulating osteoblastogenesis and osteoclastogenesis [8,17-20].Therefore, analyzing and anticipating the response of DSCs to mechanical forces can be valuable in enhancing therapeutic strategies aimed to accelerate tooth movement and prevent tooth overload.
Additionally,DSCs derived from various sources along with scaffolds and growth factors can be applied for bone regeneration [21,22].The main role of growth factors is to induce osteogenic differentiation in cultivated DSCs.Such osteogenic induction may also be created by applying mechanical forces.For instance, cells cultivated on the scaffold can face tensile force, which increases the osteogenic differentiation, and then be implanted in the intended site [23].Therefore, the response of each type of DSCs to mechanical forces can also be useful in regenerative medicine.
Recently,many in vitro models have been designed to investigate the role of different mechanical stimuli on the behavior of DSCs[24].Mechanical forces such as strain,stretch,compression and microgravity can affect proliferation, osteogenic/chondrogenic differentiation, migration and adhesion of DSCs [8,9].However, some results are controversial and it has been shown that the response of stem cells varies based on the type of stem cells and parameters of the applied mechanical force [25,26].Therefore, the main question of this scoping review was “What is the effect of mechanical forces on the behavior of DSCs in-vitro considering the type of DSCs, mechanical stimulation and cell culture system?”
2 Material and Method
Study designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR)guideline.
2.1 Information Source and Search Strategy
The search strategy was designed based on the PICOS Question:“What is the effect of mechanical forces (I) on the behavior (O) of DSCs (P) in-vitro(S)?” Considering the fact that precise analyses concerning the behavior of stem cells are performed in-vitro conditions, this scoping review has been performed on in-vitro studies.Pub-Med and Scopus electronic search were done based on the combination of relevant keywords (Tab.1).Besides, reference lists of the included articles were manually searched to find possible related studies
2.2 Eligibility Criteria
2.2.1 Type of Studies
All in vitro studies which stimulate DSCs through mechanical force were included.Only English studies published before October 2020 were included.Abstracts,letters, and reviews were excluded.
2.2.2 Types of Participants and Interventions
All types of human DSCs including DPSCs, PDLSCs, SCAPs, human exfoliated deciduous teeth and dental follicle stem cells were considered [3].Studies that stimulate DSCs with mechanical forces mentioned in Tab.1 were included.Studiesmerely assessed the effect of medium mechanical features or subjected stem cells to non-mechanical stimulations were excluded.Studies which did not perform the stem cell identification tests (i.e., multilinear differentiation capacity and cell surface marker analyses)were excluded.
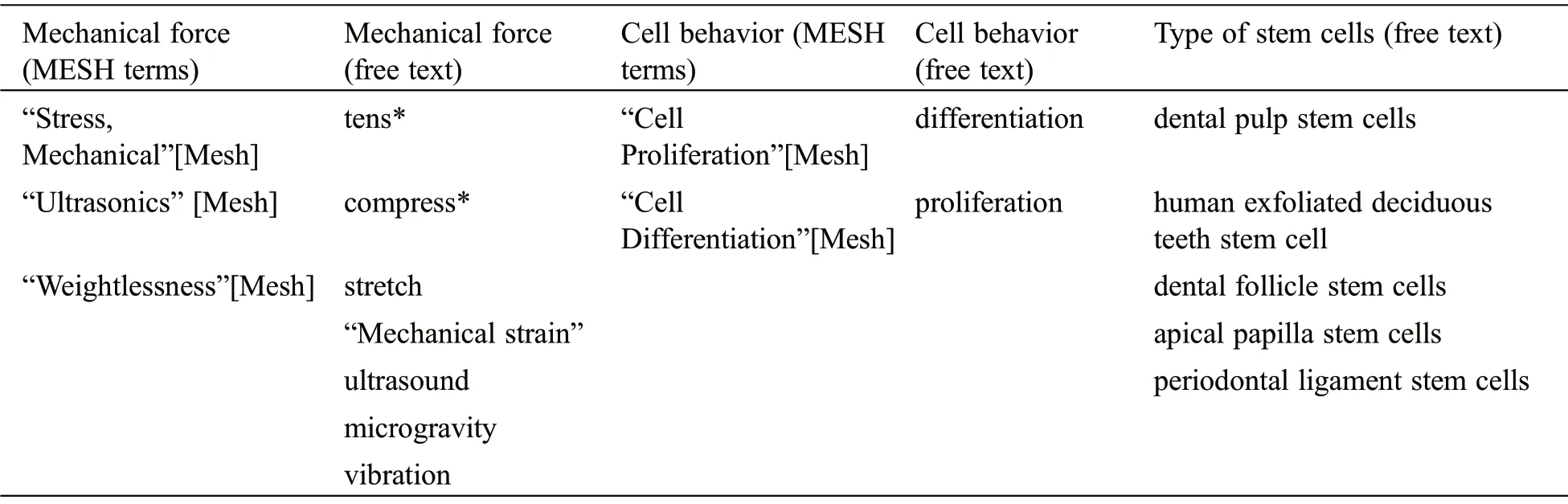
Table 1:Key words
2.2.3 Type of Outcome Measurement
Studies which assessed the effect of mechanical stimulation on stem cell behaviors such as proliferation,differentiation, migration and adhesion were included.Studies performed proliferation-related tests such as MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) assay, Bromodeoxyuridine/5-bromo-2'-deoxyuridine (BrdU) and cell counting kit or studies including osteogenic/odontogenic differentiation tests such as Alizarin Red staining, reverse transcription-polymerase chain reaction (RT-PCR)and western blot assessments were included.
2.3 Selection of Sources of Evidence and Data Charting Process
Two reviewers performed electronic search, study selection and data extraction independently and a third independent expert resolved disagreements.Initial screening of titles and abstracts was done according to the mentioned eligibility criteria.Then a complete evaluation of the full text of the chosen articles was performed.
2.4 Data Items
The following information was extracted from each of the included articles:1)types of DSCs;2)types of the mechanical stimulation; 3) force generation device and some details about the magnitude and frequency of mechanical force; 4) types of the medium used to culture stem cells; 5) the type of cell behaviors which were assessed,6) results (response of the DSCs to the mechanical forces).
2.5 Critical Appraisal
Data were evaluated based on modified CONSORT [27] including the following items:structured summary (yes/no); specification of background, explanation and objective/or hypothesis; sufficient details about interventions were done for each group (yes/no); usage of suitable statistical methods (yes/no);expressing the result of each group and the estimated size of the effect and its precision (yes/no);discussing the limitations, potential bias, imprecision and multiplicity of analyses (yes/no) and identify the sources of funding (yes/no).If more than one item was not met, the study was considered as high risk of bias (ROB).
3 Results
Electronic and hand search resulted in 425 studies out of which 18 articles were included(Fig.1).The effect of mechanical forces on PDLSCs, DPSCs and SCAP were assessed in nine, eight and one studies,respectively.All included studies had low risk of bias.Among all included studies, merely two studies analyzed the effect of mechanical force on the adhesion of DSCs [28,29].No study was found concerning the response of human exfoliated deciduous teeth and dental follicle stem cells to mechanical stimulation(Fig.2).Besides,studies which applied shear stress to dental cells did not met our inclusion criteria.
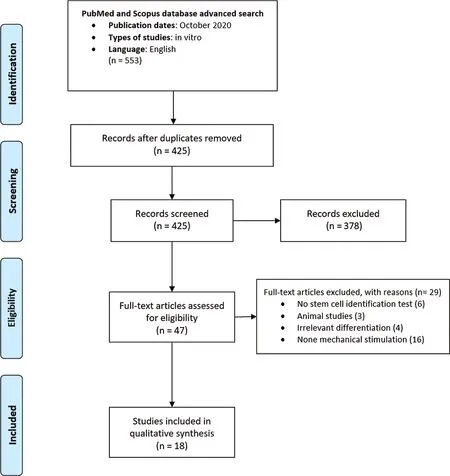
Figure 1:Flow diagram

Figure 2:Distribution of the studies based on the type of force and cells
DSCs were cultivated in different culture systems.Among the included studies, one study used polyethylene plastic dish [12], one study silicon membrane [30], one study plasma coated silicon membrane [31], one study microcarrier beds, one study glass cover lips [32], two studies silk scaffolds[33,34], one study bioactive glass scaffold, one study PLGA scaffold [35] and nine studies used plates.Among the studies cultured cells on plates, four studies [36-39] coated plates with type one collagen.Merely one study analyzed the effect of carrier features on the efficiency of the mechanical force.Wang et al.[40] analyzed the effect of 3D-stress on PDLSCs cultivated on scaffolds with different ratios of BAG (10%, 20%, 30%, 40%, 50%) and showed that the highest osteogenic differentiation occurred when 30%BAG scaffolds were used.
3.1 PDLSCs
Among 9 PDLSCs articles,cyclic tensile stress[23],low magnitude(magnitude <1 g)high frequency(frequency >20 Hz) vibration (LMHF) [41,42], static mechanical strain [37], compressive force [24],equiaxial strain [39], three-dimensional stress [40], simulated microgravity [43] and Cyclic tension force[38] were considered as mechanical stimulation (Tab.2).Five articles [37,40-43] assessed the effect of stimulation on both differentiation and proliferation while one article [24] evaluate merely proliferation and three articles [23,38,39]merely performed differentiation tests.
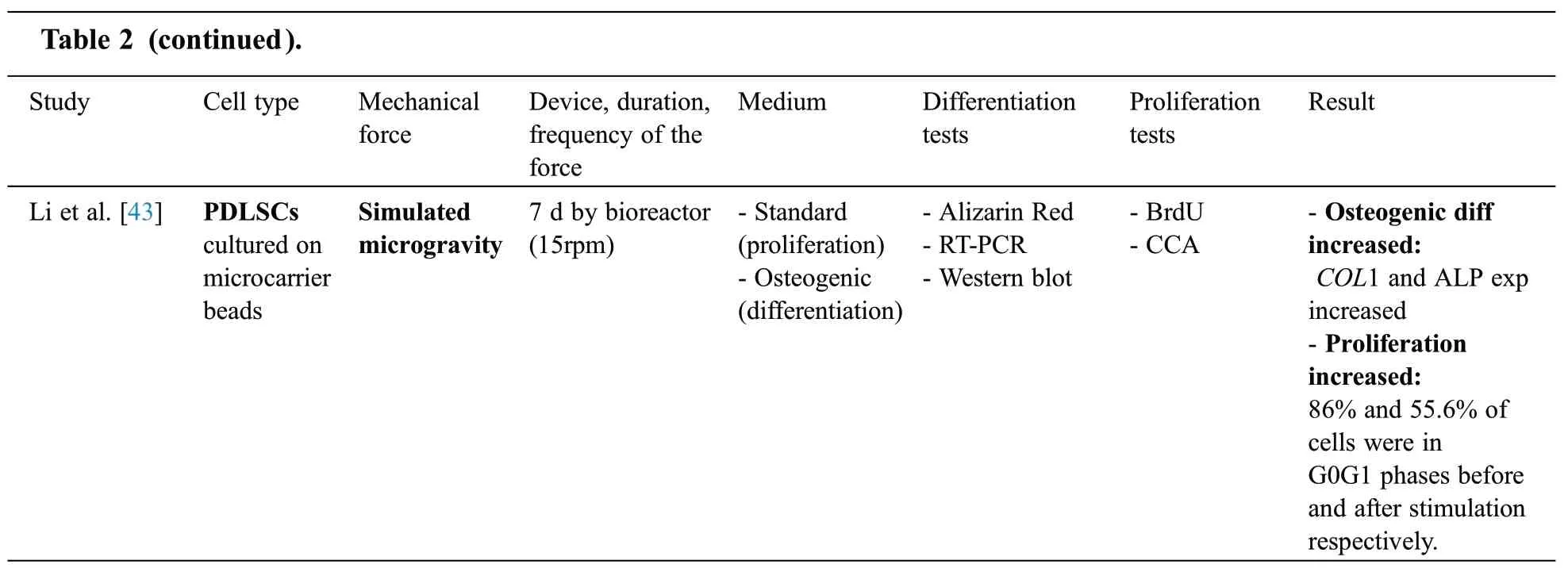
Note:PDLSCs:Periodontal ligament stem cells; Bf:Bioflex; h:Hour; RT-PCR:Reverse transcription polymerase chain reaction; N/A:not applicable; diff:differentiation; SATB:special AT-rich sequence-binding protein; RUNX2:Runt-related transcription factor2; OSX:Osterix; exp:expression; g:gram; min:minute;CCK:Cell Counting Kit; COL:Collagen;ALP:Alkaline Phosphatase;OCN:Osteocalcin; Hz:Hertz; ELISA:Enzyme-linked Immune Sorbent Assay; CCA:Cell Cycle Assay; ASB:amino silicone-bottom; BSP:Bone sialoprotein; BAG:Bioactive glass;OPN:Osteopontin; FB:flexible bottomed; rpm:rounds per minute; BrdU:Bromodeoxyuridine
Among all PDLSCs articles,in three studies[24,37,40]proliferation assessed through MTT assay and in two experiments [41,42] cell counting kits were used.In addition, in one article [43] BrdU assay was performed to evaluate proliferation and in two studies [43,41] flow cytometry was used to analyze cell cycle distribution.Two studies [41,42] showed that proliferation of PDLSCs decreased following LMHF vibration.However, static mechanical strain [37], three-dimensional stress [40] and simulated microgravity[43]promoted the proliferation of PDLSCs.
Panchamanon et al.[24]emphasized that the effect of mechanical forces on PDLSC is dependent on its magnitude [24].They showed that 49.03 Pa compressive force decreased the proliferation of PDLSCs.However,147.09 Pa and 196.13 Pa compressive force for 24h had no effects on the cell viability of PDLSCs.
All of the articles which assessed the differentiation of PDLSCs used RT-PCR test to analyze osteogenic/odontogenic differentiation through evaluating various osteogenic (ALP, OPG, BMP-2, OCN, BSP, OPN,Nestin, and RUNX2) or odontogenic genes (DSPP, DMP-1).Moreover, six articles [23,39,41-44] used western blot analyses along with RT-PCR.It has been shown that low magnitude high frequency vibration [41,42], all types of tension [39,44-46], three-dimensional stress [40] and simulated microgravity[43]promoted osteogenic differentiation of PDLSCs.
Wei et al.[39]and Panchamanon et al.[24]were the only studies which did not compare the effect of periods and magnitudes of mechanical force on the osteogenic differentiation of PDLSCs.Despite Shen et al.[38] and Tang et al.[23] showed that osteogenic differentiation of PDLSCs increased as the duration of tension application increased (i.e., from 3 h to 24 h force application).In addition, Li et al.[43] showed that SMG application for 72 h increased the osteogenic differentiation of PDLSCs to higher levels compared to 24 h of force application.Liu et al.[37] analyzed different magnitudes of tension (i.e., 6%,8%, 10%, 12%, 14%) and showed that the highest osteogenic differentiation of PDLSCs occurred when 12% tensile force applied.Zhang et al.[42] analyzed the effect of different vibration magnitudes and showed that the osteogenic differentiation of PDLSCs increased from 0.05 g to 0.3 g vibration and decreased in the vibrations of higher than 0.3 g.In another study [41], they analyzed different frequencies of vibration and showed that cells faced 50 Hz of vibration had the optimal osteogenic differentiation.
3.2 DPSCs
Among 8 articles assessing the effect of mechanical stimuli on DPSCs(Tab.3),seven articles analyzed the differentiation of DPSCs while four[32,34-36]assessed the proliferation.Dynamic mechanical tension[36], dynamic hydrostatic pressure [32], equiaxial strain [30], cyclic tensile stretch [12,33], compression[31], combined mechanical stimulation (i.e., shear stress, tension and compression) [34] and microgravity[35] were applied(Tab.3).
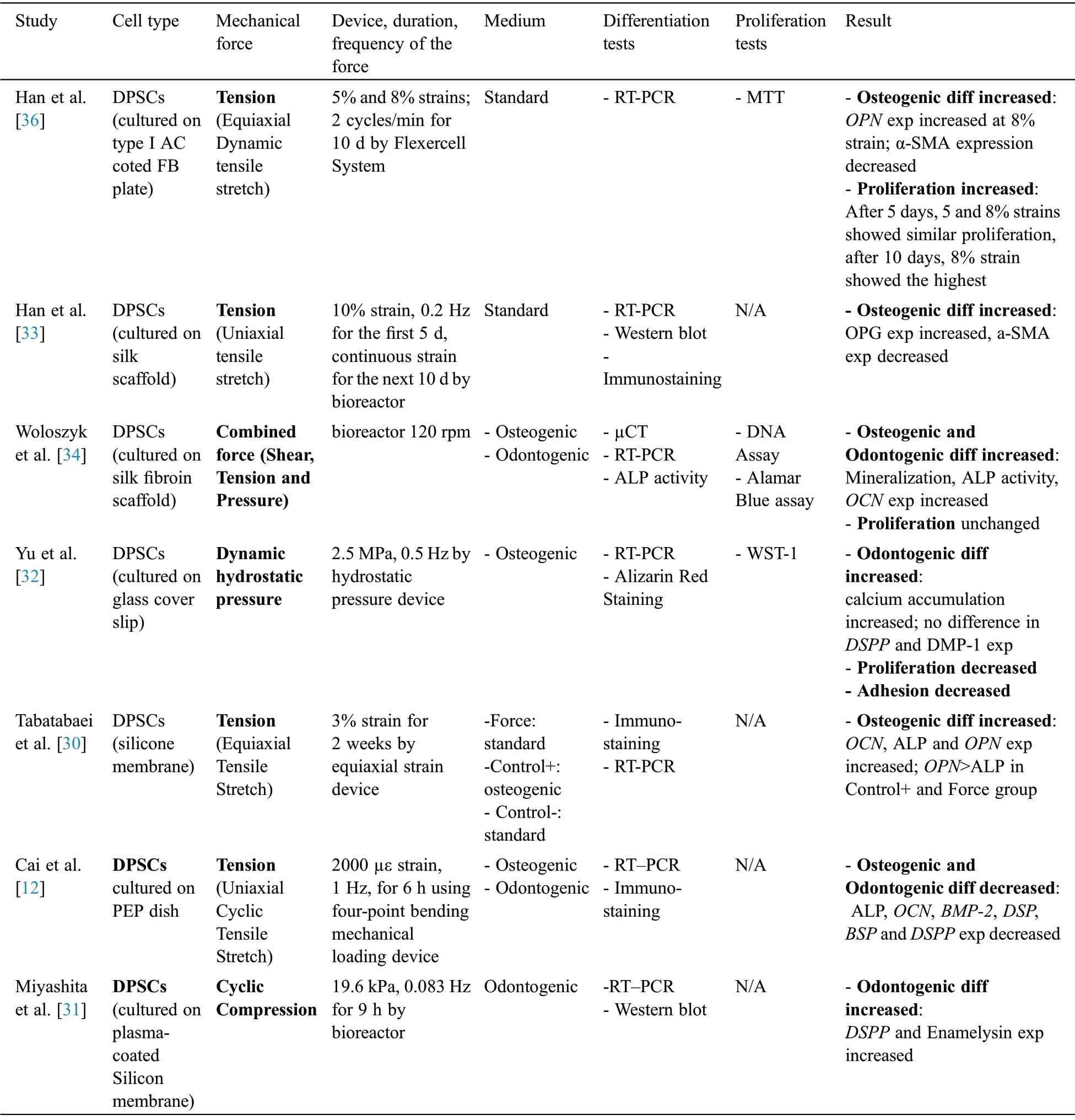
Table 3:Comparison of studies (DPSCs)
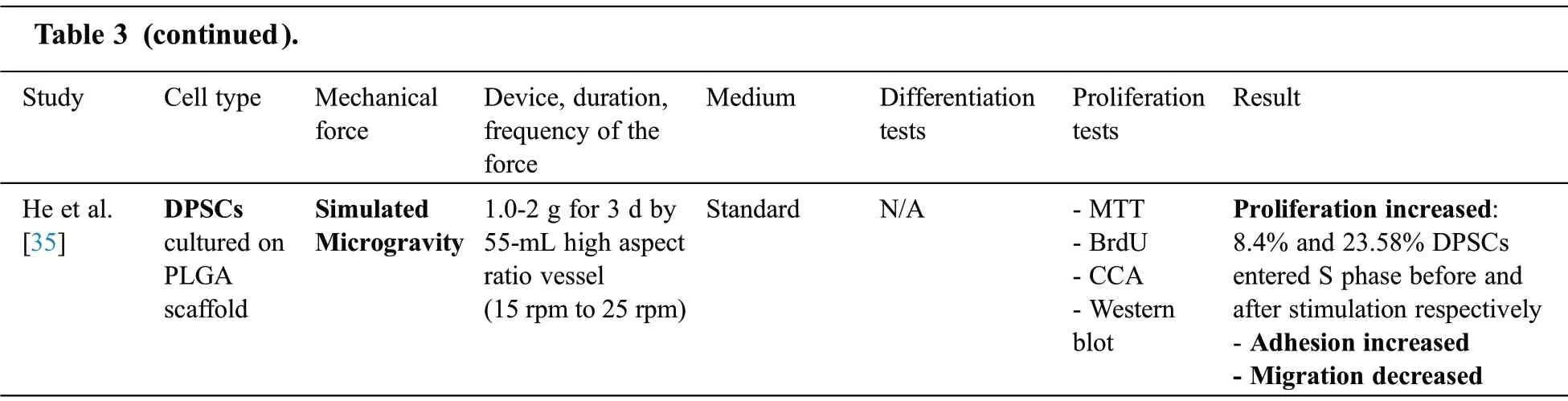
Note:DPSCs:Dental Pulp Stem Cells;Bf:Bioflex;h:Hour;RT-PCR:Reverse transcription polymerase chain reaction;N/A:not applicable;diff:differentiation;SATB:special AT-rich sequence-binding protein;RUNX2:Runt-related transcription factor2;OSX:Osterix;exp:expression;g:gram;min:minute;CCK:Cell Counting Kit;Col:Collagen;ALP:Alkaline Phosphatase;OCN:Osteocalcin;Hz:Hertz;ELISA:Enzyme-linked Immune Sorbent Assay; CCA:Cell Cycle Assay; ASB:amino silicone-bottom; BSP:Bone sialoprotein; BAG:Bioactive glass; OPN:Osteopontin; FB:flexible bottomed; rpm:rounds per minute; BrdU:Bromodeoxyuridine; kPa:Kilopascal; PLGA:poly lactic-co-glycolic acid; DSPP:Dentin Sialo Phospho-Protein; PEP:polyethylene plastic; BMP-2:Bone Morphogenic Protein-2; DSP:Dentin Sialo Protein; BSP:Bone Sialo Protein;DMP:Dentin Matrix Acidic Phosphoprotein; SMA:Smooth muscle actin.
MTT assay was used to assess proliferation in two experiments[36,35].He et al.[35]used BrdU assay and flowcytometry beside MTT to analyze DPSC proliferation and cell cycle, respectively.In addition,Wolozyk et al.[34] used DNA assay kit and Almar Blue assay to evaluate proliferation.Although proliferation increased after microgravity [35]and tension[36] exertion, Wolozyk et al.[34] demonstrated that the combined mechanical force had no significant effect on the proliferation of DPSCs.
All of the articles which assessed differentiation performed RT-PCR tests.In addition, western blot analysis [31,33], alizarin red staining [32] and immunostaining [12,30,33] were also performed to analyze osteogenic/odontogenic differentiation.Among reviewed articles, Cai et al.[12] was the only study which showed a decrease in DPSCs odontogenic differentiation following tension.However, it has been shown that combined mechanical forces [34], dynamic hydrostatic pressure [32] and compression [31] promote odontogenic differentiation of DPSCs.In addition, it has been shown that osteogenic differentiation of DPSCs increased following tension[47-49]and combined mechanical force[34].
A total of two studies assessed the effect of mechanical forces on adhesion.Yu et al.[32]showed that hydrostatic pressure decreased the adhesion of DPSCs.However,He et al.[35]showed the positive effect of microgravity on adhesion.
In the case of the medium condition,four articles[12,30-32]used inducing mediums to evaluate DPSCs differentiation while in others [33,36] cells were cultured in standard(non-inducing)medium.
Yu et al.[32],Woloszyk et al.[34],Cai et al.[12],Han et al.[33],He et al.[35]and Tabatabaei et al.[30]did not compare the effect of different time points and magnitudes of mechanical stimulation on the osteogenic/odontogenic differentiation of DPSCs.However, Han et al.[36] showed that increasing the magnitude of tension enhanced the osteogenic differentiation of DPSCs.Besides, Miyashita et al.[31]analyzed different magnitudes of compression and showed that the highest odontogenic differentiation occurred when 19.6 kPa force applied.
3.3 SCAP
Mu et al.[50] analyzed differentiation and proliferation followed by mechanical stimuli in SCAP(Tab.4).They applied mechanical strain for 30 min using a centrifuge.Differentiation was assessed through Alkaline phosphatase (ALP) activity assay, Alizarin red staining, RT-PCR and western plot.Proliferation evaluated via MTT and cell cycle analysis.It was demonstrated that although mechanical stimulation increased osteogenic and odontogenic differentiation, SCAPs proliferation was decreased following the application of mechanical force.
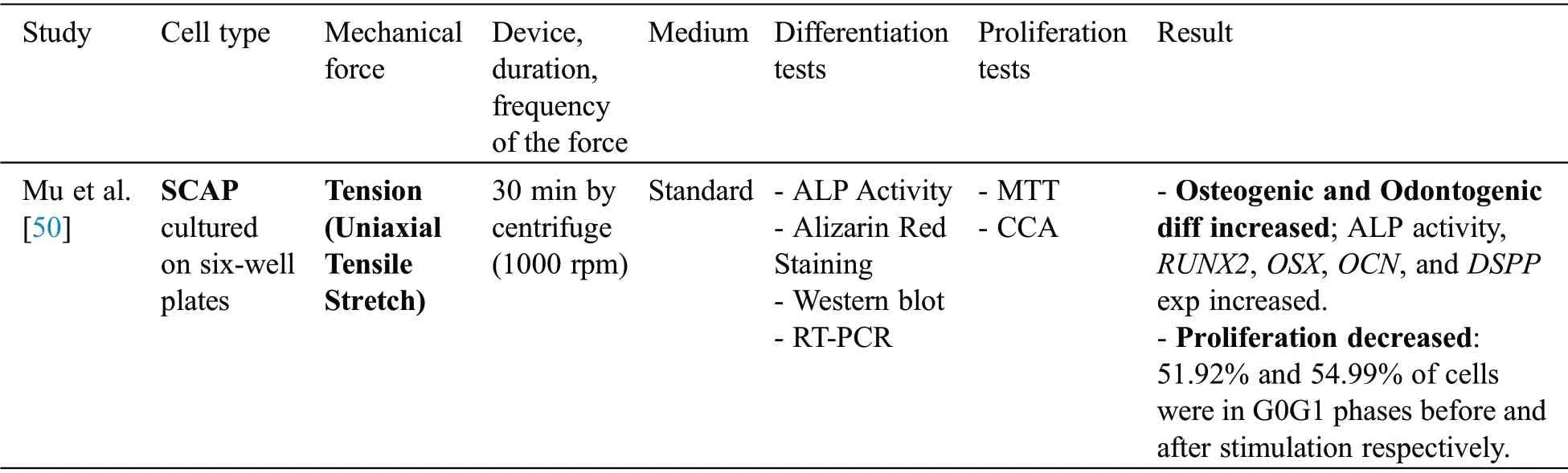
Table 4:SCAP studies
4 Discussion
It has been shown that the rate of differentiation and proliferation of DSCs can be altered by mechanical stimulation based on the type of force[12,30,31,35].The main purpose of this scoping review was to analyze studies applied mechanical forces to DSCs comprehensively.The osteogenic/odontogenic differentiation increased in all of the studies but one which applied uniaxial tensile force to DPSCs [12].In addition,results of the included studies showed that the effect of mechanical stimulation on the proliferation of DSCs differs based on the type,magnitude and frequency of the mechanical force.
4.1 Tension
During orthodontic tooth movement, osteoclastic and osteoblastic differentiation occurs in the compression and tension sides, respectively [51-54].Our study showed that tension (without any other chemical stimulation) can stimulate the osteogenic differentiation of DSCs.This is a different route from pressure-tension theory that states changes in the blood flow of periodontal tissue which occurs following the application of forces, result in the release of chemokines stimulate osteoblastic and osteoclastic differentiation [55-57].
The effect of tensile force on the differentiation of DSCs can be altered based on the force type and magnitude as well as the cell culture system and the presence of inflammatory factors [12,24,37].
Different types of mechanical tensions including cyclic tensile stretch [23], static mechanical stretch[37], equiaxial stretch [39] and uniaxial cyclic tensile stretch [12] were applied to DSCs.Static forces can mimic orthodontic and soft tissue forces while cyclic stimulations can mimic mastication forces [30].In-vivo applied tensile forces can be uniaxial or equiaxial which depends on the location of the cells.For instance, cells may face equiaxial forces due to the complex structure of cells located in the apical region.Both types of tensile force have been analyzed in the included studies.The included studies showed that mechanical tension increased the osteogenic differentiation of PDLSCs and SCAPs, regardless of the type of tensile force [39,44-46,58-62].However, the response of DPSCs to tension was based on the type of force.Uniaxial tensile force decreased the osteogenic/odontogenic differentiation [12], while other types of tensions increased osteogenic/odontogenic differentiation of DPSCs[47-49].
The magnitude of the tensile force was the other factor which was different between the studies.Different amounts of strain forces are needed for the horizontal and intrusive displacement of teeth.It has been shown that the maximal strain force which is needed for horizontal orthodontic tooth movement and intrusion is about 8%-25% and 6%-7% tensile strength, respectively [63].All of the included studies applied tensile forces in the mentioned range.Flexor tension and four-point bending systems were the most common methods to apply tension.In order to simulate the in-vivo condition of tension force (i.e., during orthodontic force application), a 3-dimensional environment is needed.Indeed, different types of tensile force must be applied to cells located in different parts of the environment to mimic the in-vivo condition accurately.However, it is not feasible to separate cells cultivated on different parts and analyze their behavior independently.Therefore, it is recommended to identify the specific type of force which is applied to stem cells located in different parts of the teeth and analyze the effect of each of the identified forces using a separate setup.
Cell culture system is another factor which may affect the response of DSCs to tensile force.However,cells cultured in 2-dimensional(2-D)and 3-dimensional(3-D)environments showed the same differentiation potential[33,36].Three-dimensional environments can mimic the in-vivo niche of stem cells more accurately[64].Han et al.[33] applied mechanical tension using bioreactors.They cultivated cells on 3-D silk scaffold prior to mechanical stimulation.They also performed another study which applied mechanical tension using the Flexercell system and cultivated cells on Bioflex 2-D plates [36].Both studies demonstrated that mechanical stimulation increased COL-1 and Osteoprotegerin expression.However, the expression of α-SMA decreased in both studies [33,36].There were no studies which compared the response of DSCs to mechanical force in 2D and 3D conditions.However, it must be considered that the composition of scaffold affects the differentiation of stem cells which may impact the response of cell to mechanical stimulation.
DSCs can be cultivated in inducing or non-inducing mediums.Considering the high proliferation capacity of DSCs, inducing mediums were used in some of the included studies to evaluate the differentiation capacity of DSCs [12,31,30-32].However, comparison of the results of studies showed no significant difference in using inducing or non-inducing mediums.Tang et al.[23] and Shen et al.[38]applied cyclic tensile force to PDLSCs [23,38].Although the condition of the medium was different between studies, they both mentioned higher expression of RUNX2 and OSX following the mechanical stimulation.In addition, Tabatabaei et al.[30] compared the inducing and non-inducing medium in their study [30].They revealed that cells cultured on inducing mediums had higher OPN expression while the expression of ALPL was lower in inducing conditions.
All of the included studies used healthy DSCs to evaluate the effect of mechanical stress.However,Liu et al.[37]compared the behavior of PDLSCs isolated from healthy and inflammatory periodontal tissue[37]and showed that periodontitis PDLSCs(PPDLSCs)were more sensitive to mechanical stretch[37].Indeed,8% strain was the best condition for osteogenic differentiation of PPDLSCs while it was 12% for healthy PDLSCs (HPDLSCs).Applying strain force at higher levels than mentioned amounts led to a decrease in the cell viability and differentiation capacity of HPDLSCs and PPDLSCs.The reason behind the different responses of PPDLSCs and HPDLSCs to mechanical stimulation is still unclear[37].
The proliferation of PDLSCs and DPSCs, on the other hand, increased after the application of tensile force.However, tension decreased the proliferation of SCAPs [50].Although it is expected that different types of DSCs respond in the same manner to the mechanical forces, the decrease of the proliferation rate of SCAPs following tensile stimulation can be justified by considering the cell cycle and relation between proliferation and differentiation.In fact, osteoblast development can be divided into three phases [65].The first phase is proliferation, in which the proliferation rate starts to decrease from its maximum level and the differentiation rate starts to increase.In the second phase, the extracellular matrix development phase, the proliferation has stopped and the expression of early differentiation genes (e.g., osteopontin,alkaline phosphatase) increases.At the final stage, Mineralization, the expression of osteocalcin and hydroxyapatite increase [65].Therefore, the decrease in the proliferation rate may be due to the onset of osteoblast development and cell entry to the first phase of osteoblast development.For instance, Mu et al.[50] showed that the MTT results of SCAPs were the same as the control group for the first three days.However,after that,osteoblast development started and cells that faced tensile force had lower cell count and higher differentiation.
It must also be considered that stem cells may respond differently to a specific magnitude of tensile force overtime.Han et al.[36]showed that after ten days,the highest level of proliferation was caused by 8%strain[36].However,in the short-term(five days),there were no significant differences between 5%and 8%strain.
4.2 Hydrostatic Pressure and Compression
Pressure and compression were considered synonyms in the included studies[31].However,hydrostatic pressure is a type of pressure that is applied through incompressible liquids and is thoroughly different from compression.Studies showed that both compression and hydrostatic pressure increased the osteogenic/odontogenic differentiation of DSCs.Besides, it has been shown that compression had magnitude dependent effect on the proliferation of DSCs [24] while hydrostatic pressure decreased the proliferation of DSCs [66].It must be considered that in the latter study the effect of different magnitudes was not analyzed and 2.5 MPa force was considered as optimum magnitude based on their cell attachment and morphology results.
Dynamic hydrostatic pressure mimics the sustained pulpal pressure,in-vivo.In-vitro results showed that hydrostatic pressure increased the odontogenic differentiation of DSCs which imitate the behavior of DSCs facing hydrostatic pressure during pulp inflammation [67].It has been shown that dental pulp faces mechanical forces during tooth eruption [68,69], mastication [70,71] and traumas.In the reparative dentinogenesis procedure, previous odontoblasts are dead due to substantive damage of pulp tissue.Thus,DPSCs will differentiate into odontoblasts due to mechanical forces to replace old cells and create tertiary dentin [72-74].In addition, the formation of pulp stones, root canal calcification and calcified nodules following abnormal occlusal and parafunctional forces can be justified based on the differentiation of DSCs to odontoblast-like cells [32,75].Another example of odontoblastic differentiation of DPSCs following mechanical force is the production of thick dentin (secondary dentin) in teeth that are in occlusion.In confirmation of the hypothesis, since impacted teeth are not used during mastication and their DPSCs do not face mechanical forces,no secondary dentin can be found in impacted teeth [25].
4.3 Microgravity
The application of microgravity can be beneficial in enhancing the proliferation and differentiation capacity of stem cells.Results of included studies showed that simulated microgravity increased the proliferation rate of DSCs [35,43].In addition, it has been shown that the osteogenic differentiation of DSCs increased following simulated microgravity which was applied through bioreactors [43].This can potentially result in producing engineered grafts ex-vivo which its cells are activated by microgravity environment and have more regeneration capacity.
4.4 Vibration
This stimulation can mimic the physiological forces of mastication[76].Among all included studies,two studies applied low magnitude high-frequency vibration to PDLSCs[41,42].The magnitude and frequency of vibration differed among the mentioned articles.It has been shown that vibration decrease the proliferation of DSCs.On the contrary, vibration enhanced the osteogenic differentiation of PDLSCs [41,42].The positive effect of vibration on the differentiation of PDLSCs peaked at 50 Hz and decreased at the higher frequencies and even become lower than control group at 180 Hz.In addition, among all of the analyzed genes, OSX expression was lower than control group in the majority of tested frequencies.The lowest gene expression among osteogenic genes belonged to OSX while RUNX2 had the highest.
4.5 Shear Stress
DSCs located in the periodontal tissue may face shear stress during the fluid transfer which occurs as a result of mastication or other forces applied to teeth and surrounding structures[77].However,none of the included studies analyzed the effect of shear stress on the behavior of dental stem cells.Nevertheless,it has been shown that this force can increase the osteogenic differentiation of other types of stem cells including adipose derived stem cells [78]and bone mesenchymal stem cells [79].
4.6 Limitation and Suggestions for Future Studies
The effect of cell-cell interaction,body fluid and surrounding tissue are not considered in vitro studies.Although these studies are beneficial to analyze the behavior of DSCs independently,in-vivo studies can be done to overcome the aforementioned limitations.Considering that DSCs may face different types of mechanical forces in the oral tissue, it is recommended to analyze the effect of the combined mechanical stimulation (i.e., tension, compression and shear) on the behavior of stem cells as it did in a study by Woloszyk et al.[34].However, in this study [34], the effect of each of the stimulations as well as impact of intercellular interaction were not analyzed.In addition, it is not feasible to categorize applied mechanical stimuli into pathological, physiological and therapeutic forces.Besides, more studies are needed to reveal; the intracellular mechanism regarding the effect of mechanical stimuli on DSCs.
5 Conclusion
Although most types of mechanical forces increased the osteogenic/odontogenic differentiation of DSCs, there were controversial results regarding the effect of uniaxial tension on DSCs.This force increased the osteogenic differentiation of PDLSCs while decreased the osteogenic/odontogenic differentiation of DPSCs.Besides, compression showed magnitude dependent effect on the proliferation of DSCs, while hydrostatic pressure and vibration decreased the proliferation of DSCs.The effect of tension on the proliferation of DSCs differs based on the type of cell.Indeed, tension increased the proliferation of DSCs but SCAPs.
Funding Statement:The current study was funded by Dentofacial Deformities Research Center,Research Institute of Dental Sciences,Shahid Beheshti University of Medical Sciences,Tehran,Iran.
Conflicts of Interest:The authors declare that they have no conflicts of interest to report regarding the present study.
杂志排行
Molecular&Cellular Biomechanics的其它文章
- Determination of Cup to Disc Ratio Using Unsupervised Machine Learning Techniques for Glaucoma Detection
- Motion Features of Legs in Volleyball Block Jump Based on Biomechanical Analysis
- Study on the Effect of PNF Method on the Flexibility and Strength Quality of Stretching Muscles of Shoulder Joints of Swimmers
- The Differences in Lower Extremity Joints Energy Dissipation Strategy during Landing between Athletes with Symptomatic Patellar Tendinopathy (PT) and without Patellar Tendinopathy (UPT)
