Hierarchically Inverse Opal Porous Scaffolds from Droplet Microfluidics for Biomimetic 3D Cell Co-Culture
2021-04-22ChangminShaoYuxiaoLiuJunjieChiFangfuYeYuanjinZhaoa
Changmin Shao, Yuxiao Liu, Junjie Chi, Fangfu Ye*, Yuanjin Zhaoa,b,,*
a Department of Rheumatology and Immunology, Institute of Translational Medicine, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing 210008, China
b Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision and Brain Health), Wenzhou 325038, China
c Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou 325016, China
d State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China
e Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China
Keywords:Microfluidics Inverse opal Cell culture Droplet Biomaterial
ABSTRACT With the advantages of better mimicking the specificity of natural tissues, three-dimensional (3D) cell culture plays a major role in drug development, toxicity testing,and tissue engineering. However, existing scaffolds or microcarriers for 3D cell culture are often limited in size and show suboptimal performance in simulating the vascular complexes of living organisms. Therefore, we present a novel hierarchically inverse opal porous scaffold made via a simple microfluidic approach for promoting 3D cell co-culture techniques.The designed scaffold is constructed using a combined concept involving an emulsion droplet template and inert polymer polymerization.This work demonstrates that the resultant scaffolds ensure a sufficient supply of nutrients during cell culture,so as to achieve large-volume cell culture.In addition,by serially planting different cells in the scaffold, a 3D co-culture system of endothelial-cellencapsulated hepatocytes can be developed for constructing certain functional tissues. It is also demonstrated that the use of the proposed scaffold for a co-culture system helps hepatocytes to maintain specific in vivo functions.These hierarchically inverse opal scaffolds lay the foundation for 3D cell culture and even the construction of biomimetic tissues.
1. Introduction
Three-dimensional (3D) cell culture is an effective technique that provides cells with a microenvironment during their culture that is closer to their living conditions in vivo [1–5]. The cells in 3D culture can establish connections between cells and between cells and extracellular matrices through tight junctions or gap junctions to form a 3D structure, which is similar to cell growth in vivo [6–9]. Therefore, 3D cell culture can not only preserve the material structure basis of the cell microenvironment in vivo, but also reflect the intuitiveness and conditional controllability of cell culture. These advantages have resulted in the rapid development of 3D cell culture and its wide use in various fields, such as tissue engineering, biopharmaceutical processing, toxicity testing, and more [10–16]. At present, the biggest limitation of 3D cell culture is that the cells in the interior are prone to necrosis during the process of stacking due to an excessive cultivation area and insufficient nutrition supply, whereas the 3D growth of such cells is normal in vivo because of abundant micro-vessels and microcirculation. Inspired by this phenomenon, many porous materials or micro-channels have been constructed for 3D cell culture [17–22].However,most porous materials are typically too small in size to prevent cell necrosis due to a lack of effective nutrient transport channels [23–25]. In addition, conventional porous materials cannot effectively simulate the 3D structure of vascular complexes in living organisms. Thus, the development of novel porous materials with more complicated structures is still anticipated in order to achieve 3D cell culture.
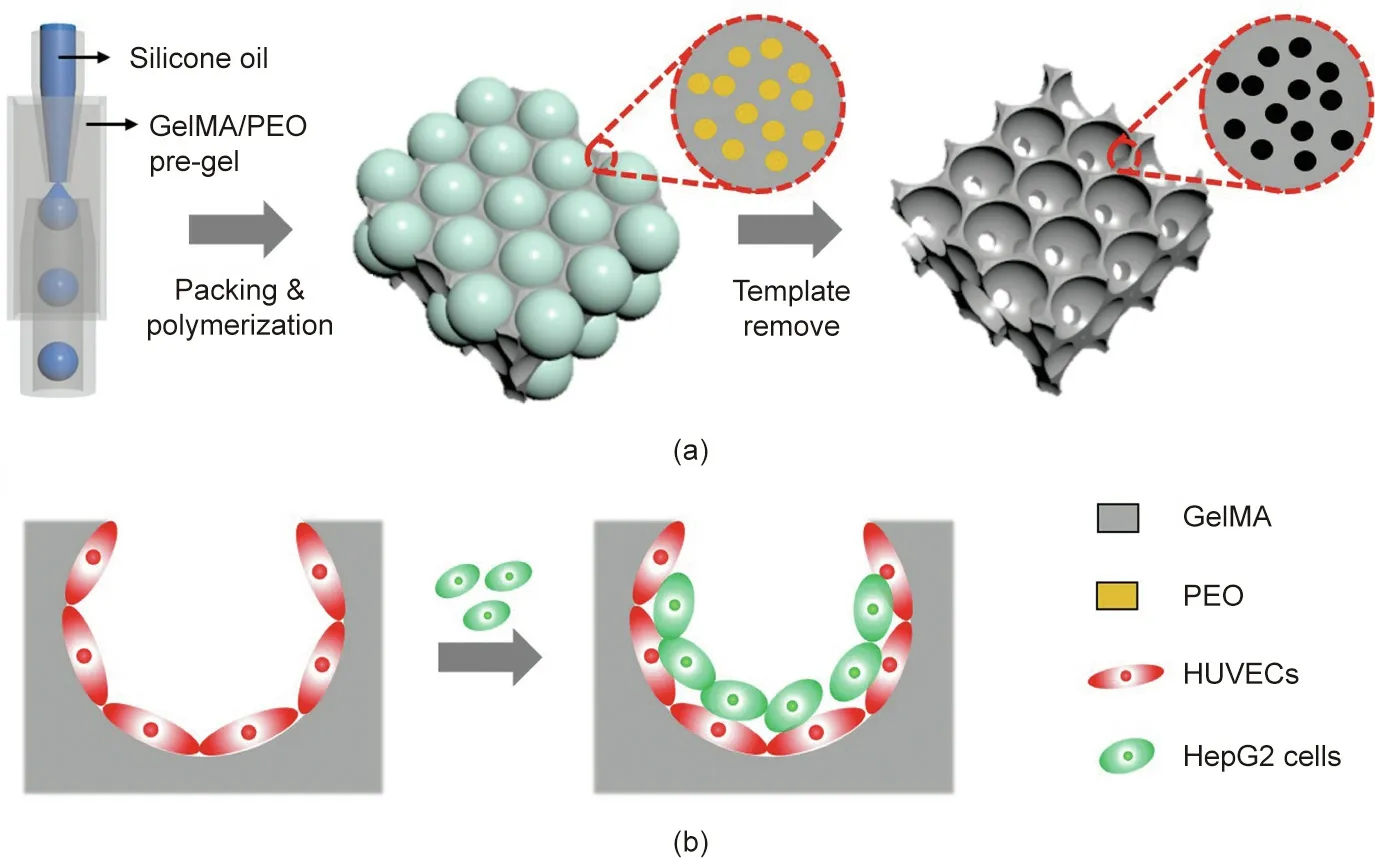
Fig.1. Schematics of(a)the fabrication of the hierarchically inverse opal porous scaffold and(b)3D cell co-culture.GelMA:gelatin methacrylate;PEO:poly(ethylene oxide);HUVECs: human umbilical vein endothelial cells; HepG2: human hepatocarcinoma.
In this study, we present a novel hierarchical inverse opal porous scaffold with the desired features for cell culture made using a simple droplet microfluidic approach, as shown schematically in Fig. 1. Inverse opal scaffolds have received considerable attention due to their controllable pore sizes and the uniform pores they inherit from the template, which consists of closely packed monodispersed microsphere or droplet lattices [26–29].Coincidently, thanks to their excellent flow-control capability,microfluidics enables the formation of highly monodisperse emulsions for different applications, such as templates for the microsphere or droplet lattices of inverse opal scaffolds [30–36]. Based on their 3D structural features, inverse opal scaffolds have found widespread application as optical materials, biosensors, cell scaffolds, and so forth [37–39]. However, due to their simple void packing structures and single composition of the scaffolds,current research on the use of inverse opal scaffolds for 3D vascularization remains rare.
Therefore,by means of a combined concept involving an emulsion droplet template and inert polymer polymerization,we herein constructed a hierarchically inverse opal scaffold for realizing 3D vascular integration.In this paper, we demonstrate that the resultant scaffolds ensure a sufficient supply of nutrients during cell culture, so as to achieve large-volume cell culture. In addition, by serially planting different cells in the scaffold,a 3D co-culture system of endothelial-cell-encapsulated hepatocytes can be developed for constructing certain functional tissues. This co-culture system promotes the secretion of higher levels of albumin and cytochrome P450 (CYP450), which indicates that the co-culture system in the scaffold helps to maintain hepatocyte activity and function.These characteristics make the hierarchically inverse opal scaffold described here ideal for tissue engineering and other biological applications.
2. Materials and methods
2.1. Materials
Gelatin methacrylate (GelMA) was self-synthesized in the lab according to the reported method [40]. Gelatin (porcine skin),methacrylic anhydride, poly(ethylene oxide) (PEO; average molecular weight of 900 000), photoinitiator 2-hydroxy-2-methylpropiophenone (HMPP), the hydrophobic reagent octadecyltrichlorosilane(OTS),poly(ethyleneglycol)-block-poly-(propylene glycol)-block-poly(ethylene glycol) (F108), and sodium dodecyl sulfate (SDS) were all obtained from Sigma-Aldrich, USA.Deionized water was acquired from a Milli-Q pure water machine.Other reagents were of analytical grade.
2.2. Fabrication of hierarchically inverse opal scaffolds
Hierarchically inverse opal scaffolds were fabricated by removing droplet templates generated by microfluidics. Simply put, a microfluidic device was obtained by assembling inner and outer tubes in a square tube. An inner tube with a tip (inner diameter:80 μm) was produced by a microelectrode puller (P-97, Sutter,USA) and treated with OTS for hydrophobic treatment. This microfluidic device was reinforced and sealed with epoxy resin AB glue after assembly. The inner and outer phase were methyl silicone oil and GelMA/PEO solution,respectively. The typical flow rates of the inner and outer phase were 0.4 and 2 mL·h-1, respectively. The outer phase solution was prepared by mixing GelMA(20%) and PEO (1.5%) aqueous solution in a volume ratio of 4:1 before the experiment. Simultaneously, SDS (2%, surfactant),F108 (2%, surfactant), and HMPP (1%, photoinitiator) were also added to the outer phase solution.The corresponding liquids were then introduced into the device by micro-peristaltic pumps (Harvard PHD 2000, Harvard Apparatus, USA), and the methyl silicone oil was sheared into droplets by the GelMA/PEO aqueous solution at the orifice of the inner tube. The outer phase was polymerized with ultraviolet (UV) light after the droplets self-assembled into a hexagonal close-packed structure. The first hierarchical architecture of the hierarchically inverse opal GelMA/PEO scaffolds was ultimately generated by ultrasonically removing the oil droplets by means of n-hexane and ethanol at least five times. To obtain the second hierarchical structure, the generated GelMA/PEO scaffolds were immersed in phosphate-buffered saline (PBS; Gibco,USA) for more than 48 h to remove the PEO phase from the photo-crosslinked GelMA hydrogel.In order to observe the exudation of PEO in the hydrogel, a PEO leaching test was performed by conjugating PEO molecules to fluorescein isothiocyanate (FITC)molecules. In brief, a mixed solution of FITC (10 mmol·L-1) and 1.5%PEO solution was dialyzed for one week and then lyophilized.The polymerized GelMA/PEO hydrogel was obtained according to the above method and was then placed in PBS. The absorbance of the released PEO from the hydrogel was determined using a microplate reader (Synergy HT, BioTek, USA). Finally, the secondary porous structure was produced after the PEO oozed out of the scaffold.
2.3. Cell culture
Human hepatocarcinoma (HepG2) cells (Cell Bank, China) and human umbilical vein endothelial cells (HUVECs; Tongpai Biological Technology Co.,Ltd.,China)were incubated in Dulbecco’s modified eagle medium (DMEM; Gibco) accompanied by 10% fetal bovine serum(Gibco),and 1%penicillin and streptomycin (Gibco).The cells were cultured in a carbon dioxide(CO2)incubator(Heracell 150,Thermo,USA)with 5%CO2at 37°C.Next,GelMA/PEO scaffolds were immersed in 75%alcohol and placed in a clean bench for more than 4 h with UV sterilization. The scaffolds were then washed three times with PBS and transferred to a six-well plate(Corning,USA).Fifty microliters of HUVEC suspension at a concentration of 6×105cells·mL-1was seeded on the top of the scaffolds,and then 2 mL of media was slowly added.One milliliter of the culture media was renewed every day. Cell growth was observed by staining cells with 5 μmol·L-1of calcein-AM (Molecular Probes,USA). For the cell co-culture experiment, HUVECs (6×105cells·mL-1) were first planted in the scaffold; then, HepG2 cells(5×105cells·mL-1) were seeded into the scaffold for cocultivation. To examine the distribution of cells in the scaffolds,the HepG2 cells and HUVECs were stained with 5 μmol·L-1of calcein-AM and 5 μmol·L-1of DiD(Molecular Probes),respectively.The cell proliferation rate was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Simply put,the cultured scaffolds were placed in a 24-well plate,and medium with 10%MTT was added.After incubating for 4 h in the incubator, the solutions were carefully aspirated; then, 500 μL of dimethyl sulfoxide was added to dissolve the crystals in the wells.The optical density(OD)value was determined using a microplate reader. The albumin content was measured by a Rat Albumin enzyme-linked immunosorbent assay (ELISA) kit (Abcam, UK).CYP450 activity was detected using a human CYP450 3A4 ELISA kit (Nanjing Jiancheng Bioengineering Institute, China).
2.4. Characterization
Microfluidic droplet generation was recorded by microscope(AE2000, Motic, China) accompanied by a camera (S-PRI F1, AOS Technologies AG, Switzerland). The structures of the generated scaffolds were characterized by means of optical microscopy(Olympus BX51, Japan) and field-emission scanning electron microscopy (SEM; S-300N, Hitachi, Japan). Fluorescent images of cells and 3D reconstruction images were snapped using a confocal laser-scanning microscope (CLSM; Olympus FV10i).
3. Results and discussion
In a typical experiment,the oil-in-water(O/W)single-emulsion droplets generated by microfluidics were employed as templates for the fabrication of hierarchically inverse opal scaffolds, as illustrated in Fig. 1. A mixture of GelMA and PEO was selected as the skeleton material for the hierarchically inverse opal scaffolds.GelMA is a kind of biocompatible hydrogel with wide applications in the fields of 3D cell culture and tissue engineering [41–43].Similarly, PEO is often used as a porogen in the preparation of hydrogel scaffolds due to its biocompatibility, inertness, and ease of modification[44,45].Based on these backgrounds,we fabricated the hierarchically inverse opal scaffolds by using the mixture of GelMA and PEO in a microfluidic device and then removing the PEO component so as to make the hierarchical porous structure.
In our experiments, a glass microfluidic device was fabricated by coaxially assembling glass tubes on a single glass slide.The inner capillary was used to introduce the dispersion phase(e.g.,methyl silicone oil),the outer capillary was used to introduce the continuous phase (e.g., the mixture of GelMA and PEO solution), and the outermost square capillary was employed to record the online generation process of the droplets. As shown in Fig. 2(a), methyl silicone oil was emulsified into droplets by the GelMA/PEO solution at the outlet of the inner tube. The generated droplet sizes and the corresponding apertures of the scaffold could be controlled by adjusting the flow rate of the two-phase solution.It was found that the diameter of the droplets clearly increased with an increasing rate of the dispersion phase, while an opposite relation was found between the rate of the continuous phase and the droplet diameter (Fig. 2(b)).
As shown in Figs. 2(c) and (d), the generated droplets had uniform size,good sphericity,and high monodispersity,making them an ideal template for the inverse opal scaffolds. After the droplets had assembled into a hexagonal close-packed arrangement, and the GelMA pre-gel solution was subsequently polymerized, the first hierarchical architecture (i.e., the homogeneous macroporous structure) of the hierarchically inverse opal scaffolds was finally generated by washing away the oil phase with n-hexane and ethanol.The resultant GelMA/PEO scaffolds were observed using a light microscope and SEM, as described in Fig. 3. It was observed that the GelMA/PEO scaffolds had a uniform and interconnected macroporous structure (Fig. 3(a)). Moreover, the adjacent holes were interconnected by multiple uniform windows, which is beneficial for long-term cell culture because it can enhance the transport of nutrients or wastes through the entire scaffold structure(Fig.3(b)).
After the first hierarchical architecture of the inverse opal scaffolds was obtained through the abovementioned method, the second hierarchical structure was formed by immersing the generated GelMA/PEO scaffolds in PBS for more than 48 h to remove the PEO from the cured GelMA hydrogel.In order to observe the exudation of PEO in the hydrogel,a PEO leaching test was performed by conjugating PEO molecules with FITC molecules.It was found that the PEO gradually oozed out over time, and the change of absorbance tended to be flat after 48 h (Fig. S1 in Appendix A). The resultant porous microstructure was observed by SEM and through 3D reconstruction of the CLSM.The results showed that the secondary microporous structure was produced after the PEO oozed out(Figs. 3(c) and (d)). The porous network structure was capable of reducing the distance between opposing surfaces of the pores from a few hundred microns to tens of microns to match the transverse dimension of the stretched cells,which would ensure the adequate transportation of oxygen and nutrients in large-area and long-term cell culture.
To mimic the structure of the blood vessel network in the body,the resultant scaffolds were employed to culture HUVECs.First,we investigated the biocompatibility of the material, which was an essential element to be considered. More specifically, the HUVECs were co-cultured with GelMA hydrogel and GelMA/PEO composite membranes,respectively,for 1,3,5,and 7 days,and then observed using CLSM by staining with calcein-AM. As shown in Fig. S2 in Appendix A,we found that the HUVECs were able to grow and proliferate well in the GelMA hydrogel and GelMA/PEO composite membranes. A consistent result was also obtained from the corresponding quantitative experiments carried out by MTT assay.These findings indicated the ideal cell biocompatibility of our prepared scaffold materials.
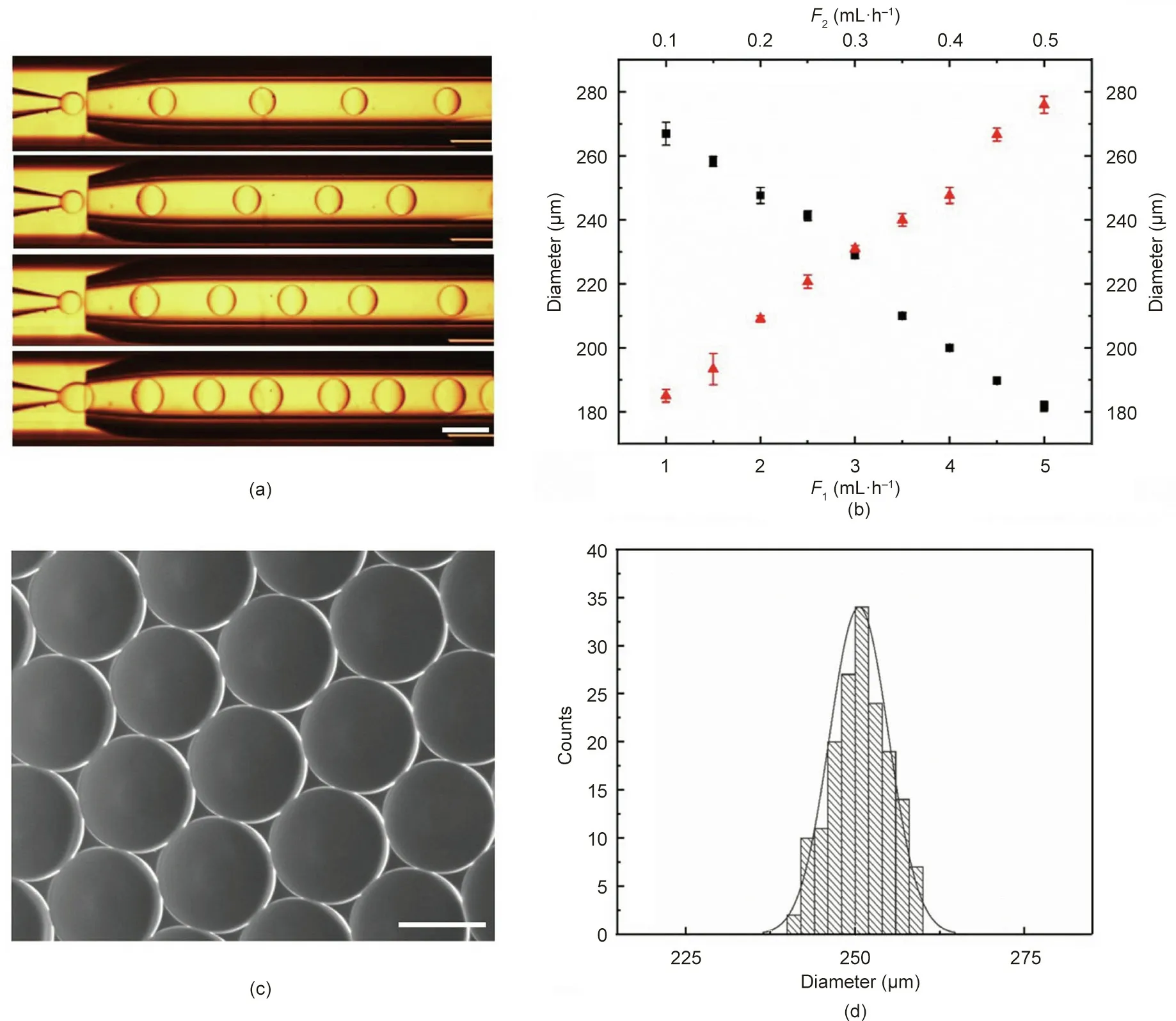
Fig. 2. Droplet templates generated by means of microfluidics. (a) Online generation images of droplets with different flow rates of the inner phase (scale bar: 500 μm).(b)Relationship between droplet diameter and flow rate(n=3)(F1:outer phase flow rate,black squares;F2:inner phase flow rate,red triangles).(c)Self-assembled droplet templates (scale bar: 200 μm). (d) Size distribution of droplets (coefficient of variation is 4.35%).
Later,to demonstrate the performance of the scaffolds in 3D cell culture,the proliferations of HUVECs in the scaffolds derived from the abovementioned two types of materials were qualitatively observed by CLSM and quantitatively assessed by MTT assays on Days 1,3,5,7,and 15(Fig.4).It was apparent that the cells proliferated well in both scaffolds in the first three days. However, the proliferations of cells in the GelMA scaffolds were significantly smaller than those in the GelMA/PEO scaffolds after three days.This phenomenon can be ascribed to the fact that the cells in the traditional scaffolds were prone to necrosis during long-term in vitro culture due to a lack of oxygen and nutrients in the scaffolds, whereas our hierarchically inverse opal scaffolds were able to conquer this limitation. In addition, the morphologies of the cells grown on the two scaffolds were significantly different. As shown in Fig.S3 in Appendix A,in comparison with the cells grown in the GelMA scaffolds,the cells grown in the GelMA/PEO scaffolds were more likely to adhere to the walls of the scaffolds and exhibited a spindle-shaped morphology.These results indicated that the GelMA/PEO scaffolds effectively promoted cell growth, adhesion,proliferation,and migration in comparison with the common nonporous GelMA scaffolds because the hierarchical porous microstructure was more conducive to the transportation of cellneeded oxygen and nutrients.
Based on the superior properties of the hierarchical GelMA/PEO scaffolds in 3D cell culture,a novel bionic 3D vascular network was constructed that was significant for the investigation of complex cell–cell and cell–extracellular matrix interactions in large-area cell culture. For this purpose, HUVECs were uniformly seeded into the GelMA/PEO scaffolds and observed using CLSM by staining with calcein-AM and using SEM (Fig. 5 and Fig. S4 in Appendix A).It was observed that the cells were evenly seeded into the pores of scaffolds. As demonstrated in Fig. 5, the cells were homogeneously distributed throughout the entire surface area of the scaffolds on the first day after inoculation and were primarily stretched onto the skeleton of the scaffolds.Over the duration of the culture time, the cells occupied most of the void spaces of the pores and formed a 3D cellular network. Moreover, the viability of the cells in the center of the pores was observed using CLSM in different Z-planes(Fig.S5 in Appendix A).It was found that the cells grown in the pores of the scaffolds had high viability at different depths and locations, which differed from the situation in the traditional culture scaffold, where the cells in the interior easily became necrotic due to a lack of oxygen and nutrients in large-area cell culture. Thus, improving the microstructural design of the scaffolds allowed the cells to be homogeneously distributed throughout the hierarchical GelMA/PEO scaffolds,while preventing cell necrosis inside the scaffolds and promoting cell–cell communication.
In order to further develop the functions of the designed hierarchical GelMA/PEO scaffolds, an unprecedented 3D multilayer coculture system of endothelial-cell-encapsulated hepatocytes was achieved by sequentially seeding different cells into the scaffolds(Fig. 6). More specifically, HUVECs and HepG2 cells were seeded into the scaffolds and cultured. In order to examine the growth state of the cells in the scaffold,the HUVECs and HepG2 cells were respectively stained with DiD cell-labeling solution(red color)and calcein-AM(green color).Images of the cells after co-culture in the scaffolds were obtained by means of CLSM (Fig. 6(a)). The results indicated that both of the cell types inoculated into the scaffolds exhibited good activity during the co-culture. A side view of the cell co-culture system also indicated that the two cells were layered.
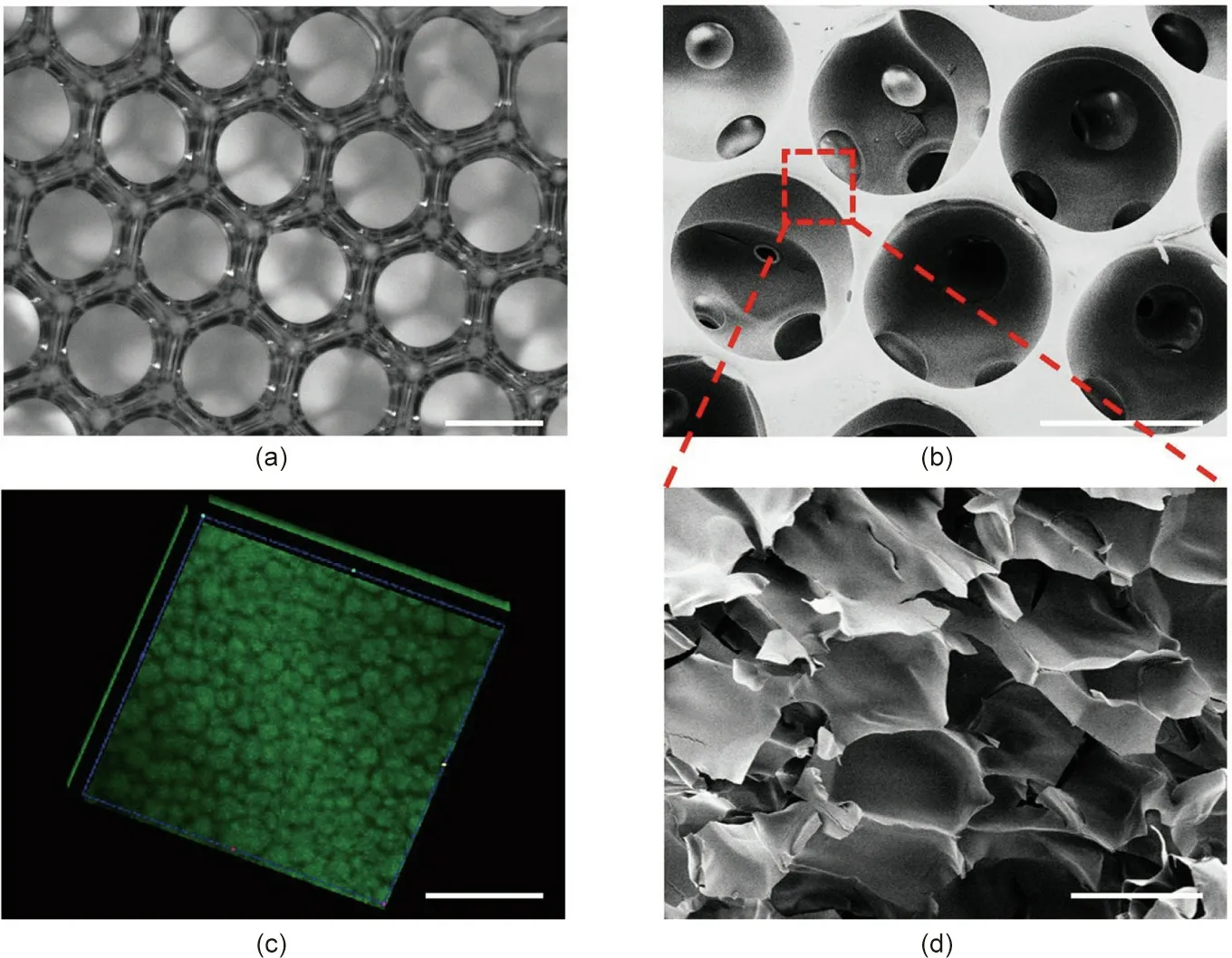
Fig.3. The structure of the hierarchically inverse opal porous scaffolds.(a)Optical microscope pictures of the first hierarchical structure of the scaffolds.(b)SEM image of the first hierarchical structure of the scaffolds. (c) 3D reconstruction fluorescent images of the second hierarchical structure of the GelMA/PEO hydrogels. (d) SEM image of the second hierarchical structure of the GelMA/PEO hydrogels. The scale bars in parts (a)–(d) are 200, 200, 100, and 10 μm, respectively.

Fig. 4. (a–d) 3D reconstruction of CLSM fluorescent images of HUVECs grown in (a, b) GelMA and (c, d) GelMA/PEO scaffolds on Days 7 and 15, respectively (scale bar:200 μm). (e) Proliferation profile of HUVECs cultured in GelMA and GelMA/PEO scaffolds, as determined by MTT assay (***p <0.001, n=3).
The secretion level of liver-associated enzymes was then detected on the hepatocytes after co-culture with HUVECs in terms of albumin and CYP450, which are the key parameters of cell physiology in hepatocyte culture. These results showed that the co-culture of hepatocytes and HUVECs secreted higher levels of albumin and CYP450 in the GelMA/PEO scaffolds than in the common GelMA scaffolds (Figs. 6(b) and (c), Fig. S6 in Appendix A).To be specific, the albumin secretion and CYP450 expression of the hepatocytes co-cultured in the GelMA/PEO scaffolds were about 1.36- and 1.17-fold higher than those of the hepatocytes co-cultured in the common GelMA scaffolds for seven days. These results showed that the co-cultured hepatocytes in the GelMA/PEO scaffolds were able to maintain enzyme activity during the cultivation process and exhibited higher enzymatic activity than those in the GelMA scaffolds. This feature of the designed GelMA/PEO scaffolds makes them a promising application prospect in the field of in vitro tissue generation,which would benefit tissue engineering.
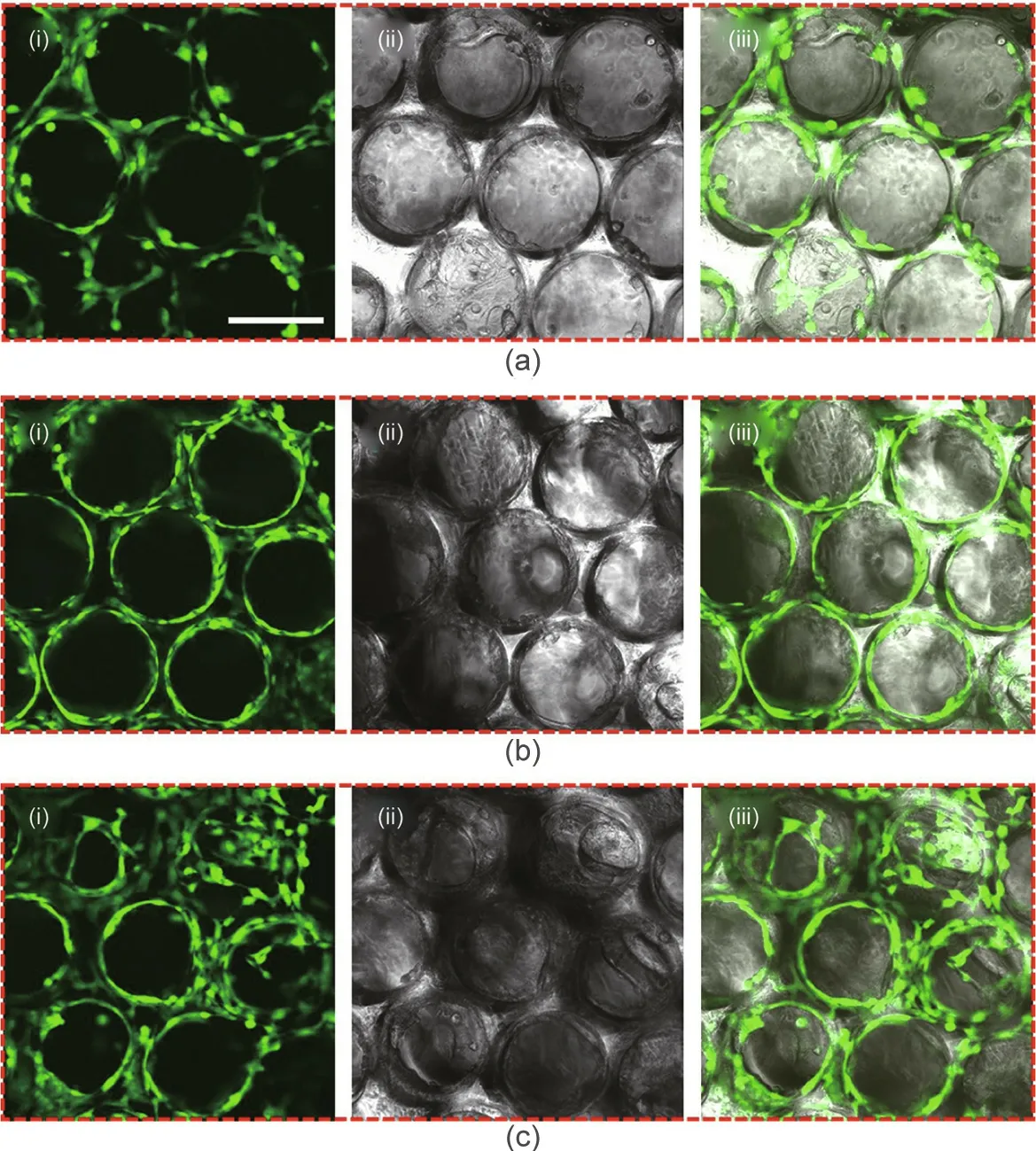
Fig.5. CLSM images of HUVECs cultured in GelMA/PEO scaffolds on(a)Day 1,(b)Day 3,and(c)Day 7:(i)fluorescent images;(ii)bright field images;and(iii)merged images(scale bar: 200 μm).
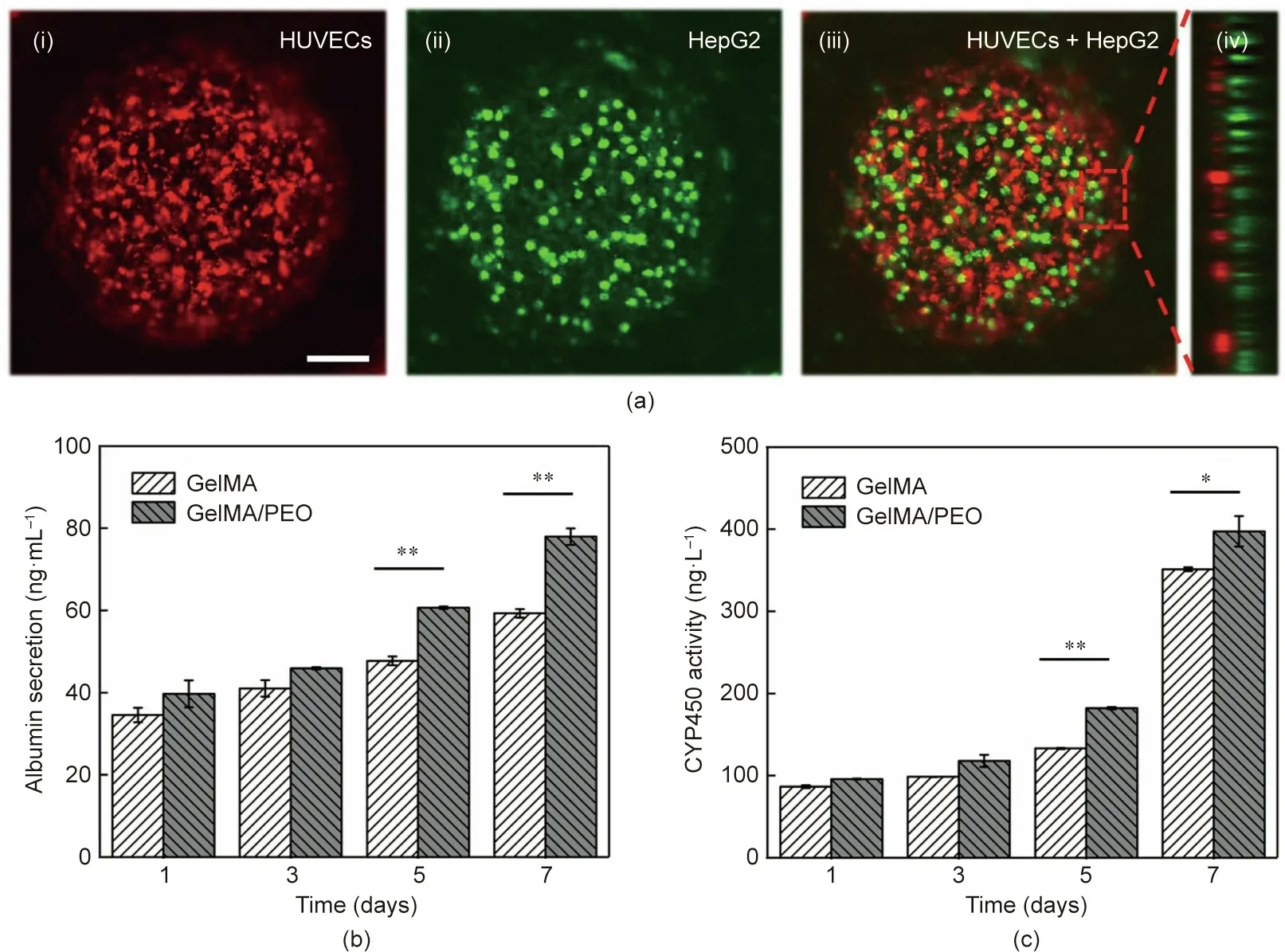
Fig. 6. (a) CLSM images of the cell co-culture system in the GelMA/PEO scaffolds containing HUVECs (red) and HepG2 cells (green) after 7 days’ culture: (i) HUVECs;(ii)HepG2 cells;(iii)merged images;and(iv)side view(scale bar:100 μm).(b)Albumin secretion and(c)CYP450 expression of HepG2 cells co-cultured with HUVECs in the GelMA and GelMA/PEO scaffolds (*p <0.05, **p <0.01, n=3).
4. Conclusions
In conclusion, we have presented a type of hierarchically inverse opal scaffold for promoting 3D cell culture through a simple microfluidic method. The scaffolds were constructed using a composite concept involving an emulsion droplet template and inert polymer polymerization. It was demonstrated that the designed scaffolds ensure a sufficient supply of nutrients during cell proliferation,so as to achieve large-volume cell culture.Moreover, by serially seeding different cells in the scaffold, a novel 3D co-culture system of endothelial-cell-encapsulated hepatocytes was developed for constructing certain functional tissues. This co-culture system promotes the secretion of higher levels of albumin and CYP450,which indicates that the co-culture system in the scaffold can help to maintain hepatocyte activity and function.We expect that these designed scaffolds will provide new ideas for the construction of organoids in vitro and will ultimately benefit tissue engineering.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFA0908200), the National Natural Science Foundation of China (52073060, 32101159, and 61927805), the Shenzhen Fundamental Research Program(JCYJ20190813152616459),and the Wenzhou Institute,University of Chinese Academy of Sciences (WIUCAS)’ startup fund(WIUCASQD2019007).
Compliance with ethics guidelines
Changmin Shao, Yuxiao Liu, Junjie Chi, Fangfu Ye, and Yuanjin Zhao declare that they have no conflict of interest or financial conflicts to disclose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2020.06.031.
杂志排行
Engineering的其它文章
- The Intelligent Beijing–Zhangjiakou High-Speed Railway
- Mechanisms of Steatosis-Derived Hepatocarcinogenesis: Lessons from HCV Core Gene Transgenic Mice
- Microneedle Makers Seek to Engineer a Better Shot
- Battery Recycling Challenge Looms as Electric Vehicle Business Booms
- Global Top Ten Engineering Achievements 2021
- Biomedical Engineering: Materials, Devices, and Technological Innovation Continue to Build a Better Future for Humankind
