Hepatitis E in solid organ transplant recipients: A systematic review and meta-analysis
2021-04-17PanupongHansrivjitAngkawipaTrongtorsakMaxPuthenpuraBoonphiphopBoonphengCharatThongprayoonKarnWijarnpreechaAvishekChoudhuryWisitKaewputShennenMaoMichaelMaoCarolineJadlowiecWisitCheungpasitporn
Panupong Hansrivjit, Angkawipa Trongtorsak, Max M Puthenpura, Boonphiphop Boonpheng, Charat Thongprayoon, Karn Wijarnpreecha, Avishek Choudhury, Wisit Kaewput, Shennen A Mao, Michael A Mao,Caroline C Jadlowiec, Wisit Cheungpasitporn
Abstract
BACKGROUND Hepatitis E virus (HEV) infection is underdiagnosed due to the use of serological assays with low sensitivity. Although most patients with HEV recover completely, HEV infection among patients with pre-existing chronic liver disease and organ-transplant recipients on immunosuppressive therapy can result in decompensated liver disease and death.
AIM To demonstrate the prevalence of HEV infection in solid organ transplant (SOT)recipients.
METHODS We searched Ovid MEDLINE, EMBASE, and the Cochrane Library for eligible articles through October 2020 . The inclusion criteria consisted of adult patients with history of SOT. HEV infection is confirmed by either HEV-immunoglobulin G, HEV-immunoglobulin M, or HEV RNA assay.
RESULTS Of 563 citations, a total of 22 studies (n = 4557 ) were included in this metaanalysis. The pooled estimated prevalence of HEV infection in SOT patients was 20 .2 % [95 % confidence interval (CI): 14 .9 -26 .8 ]. The pooled estimated prevalence of HEV infection for each organ transplant was as follows: liver (27 .2 %; 95 %CI:20 .0 -35 .8 ), kidney (12 .8 %; 95 %CI: 9 .3 -17 .3 ), heart (12 .8 %; 95 %CI: 9 .3 -17 .3 ), and lung (5 .6 %; 95 %CI: 1 .6 -17 .9 ). Comparison across organ transplants demonstrated statistical significance (Q = 16 .721 , P = 0 .002 ). The subgroup analyses showed that the prevalence of HEV infection among SOT recipients was significantly higher in middle-income countries compared to high-income countries. The pooled estimated prevalence of de novo HEV infection was 5 .1 % (95 %CI: 2 .6 -9 .6 ) and the pooled estimated prevalence of acute HEV infection was 4 .3 % (95 %CI: 1 .9 -9 .4 ).
CONCLUSION HEV infection is common in SOT recipients, particularly in middle-income countries. The prevalence of HEV infection in lung transplant recipients is considerably less common than other organ transplants. More studies examining the clinical impacts of HEV infection in SOT recipients, such as graft failure,rejection, and mortality are warranted.
Key Words: Hepatitis E virus; Hepatitis E virus infection; Solid organ transplant;Prevalence
INTRODUCTION
Hepatitis E virus (HEV) results in approximately 20 million infections each year[1]. This virus is endemic to mostly developing countries in Asia, Africa, and Central America.There are additional cases of the disease manifesting in developed countries without patients having traveled to endemic areas[1,2]. As HEV is transmitted by the fecal-oral route, infection is more prevalent in areas with poor water quality and food contamination[1]. Patients typically demonstrate symptoms of fevers, gastrointestinal complaints, and jaundice within weeks of infection that self-resolve with supportive care[1]. Although most patients with HEV recover completely, HEV infection among patients with pre-existing chronic liver disease, pregnant women and organ-transplant recipients on immunosuppressive therapy can result in fulminant hepatitis with a 10 %-30 % mortality rate[3].
HEV has been noted to impact solid organ transplant (SOT) recipient outcomes.HEV infection has been cited to cause graft cirrhosis and subsequent failure in liver graft recipients secondary to chronic infection[4]. Furthermore, heart transplant recipients have been noted to have secondary liver infection and subsequent fibrosis[5].In contrast, renal transplant allografts were found to have similar rejection and twoyear graft survival between HEV seropositive and negative recipients, thus demonstrating HEV does not always impact non-liver allografts[6]. HEV reactivation from infected SOT allografts remains a risk as well, with case reports describing this occurrence in liver transplant recipients who receive an allograft with latent disease[7].However, little evidence has demonstrated cases of HEV reactivation in renal transplant patients[7]. HEV viremia has also been found in non-SOTs such as hematopoietic stem cell transplant[8]. This suggests possible transmission of the virus through bone marrow products as well as SOT. Once infected with the virus,transplant recipients are at risk for developing chronic liver disease, especially with HEV genotype 3 [9].
With the majority of this evidence being limited to case reports and some retrospective studies, there is very limited conclusive evidence illustrating the true HEV association, its related risk profile, and the clinical outcomes in transplant patients. Pooling the aggregate data of current studies will help elucidate the extent of risk and help stratify the clinical outcomes. We conducted this systematic review and meta-analysis to describe the prevalence of HEV infection in SOT patients. Our study is the first meta-analysis to emphasize the burden of HEV infection in SOT recipients.
MATERIALS AND METHODS
Search strategy
This manuscript follows the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)[10]statement as well as Meta-analysis of Observational Studies in Epidemiology (MOOSE)[11]guidelines. A systematic search was conducted through the Ovid MEDLINE, EMBASE, and Cochrane Library from database inception to October 2020 using the following search terms: (“hepatitis E” OR “HEV”) AND(“transplant” OR “transplantation”) AND (“outcome*” OR “mortality” OR “graft loss”OR “graft function” OR “incidence” OR “death”). The detailed search strategy for each database is summarized in Supplementary search strategy. No language restrictions were applied.
Inclusion criteria
The eligibility of each study was determined by the following inclusion criteria: (1 )The nature of study is observational or conference abstract; (2 ) Study population consisted of SOT recipients; and (3 ) The prevalence of HEV infection was reported as one of the outcomes of interest. Exclusion criteria consisted of pediatric patients,hematopoietic stem cell transplant recipients, and studies with a total sample size of less than 50 patients. The latter was to avoid inter-study variance. Study eligibility was independently evaluated by two investigators (PH and AT). Any disagreements were resolved by mutual consensus. The quality of each study was appraised using the Newcastle–Ottawa quality scale[12 ], which assesses six components including (1 )Representativeness of the subjects; (2 ) Ascertainment of the exposure; (3 )Demonstration of outcome of interest was not present at start of study; (4 ) Assessment of outcome; (5 ) Follow-up duration period was long enough for outcome to occur; and(6 ) Adequate follow-up duration.
Review process and data extraction
The titles and abstracts of all discovered studies were screened (PH and AT) prior to a full-text review. The full-text of the screened articles was reviewed to determine their eligibility for inclusion into the systematic review and meta-analysis. We created a standardized data collection form to extract the following information from the included studies: First author’s name, year of publication, country of origin, study design, subject(s), sample size, transplanted organ (heart, lung, liver, kidney, and undifferentiated), age, male sex, ethnicity, prevalence of HEV, confirmatory test used to diagnose HEV infection, prevalence of acute HEV infection, death, other reported outcomes and follow-up duration. Country of research origin was classified into highincome and middle-income based on the definition by the World Bank[12]. De novo HEV infection is defined by post-transplant HEV infection in patients with negative pre-transplant HEV-immunoglobulin G (IgG), HEV-immunoglobulin M (IgM) or HEV-RNA. Acute HEV infection is determined by positive post-transplant HEV-IgM with or without positive HEV-RNA.
Measurements
The prevalence of HEV infection, prevalence of de novo HEV infection, and prevalence of acute HEV infection underwent meta-analysis and the results were reported in percentage along with 95 % confidence interval (CI). Forest plot of each analysis was created. Results were presented in percentage for categorical data and in mean ± SD or median (interquartile range) for continuous data.
Network association
The prevalence of HEV infection in each organ transplant (heart, lung, liver, kidney,and undifferentiated) were individually compared using mixed-effects model. The association of each couple comparison was assessed with Cochrane’s Q-test and its P value. P values less than 0 .05 were considered statistically significant.
Subgroup analysis, meta-regression analysis, and publication bias
Subgroup analyses based on the following variables were performed: study year,country of origin, study design, sample size, mean age, male proportion, number of confirmatory tests used in the study, antibody assay, and follow-up duration. Mixedeffects model of analysis was used in subgroup analyses. Publication bias was evaluated by (1 ) Funnel plot (if the total number of studies was greater than ten[13]);and (2 ) Egger’s regression intercept. An intercept P value of less than 0 .05 was considered significant for potential publication bias. The quality of each study was appraised using the Newcastle-Ottawa quality scale[14].
Statistical analysis
All statistical analyses were performed by the Comprehensive Meta-analysis version 3 software (Eaglewood, NJ, United States) and SPSS version 23 .0 (IBM Corp., Armonk,NY, United States). Statistical heterogeneity of the included studies was assessed using the Cochran's Q-test and I2statistics. An I2 value of ≤ 25 % represents insignificant heterogeneity, 25 %-50 % represents low heterogeneity, 50 %-75 % represents moderate heterogeneity, and > 75 % represents high heterogeneity[15 ]. For analyses with I2 > 50 %,the results were analyzed by random-effects model to minimize the heterogeneity and external variance[16 ]. A P value of less than 0 .05 represents statistical significance.
RESULTS
Study characteristics
Of 563 citations, a total of 20 studies consisting of 5842 subjects were included in this meta-analysis and systematic review. Figure 1 provides a flowchart of the literature search and study selection for this meta-analysis. Included studies were published from 2011 to 2020 . The study design was retrospective (66 .7 %) and prospective(33 .3 %). The median age was 52 .0 (11 .9 ) years, 68 .5 % were male, and 27 .7 % were Caucasian. The median duration of follow-up was 13 .7 (14 .0 ) mo.
Prevalence of HEV infection
For the prevalence of HEV infection, a total of 18 studies (n = 4557 ) were included in the meta-analysis. Erken et al[17]was excluded as the authors only reported the prevalence of de novo HEV infection while Reekie et al[18]was excluded because of the potential risk of bias. All other articles had acceptable NOS scores (low risk of bias) for inclusion into meta-analysis for prevalence of HEV infection.
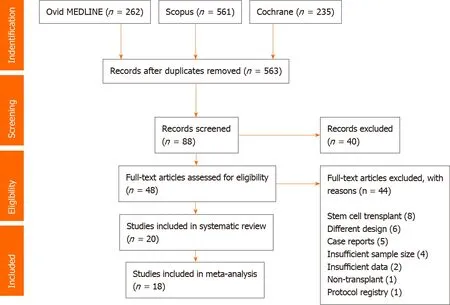
Figure 1 PRISMA flowchart of article search and selection.
The pooled estimated prevalence of HEV infection in SOT recipients was 20 .2 %(95 %CI: 14 .9 -26 .8 ; I2 = 95 .3 %; Figure 2 A). The pooled estimated prevalence of HEV infection in each transplanted organ was: Liver (27 .2 %; 95 %CI: 20 .0 -35 .8 ; n = 11 ; n =1887 ), kidney (15 .3 %; 95 %CI: 6 .6 -31 .5 ; n = 4 ; n = 1137 ), heart (12 .8 %; 95 %CI: 9 .3 -17 .3 ; n= 1 ; n = 274 ), lung (5 .6 %; 95 %CI: 1 .6 -17 .9 ; n = 3 ; n = 625 ), and undifferentiated (29 .6 %;95 %CI: 10 .1 -61 .1 ; n = 3 ; n = 634 ).
De novo HEV infection
A total of seven studies (n = 2004 ) were included in the meta-analysis of de novo HEV infection. The pooled estimated prevalence of de novo HEV infection in SOT recipients was 5 .1 % (95 %CI: 2 .6 -9 .6 ; I2 = 90 .8 %). The forest plot is illustrated in Figure 2 B.
Acute HEV infection
A total of nine studies (n = 1925 ) were included in the meta-analysis of acute HEV infection. The pooled estimated prevalence of acute HEV infection in SOT recipients was 4 .3 % (95 %CI: 1 .9 -9 .4 ; I2 = 90 .7 %). The forest plot is illustrated in Figure 2 C.
Network association analysis
We used subgroup analysis to compare the pooled estimated prevalence of HEV infection from two solid organs of interest at a time. Figure 3 depicts a diagram of the network association analysis. In brief, the prevalence of HEV infection in lung transplant was significantly lower than liver transplant patients (Q = 7 .033 , P = 0 .008 )and undifferentiated patients (Q = 4 .322 , P = 0 .038 ). There were no statistically significant associations across all other comparisons.
Subgroup analyses
Subgroup analysis results are depicted in Table 1 . Here, we analyzed the pooled estimated prevalence of HEV infection based on study characteristics. We applied mixed-effects model to minimize inter-study variance in the subgroup analyses. In brief, we found that the pooled prevalence of HEV infection was similar after adjustment for study year (< 2015 vs ³2015 ), study design (prospective vs retrospective), sample size (< 400 vs ³400 ), age (£ 50 years vs > 50 years), male proportion (£ 65 % vs > 65 %), number of confirmatory tests (> 1 marker vs single marker), and follow-up duration (£ 12 mo vs > 12 mo). Interestingly, we found that the prevalence of HEV infection was significantly higher in middle-income countries compared to high-income countries (41 .8 % vs 18 .9 %; Q = 22 .375 , P < 0 .001 ). The seroprevalence of positive anti-HEV antibodies was significantly higher in studies thatutilized Wantai assay compared to studies with other assays (28 .4 % vs 12 .3 %; Q =10 .134 , P = 0 .001 ).
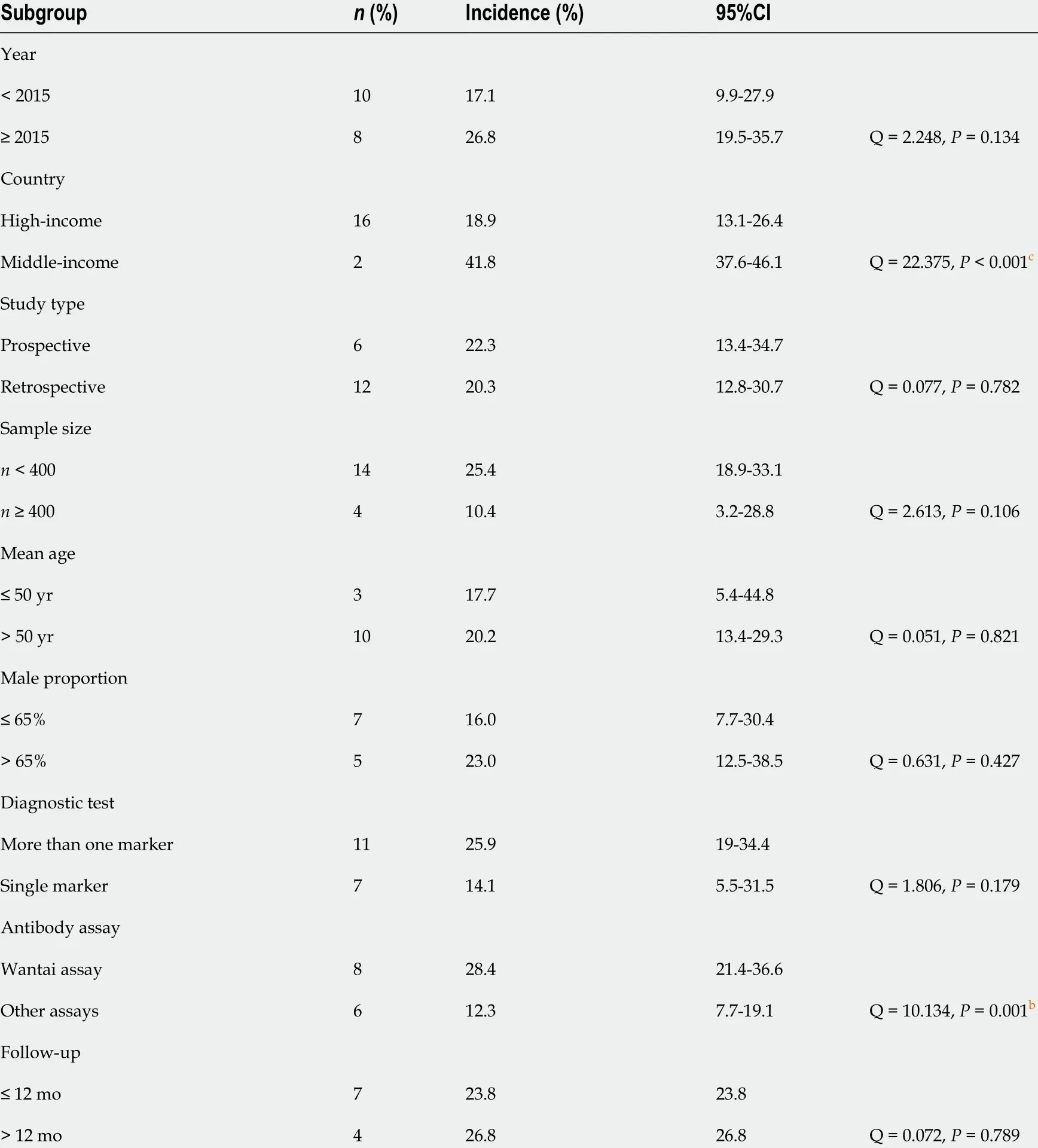
Table 1 Subgroup analyses of all variables
Evaluation for publication bias
The p-value of Egger’s regression intercept for the analysis of pooled total prevalence of HEV infection, de novo HEV infection and acute HEV infection was 0 .060 , 0 .054 ,and 0 .136 , respectively. These values indicated no potential publication bias. The funnel plot for the HEV pooled prevalence infection analysis in undifferentiated SOT recipients is illustrated in Supplementary Figure 1 .
Systemic review
Table 2 illustrates study characteristics and outcomes included in this systematic review. Kamar et al[19 ] showed that the use of tacrolimus (OR 1 .89 ; 95 %CI: 1 .49 -1 .97 )and low platelet count (OR 1 .02 ; 95 %CI: 1 .00 -1 .10 ) were associated with chronic HEV infection in SOT patients. Additionally, cirrhosis (OR 7 .6 ; 95 %CI: 4 .4 -13 .1 ), liver transplantation (OR 3 .1 ; 95 %CI: 1 .8 -5 .4 ) and Human immunodeficiency virus (HIV)infection (OR 2 .4 ; 95 %CI: 1 .3 -4 .4 ) were significant risk factors for HEV infection in the Spanish cohort[20 ]. Another study[21]demonstrated that HBV coinfection was associated with chronic HEV infection in SOT recipients (OR 7 .4 ; 95 %CI: 1 .3 -37 .0 ), and patients with positive HEV-IgG had higher odds of developing hepatocellular carcinoma (OR 2 .3 ; 95 %CI: 1 .1 -4 .8 ). Pischke et al[22]emphasized the prevalence of HEV infection in
heart transplant patients by demonstrating that these patients had a significantly higher seroprevalence of HEV-IgG than healthy individuals. Interestingly, in a Chinese cohort of 408 Liver transplant recipients[23 ], alcoholic cirrhosis (OR 5 .3 ; 95 %CI:1 .4 -21 .0 ) and liver failure (OR 23 .8 ; 95 %CI: 2 .8 -203 .1 ) were associated with increased de novo HEV infection during a follow-up of 3 years while graft rejection (OR 0 .22 ;95 %CI: 0 .06 -0 .74 ) was surprisingly a protective factor.

Table 2 Characteristics of studies included in the systematic review

UD: Undifferentiated; HEV: Hepatitis E virus; LFTs: Liver function test; OR: Odds ratio; LTx: Liver transplant; IgG: Immunoglobulin G; MC: Multicenter;IgM: Immunoglobulin M; KTx: Kidney transplant; HCC: Hepatocellular carcinoma; HIV: Human immunodeficiency virus; ALT: Alanine aminotransferase;R: Retrospective; NAFLD: Non-alcoholic fatty liver disease; MP: Methylparaben.

Figure 2 Forest plots of meta-analysis. A: The pooled prevalence of hepatitis E virus (HEV) infection (I295 .3 %; Egger’s intercept 0 .060 ); B: The pooled prevalence of de novo HEV infection (I2 90 .8 %; Egger’s intercept 0 .054 ); C: The pooled prevalence of acute HEV infection (I290 .7 %; Egger’s intercept 0 .136 ). CI:Confidence interval; HEV: Hepatitis E virus.
DISCUSSION
The meta-analysis revealed prevelance of HEV in SOT recipients is 20 %. De novo HEV infection and acute HEV infection accounted for less than 5 % of infections. A recent meta-analysis of 419 studies comprised of 519 ,872 individuals showed an estimated global seroprevalence of anti-HEV IgG of 12 .5 % and a pooled estimated anti-HEV IgM seroprevalence of 1 .5 %[24]. Although our study did not provide a direct comparison to non-transplant patients, it can be extrapolated that the prevalence of HEV infection is higher in SOT patients (20 .1 % vs 12 .5 %). The prevalence of acute HEV infection was also higher in SOT patients compared to non-transplant patients (4 .3 % vs 1 .5 %). These findings emphasize the burden of HEV infection in SOT patients. To date, the United States has not issued national guidelines for the management of hepatitis E in SOT.However, recent guidelines from the British Transplantation Society have recommended screening for HEV infection in individuals with elevated liver enzymes(evidence 1 D)[25]. Unfortunately, the evidence for this recommendation is relatively weak due to a lack of studies supporting the association between HEV infection and adverse post-transplant clinical outcomes. More studies on this particular topic are needed. Furthermore, our study indicated a high burden of de novo HEV infection and acute HEV infection in SOT patients. Whether these infections affect the posttransplant clinical outcomes different from chronic HEV infection is yet to be investigated.
It is possible that the seroprevalence of anti-HEV IgG could be affected by the assays used for antibody testing. Rossi-Tamisier et al[26]compared the positive rates of two different commercial microplate enzyme-immunoassays and found that the prevalence of seropositive IgG against HEV was higher in the Wantai assay compared to Adaltis assay[26 ]. Similarly, Li et al[24]conducted a meta-analysis and described that the seroprevalence of anti-HEV IgG was highest with the Wantai assay in comparison with other commercial assays[24]. In our subgroup analysis, we also observed that the seroprevalence of anti-HEV antibodies from studies that utilized the Wantai assay was significantly higher than other assays. Thus, the type of assay test should be taken into consideration when interpreting positive anti-HEV IgG or IgM results.
We also found that the prevalence of HEV infection was significantly higher in middle-income countries vs high-income countries. This finding is consistent with previously published. Li et al[24]suggested that the seroprevalence of anti-HEV IgG was at least two-fold higher in Africa and Asia in comparison to Europe and North America[24]. As HEV route of transmission via the fecal-oral route is similar to hepatitis A virus, patients with poor hygiene are predisposed to both hepatitis A and hepatitis E infection. Consumption of raw meat, exposure to soil, contact with dogs, residing in rural areas, and an education level attained less than elementary school is known risk factors for HEV infection[24]. However, our study did not include any articles that originated from low-income countries where the prevalence of HEV infection is anticipated to be high. This may be due to the lower rate of SOTs within this demographic. More studies from low- and middle-income countries are encouraged to reliably determine the global burden of HEV infection in SOT recipients.
We found that the prevalence of HEV infection was lowest in lung transplant recipients. It is unclear why lung transplant recipients had less HEV infection compared with liver transplant recipients. It is possible that the prevalence of HEV infection in lung transplant recipients is under-reported in the literature, given the smaller number of lung transplants annually, at least in the United States. The total number of lung transplants is three times fewer than the total number of liver transplants from the United States Organ Procurement and Transplantation Network[27]. However, it is also possible that lung transplant recipients may be predisposed to receiving ribavirin therapy for other indications, such as respiratory syncytial virus or hepatitis C virus infection. Ribavirin and interferon-a are two main antivirals that have been used to treat cases of HEV infection. There are several reports of successful use of ribavirin in chronic HEV infection to achieve overall sustained virologic response of up to 80 %[28 -30]. The underlying mechanism by which lung transplant patients had lower HEV infection should be investigated in future clinical studies.
Several risk factors for HEV infection in SOT patients have been identified from our systematic review. The use of tacrolimus (versus cyclosporine), low platelet count,cirrhosis, liver failure, human immunodeficiency virus (HIV) coinfection, and hepatitis B virus (HBV) coinfection are all significant risk factors for HEV infection.Hypothetically, tacrolimus generally delivers more immunosuppressive property than cyclosporine, which could predispose patients to contract HEV. This statement,however needs more supporting clinical evidence. Liver disease and associated manifestations including cirrhosis, liver failure, and low platelet count, are not specific to HEV infection; they may be attributed to HEV infection or one of many other etiologies of chronic liver failure. HIV and HBV coinfection raises concern for transfusion-associated HEV transmission, which has been reported in several studies worldwide[31 -34].
Our study is subjected to certain limitations. First, all studies were observational in design, making them susceptible to selection bias. We attempted to minimize this bias by performing risk of bias assessment prior to inclusion of studies into our metaanalysis and systematic review. Second, the clinical impact of HEV infection was not meta-analyzed due to limited information from the original articles. More studies investigating the association between HEV infection and clinical outcomes are needed.Third, the genotype of HEV was not reported. Although it is well perceived that HEV genotype 3 and 4 are more common in immunocompromised patients[2], the prevalence of HEV genotype 3 and 4 infection in SOT patients remains inconclusive from our study. Fourth, only the status of recipients was evaluated in our study. HEV infection profile in donors was not taken into consideration due to the limited data in the original articles. HEV transmission via transplanted liver has been reported and would potentially impact the prevalence of HEV infection in the recipients. Fifth,generalization of our findings to heart transplant patients is limited because only one study included heart transplant patients. Finally, the majority of included studies were from high-income countries. Additional cohorts from low-income and middle-income countries are highly encouraged.
The future prospects include evaluation of the impact of HEV on SOT patients and graft analysis by meta-analysis or meta-regression analysis. Once the association between HEV infection and adverse clinical outcomes is conclusive, the role of ribavirin therapy for HEV eradication should be investigated in future clinical trials.The ultimate objective of this study is to help contribute to the core knowledge of improving the clinical outcomes of SOT recipients.
CONCLUSION
In conclusion, HEV infection is common in SOT recipients and accounts for 20 .2 %. It is at least two-fold higher in middle-income countries compared to high-income countries. The prevalence of HEV infection in lung transplant recipients is considerably less common than other organ transplants. More studies demonstrating the clinical impacts of HEV infection in SOT recipients, such as graft failure, rejection,and mortality, are warranted.
ARTICLE HIGHLIGHTS

杂志排行
World Journal of Gastroenterology的其它文章
- Cytapheresis re-induces high-rate steroid-free remission in patients with steroid-dependent and steroidrefractory ulcerative colitis
- Perioperative blood transfusion decreases long-term survival in pediatric living donor liver transplantation
- Emerging wearable technology applications in gastroenterology: A review of the literature
- Primary localized gastric amyloidosis: A scoping review of the literature from clinical presentations to prognosis
- Risk stratification and geographical mapping of Brazilian inflammatory bowel disease patients during the COVID-19 outbreak: Results from a nationwide survey
- Risk perception and knowledge of COVID-19 in patients with celiac disease
