Crystallization Behavior and Kinetics of Lithium Aluminosilicate Glasses with Various Li2O Contents
2021-04-16HANJianjunHONGWeiJIANGHongHEJianxiongWANGQianchen
HAN Jianjun, HONG Wei, JIANG Hong, HE Jianxiong, WANG Qianchen,
WANG Jing1, GAO Wenkai1
(1. State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China; 2. State Key Laboratory of Special Glass, Chengmai 571924, China; 3. AVIC (Hainan) Special Glass Technology Co. Ltd., Chengmai 571924, China)
Abstract: The crystallization behavior of Li2O-Al2O3-SiO2 glasses with various concentration of Li2O were investigated by DSC, XRD, and FE-SEM. The h/l-quartz s.s. is the main crystalline phase for all of the glass with the heat treatment of 720 ℃/3 h+800℃/1.5 h while the crystallinity and crystal size increase with the increase of Li2O contents. Due to the negative thermal expansion coefficient of h/l-quartz s.s., the thermal expansion coefficient of glass-ceramics decreases with the increasing of Li2O contents. When the Li2O content is 9mol%, the near-zero CTE20-700 ℃= -0.09×10-6 ℃-1 is obtained upon the heat treatment of 720 ℃/3 h+800℃/1.5 h. The crystallization activation energy of the glass with 9mol% Li2O is about 351 kJ/mol, and the crystallization mechanism is volume crystallization.
Key words: LAS glass-ceramics; crystallization; zero thermal expansion; kinetics
1 Introduction
Zero-expansion properties are important for improving the thermal geometric stability of aerospace structures and electronic equipment. The working environment of satellite antennas and electronic devices is complicated. Uneven temperature distribution and large temperature changes cause large thermal deformation, which causes signal distortion[1]. Large temperature changes often cause large temperature stress and cause structural damage. The current zeroexpansion materials mainly include NZP ceramics,aluminum titanate composite ceramics and LAS glassceramics[2]. Among them, LAS glass-ceramics have excellent mechanical properties, high temperature resistance and transparency, and can adjust the thermal expansion coefficient of glass-ceramics in a wide range by controlling the glass composition and heat treatment, thus causing extensive research.
Karmakar Bet al[3]studied the crystallization kinetics and crystallization mechanism ofβ-quartz solid solution. The experimental results show that the Avrami index (n) is 1.5. The activation energy (Ea)is 500 kJ/mol. Feng Det al[4]studied the effect of CaF2on the properties of LAS glass-ceramics. In the study, glass can be used as a binder to wrap diamond as an abrasive. They found that the addition of CaF2can reduce the nucleation temperature and facilitate crystallization. Roy Ret al[5]found the crystal phase equilibrium of keatite at low temperatures. The upper limit of stability of eucryptite, spodumene and feldspar was 972 ℃, 500 ℃, and 680 ℃, respectively .
In this work, the LAS glass-ceramics with nearzero thermal expansion coefficient were prepared, and the effects of Li2O concentration of LAS glass on the crystallization behavour and propeties were discussed.
2 Experimental
Table 1 presents the compositions of the LAS glasses. The glass samples with the Li2O concentrations varied from 7mol% to 11mol% were labeled Li-7-Li-11. All the starting materials including SiO2,Al(OH)3, Li2CO3, ZnO, (MgCO3)4·Mg(OH)2·5H2O,P2O5, TiO2, ZrO2, and Sb2O3were mixed in stoichiometric proportions. All the chemicals were of analytical grade. The batches were melted in a platinum-rhodium crucible at 1 650 ℃ for 3 h. Then the melt was poured onto a preheated brass mold, and the as-prepared bulk glass was annealed at 650 ℃ for 2 h to eliminate the thermal stress. The LAS glass were heat treated to make the precipitation of crystals from glass matrix.
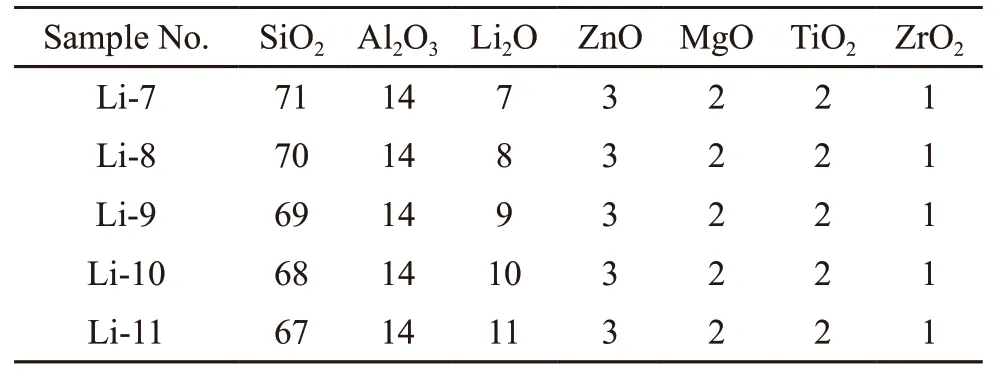
Table 1 Chemical compositions of LAS glasses/mol%
The glass transition temperature (Tg) and crystallization peak temperature (Tp) were measured with the glasses crushed into particles with the size of about 75 μm. Crushed samples of 20 mg were analyzed by differential scanning calorimeter (DSC, Netzsch STA449F1, Germany) from room temperature to 1 200 ℃ at the heating rates of 5, 10, 15, and 20 ℃/min against alumina powder as reference material.X-ray diffraction patterns of the glasses were recorded after various thermal treatments. X-ray diffractometer(D8 Advance, Bruker) measurements were performed using Cu-Ka to identify the crystalline phases. The microstructure of the cross-section of LAS glassceramics etched by 5vol% HF was observed by FESEM (S4800 Hitachi, Japan). The coefficient of thermal expansion (CTE) of glass and glass-ceramics were measured from room temperature to 1 000 ℃at the heating rate is 5 ℃/min by using dilatometer(Netzsch DIL402C, Germany).
3 Results and discussion
3.1 DSC and dilatometer analysis of LAS glasses
Fig.1 shows the DSC traces of LAS glasses with 7mol%-11mol% Li2O in the temperature range 200-1 200 ℃ with the heating rate 10 ℃/min. One exothermic peak can be seen for each sample, while.With the increasing of Li2O content from 7mol% to 11mol%, theTgdecreases from 721.8 to 648.5 ℃, and theTpdecreases from 877.5 to 818.6 ℃. Such a large decreasing inTpreveals that Li2O concentration plays an important role in the crystallization behavior of LAS glasses. The glass with 11mol% Li2O exhibits the lowestTpand sharpest exothermic peak, indicating highest crystallization rate and lowest crystallization activate energy. The coefficients of thermal expansion and the dilatometric softening temperaturesTdwere determined from the thermal expansion curves (Fig.2).Increasing content of Li2O results in decreasing ofTdand increasing of CTE. Table 2 compiles the results obtained by DSC and dilatometry.
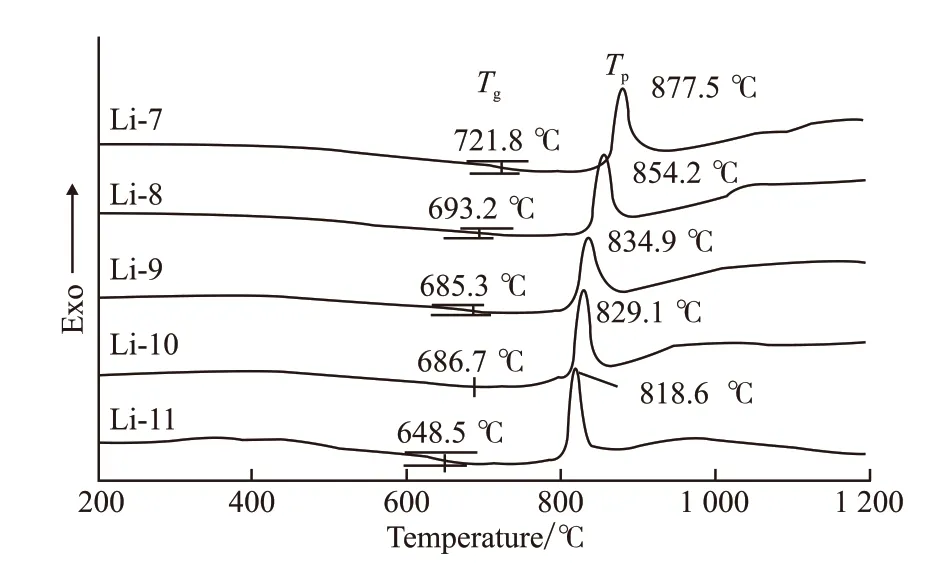
Fig.1 The DSC curves of LAS glasses with 7mol%-11mol% Li2O
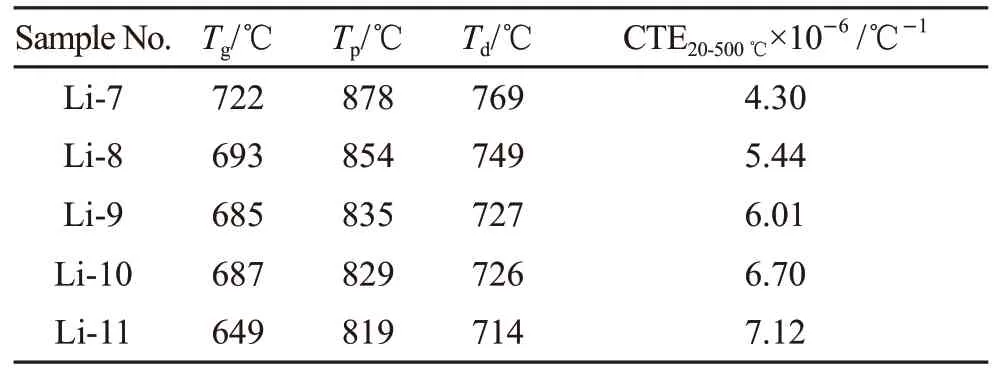
Table 2 The coefficients of thermal expansion, transition, and softening temperature of LAS glasses
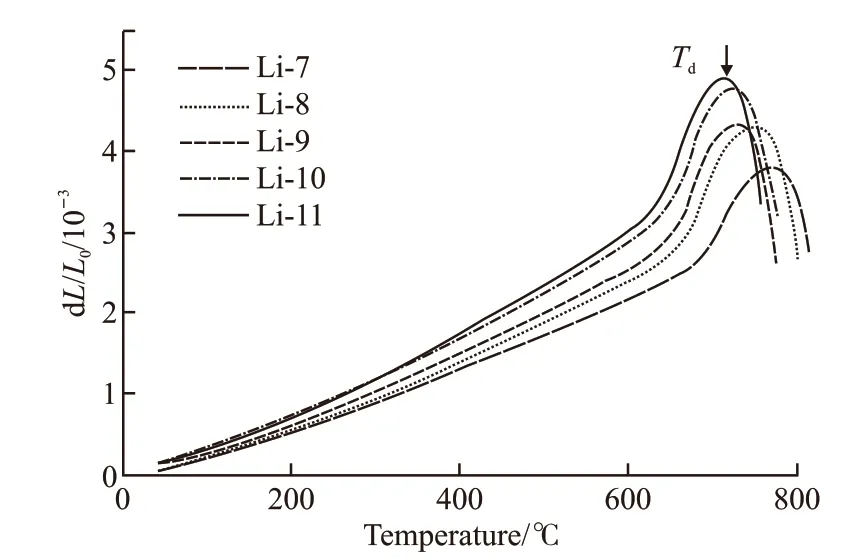
Fig.2 The thermal expansion curves of LAS glasses with 7mol%-11mol% Li2O
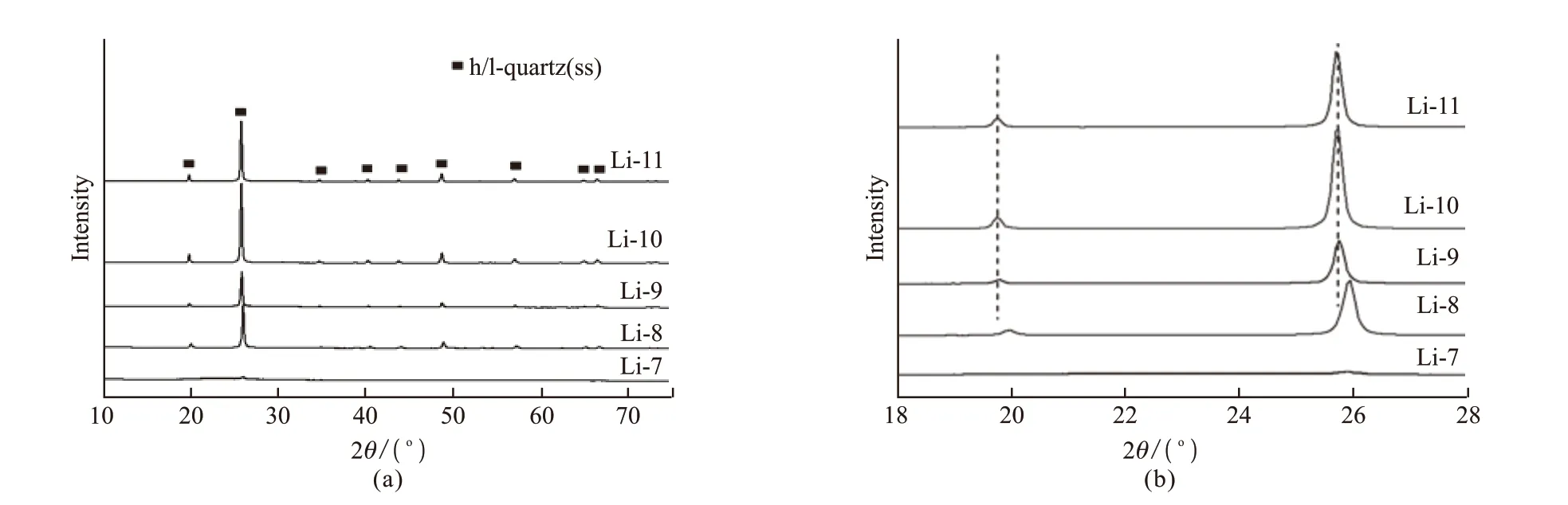
Fig.3 (a) XRD patterns of LAS glasses with the heat treatment 720 ℃/3 h+800 ℃/1.5 h; (b) The magnification of XRD patterns with the 2θ range from 18° to 28°
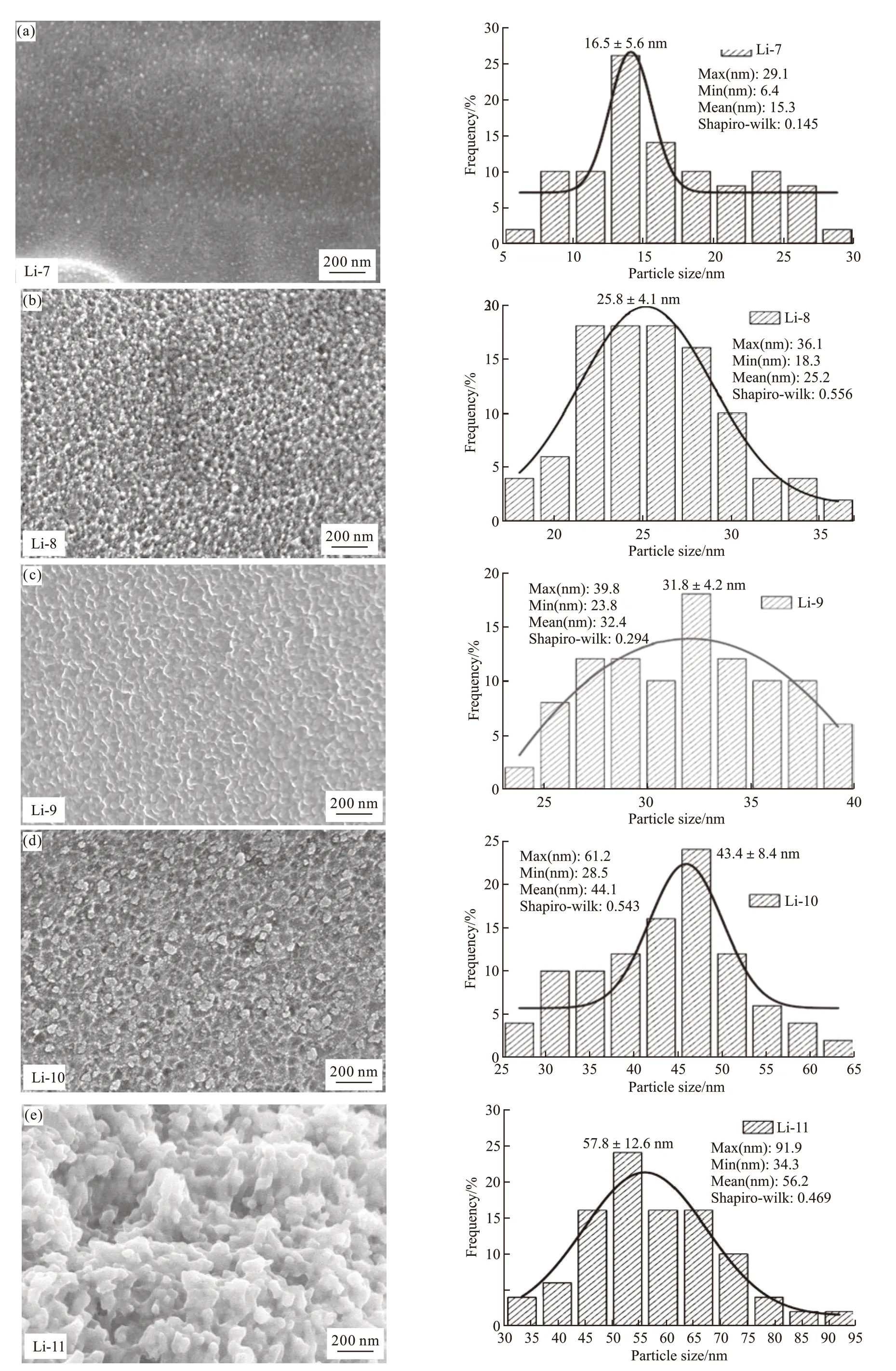
Fig.4 FE-SEM images of glass-creamic: (a) Li-7, (b) Li-8, (c) Li-9, (d) Li-10, and (e) Li-11 with the heat treatment 720 ℃/3 h+800 ℃/1.5 h
The Li2O acts as a glass network modifier and the SiO2acts as a glass network former. As a result, the substitution of Li2O for SiO2weaken the glass network connectivity and thus increase the CTE and decreaseTg,Tp, andTd.
3.2 Crystallization behavior of the LAS glasses
The LAS glasses with 7mol%-11mol% Li2O were nucleated at 720 ℃ for 3 h and then crystallized at 800℃ for 1.5 h. All the heat-treated samples are colorless and transparent. The XRD patterns of the heat-treated LAS glasses were shown in Fig.3. The glass-ceramics exhibited similar peak positions attributable to the h/l-quartz s.s. The glass-ceramics with higher Li2O concentrations showed higher intensity in XRD peaks,indicating the formation of lager size crystals and higher crystallinity. 2 ℃ range from 18° to 28° of XRD patterns is illustrated in the Fig.3(b). When the Li2O concentration increased to 9mol%, the peaks of the h/l-quartz s.s. shifted to lower 2 ℃ values. The main peak of h/l-quartz shifted from 25.9° to 25.7°, while the position did not shift anymore when further increase in the Li2O concentration to 10mol% and 11mol%.The peaks shift is due to the increasing of unit cell volume[6]. In the process of precipitation h/l-quartz s.s.,Al3+ions forms [AlO4] tetrahedral structure combines with [SiO4] tetrahedral network, and the Li+ions enter into the quartz structure to balance the charge, with the Li2O concentration increased, the more Li+ions were incoporated into quartz solid solution. The increase of solid solubility resulted in the expansion of unit cell.
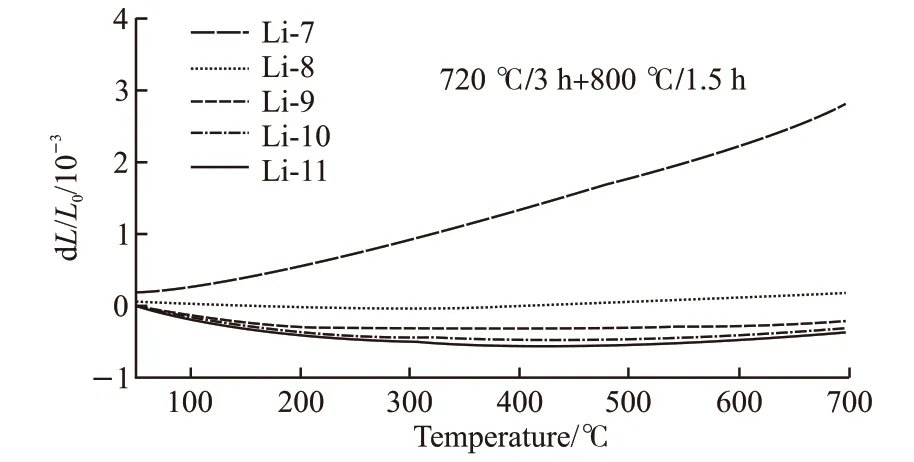
Fig.5 The thermal expansion curves of LAS glass-ceramics containing 7mol%-11mol% Li2O with the heat treatment 720 ℃/3 h+800 ℃/1.5 h
Fig.4 shows FE-SEM images of the LAS glass-ceramics with the heat treatment 720 ℃/3 h+800 ℃ /1.5 h. 50 grains were randomly selected to make grain size distribution maps, and SPSS was used to verify whether the grain size distribution conformed to the normal distribution[7]. Fig.4(a) shows that the Li-7 sample has a small and uniform grain size, and the average grain size is 16.6±5.6 nm. The average crystal size of Li-11 glass-creamic is the largest 55.7±4.6 nm (in Fig.4(e)).SEM results show that the grain size increases with the increasing of Li2O content, which is consistent with the conclusion of XRD pattern.
Fig.5 shows the diametric curves obtained from the LAS glass-ceramics heat-treated at 720 ℃/ 3 h+800 ℃/1.5 h. They exhibited nearly linear thermal expansion behavior at the temperatures from 100 to 700 ℃. The linear dilatometry curves indicated that the phase transition from high- to low-quartz do not occurred. Since the phase transformation from the highto the low-quartz ss would result 0.8% volume increase in the temperature range from 480 to 530 ℃[8-10],and their dilatometric curves may exhibit a and decrease in the CTE at around 500 ℃ but not a linear relationship. While the incorporation of Li2O, MgO and Al2O3stabilized the h-quartz ss and inhibit the phase transformation[11].
The CTE100-700℃of Li-7 glass-ceramic is around 4.17×10-6℃-1,which is close to that of unheated glass because of the low crystallinity. The CTE100-700℃of Li-8 glass-ceramics significantly decreased to 0.25×10-6℃-1, and the Li-9 displayed a most nearly zero CTE100-700℃at -0.09×10-6℃-1. The Li-10 and Li-11 exhibited negative thermal expansion at-0.52×10-6℃-1
3.3 Crystallization kinetics of LAS glasscreamic with near-zero CTE
The LAS glass with 9mol% was heat treated at 800-1 250 ℃ for 1.5 h to study the crystallization behavior, and the XRD patterns of the heat treated samples were shown in the Fig.6. When the heating temperatures (TH) were 800 and 850 ℃, the LAS kept transparent and the h/l-quartz solid solution precipitated from the glass matrix. As theTHincreased to 950 ℃,the sample was opaque and spodumene s.s. occurred,and the XRD peaks from spodumene s.s. became stronger with further increasing inTH. In addition to the main phase, small amount of spinel (JCPDS 82-1043)formed when theTHabove 950 ℃. The formation of spodumene s.s. with larger size and high crystallinity causes transparency loss, and large CTE.
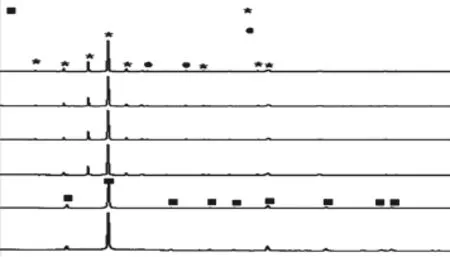
Fig.6 The XRD patterns of glass with 9mol% Li2O crystallized at 800 ℃, 850 ℃, 950 ℃, 1 050 ℃, 1 150 ℃, and 1 250 ℃for 1.5 h, respectively
The DCS curves of the Li-9 glass with the heating rates of 5, 10, 15, and 20 ℃/min are shown in Fig.7.With the increasing of heating rate from 5 to 20 ℃/min, theTpincrease from 831.8 to 871.2 ℃. According to DSC data, the crystallization activation energy can be determined by Kissinger equation, and the corresponding equations are as follows[12]:
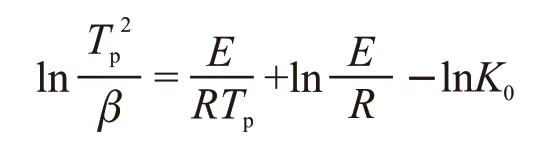
where,βis the heating rate,Tpis the crystallization peak temperature at a given heating rate andRis the gas constant,K0is the effective frequency factor. From the slope of the Kissinger plots, the activation energies for the crystallized glass samples were determined as 351.3 kJ/mol (Fig.8), and are also given in Table 3.
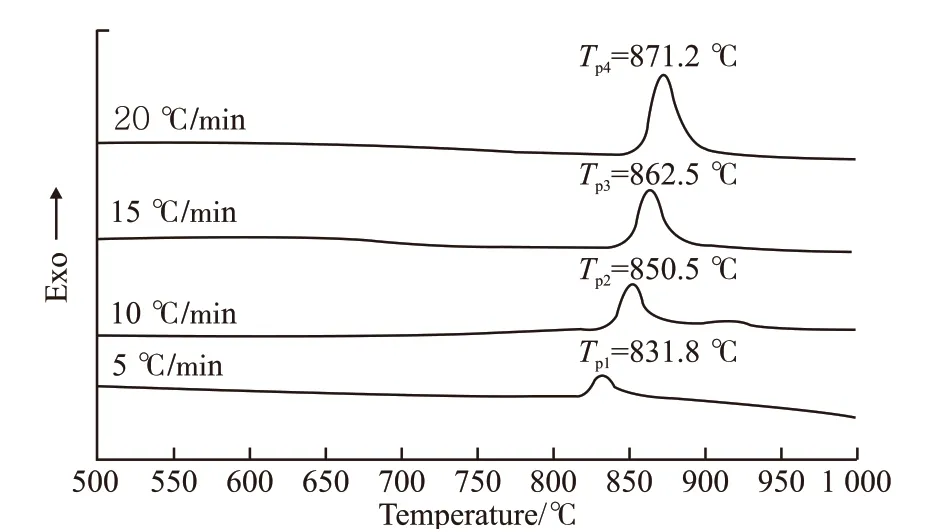
Fig.7 The DSC curves of Li-9 glass at heating rate of 5 ℃/min,10 ℃/min, 15 ℃/min, and 20 ℃/min
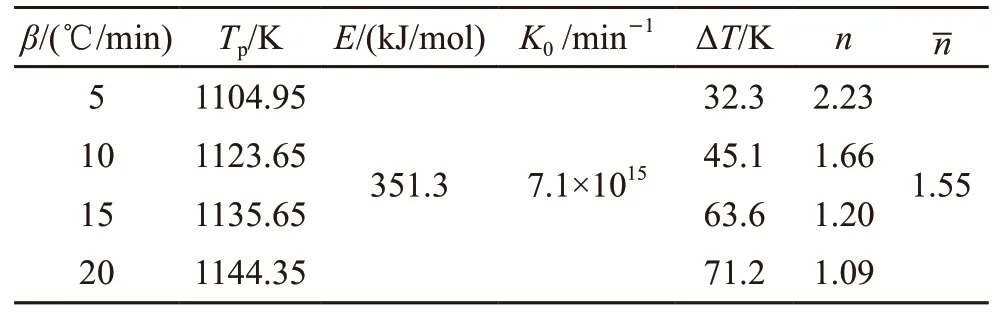
Table 3 Tp values and crystallization values from DSC curves of Li-9 glass sample at different heating rates
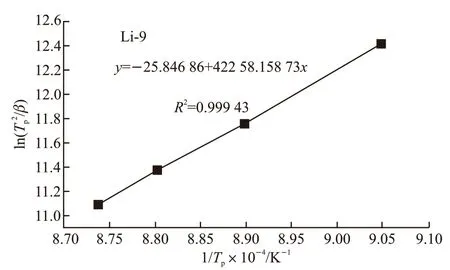
Fig.8 Relationship between ln(Tp2/β) and 1/Tp
The value of the Avrami constant,n, can be determined by the Augis Bennett equation[13]:
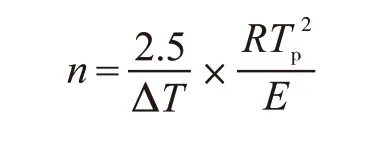
where, ΔTis the full width of the exothermic peak at the half maximum intensity. The value ofnclose to 1 means that surface crystallize dominates overall crystallization, while the value ofnclose to 2 means that two-dimension crystallization, and the value of 3 implies a bulk crystallization process[14].
According to the calculation results, the crystallization activation energyEand Avrami constantnare 351 kJ/mol and 1.5, respectively. The values of Avrami constant indicated the volume crystallization in one dimension is dominant in the produced glass.
4 Conclusions
The Li2O-Al2O3-SiO2glass-creamics with near-zero thermal expansion were prepared, and the crystallization behavior of LAS glass with 7%-11%Li2O were investigated. All the LAS glass showed h/l-quartz s.s. as the main crystallire phase upon the same heat treatment while the cystallinity and crystal size increased with the increasing of Li2O contents. The glass containing 9mol% Li2O exhibited the most close to zero thermal expansion coefficient (CTE20-700℃=-0.09×10-6℃-1),when heat treated at 720 ℃for 3 h and then 800 ℃ for 1.5 h. The crystallization mechanism of this glass is volume crystallization in one dimension, and the crystallization activation energy is about 351 kJ/mol, the Avrami constantnis about 1.5.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
