Adsorptive Behavior of Methyl Blue on Graphene Aerogel: A Mechanism Study
2021-04-16SONGXuyanHEYunluPANXiWEIMinLIRanZHOUXiaoyuZHENGYingLIJunshengTANGHaolin
SONG Xuyan, HE Yunlu, PAN Xi, WEI Min, LI Ran, ZHOU Xiaoyu,ZHENG Ying*, LI Junsheng, TANG Haolin
(1. Technology Centre of Hubei China Tobacco Industry Co., Ltd., Wuhan 430051, China; 2.College of Urban Construction, Wuchang Shouyi University, Wuhan 430064, China; 3.State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China)
Abstract: Graphene aerogel was synthesized and used for the removal of methyl blue from aqueous solutions. The effect of solution pH, temperature and adsorption time on the adsorption performance of the graphene aerogel was studied systematically. In addition, investigations were also performed to determine the nature of adsorption. The experimental results show that graphene aerogel is a highly efficient adsorbent for the treatment of methyl blue in aqueous solutions. In addition, the adsorption of methyl blue proceeds through a single layer physical adsorption on the graphene aerogel. The findings herein are useful for the future development of adsorbent for in water.
Key words: graphene aerogel; adsorption; methyl blue; mechanism
1 Introduction
Controlled adsorption of various molecules on certain substrate is an important process in many daily practical applications and substrate materials with adsorption capacity may find many applications in relating fields. For example, bio-compatible polymeric materials with adsorbed fragrance molecules can be used in food and tobacco applications; materials with high adsorption capacity for dye stuff can be used for the treatment of polluted water[1]. In principle, the adsorption characteristics of the molecules on the substrates is largely determined by the properties of the materials,such as the surface area and physical/chemical affinity between the substrate and the molecules to be adsorbed[2].
Dyeing waste water is one of the most common pollution sources of water pollution. Generally, dyeing waste water can be treated through flocculation process,biological process and separation process[3,4]. However,the above-mentioned processes suffer from their intrinsic drawbacks such as limited efficiency, high price or possibility of introducing additional pollutants[5]. As a comparison, physical adsorption of the dye stuff with high surface material is a low cost yet effective approach for the removal of dye pollutant[6]. The primary advantage of the physical adsorption process is its high adaptability for different pollutants, possibility to regenerate the adsorption substrate and simple handling process[7]. Graphene aerogel is a three-dimensionally assembled network of graphene sheets, which has relatively large surface area, high structural stability and low density[8-10]. These features make graphene aerogel ideal material for physical adsorption and removal of pollutants[11-13]. In this manuscript, graphene aerogel is synthesized and used for the adsorption of methyl blue, which is a major component in most dyeing waste water. The adsorption behavior of methyl blue on graphene aerogel was investigated systematically and the possibility of using graphene aerogel for dyeing adsorption in water was discussed.
2 Experimental
2.1 Synthesis and characterization of graphene aerogel
The synthetic protocol of graphene aerogel was adopted from previous work with minor modification[14]. In brief, graphene oxide (GO) was firstly prepared with the Hummer method. In brief, 2.5 g graphite powder was added into a flask (in an ice bath) containing mixture of sulfuric acid and phosphoric acid, into which 14 g of KMnO4was slowly added. After keeping the flask in the ice bath for 2 h, the flask was heated to 50 ℃ for 8 h to yield brown products. The products was collected and dispersed again in deionized water,followed by addition of small amount of H2O2, until the products turned yellow. The GO products were collected again and washed with HCl and deionized water until the pH of washing water reached 7. The as-synthesized GO was dispersed into deionized water with a concentration of 10 mg·mL-1. Next, the GO dispersion was mixed with 100 mg·L-1poly vinyl alcohol solution with a volume ratio of 1:1 by sonicating. Afterwards,L-cysteine was added into above solution and heated at 95 ℃ for 4 h, which leads to formation of hydrogel.The hydrogel was dialyzed in ethanol/water mixture(v/v=1/4)for 12 h to remove the residual L-cysteine in the hydrogel. To obtain graphene aerogel, the hydrogel was frozen at -20 ℃ for 12 h, followed by further freeze dried at -50 ℃ in a lyophilizer (≤1 Pa; 48 h).The morphology of the as-synthesized graphene aerogel was characterized with an Hitachi S-4800 scanning electron microscope.
2.2 Adsorption behavior of methyl blue on graphene aerogel
The concentration of methyl blue was quantified with UV-Vis measurement. A standard curve was established using the UV-absorbance at a wavelength of 664 nm with methyl blue solution with known concentration (5, 10, 15, 20, and 25 mg·L-1). By comparison of the UV-Vis absorbance of the testing solution (after dilution) with that from the standard curve, the concentration of the methyl blue could be determined. The percentage of adsorbed methyl blue by graphene aerogel (Q) was calculated with equation:
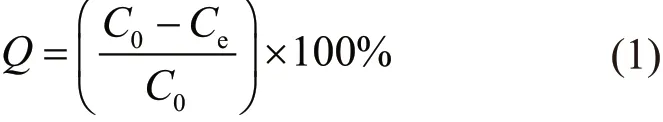
where,C0is the initial concentration of methyl blue andCeis the concentration of methyl blue after reaching adsorption equilibrium. The adsorption of methyl blue by graphene aerogel was quantified at different pH,temperature and time intervals to gain a mechanistic view on the adsorption behaviors.
3 Results and discussion
As shown in Fig.1(a), the as-synthesized graphene has an ultra-light density because of its highly porous nature. Such a low density and high porosity is beneficial for the complete infiltration of the aqueous solution into the internal pores of graphene oxide and subsequent adsorption of methyl blue onto graphene aerogel. In addition, the graphene aerogel showed good elasticity and could rapidly retain its original shape upon compressing. The excellent mechanical performance of graphene aerogel allows for its reuse in the water treatment. Since the adsorptive performance of graphene aerogel was affected by its microstructure,the morphology of the as-synthesized graphene aerogel was characterized with SEM measurement. The high porosity of graphene aerogel was confirmed by the SEM images. The graphene layers were stacked and interconnected in a three-dimensional manner, leading to well-developed porous features. In addition, the wrinkles on the graphene layers further increased the surface area of graphene aerogel, which creates more adsorption sites for methyl blue.
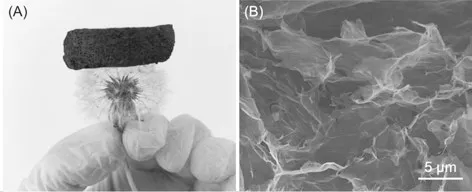
Fig.1 (a) Image of bulk graphene aerogel; (b) SEM image of the as-synthesized graphene aerogel
The effect of pH of the methyl blue solution on the adsorption characteristics of graphene aerogel was firstly investigated. The pH of the methyl blue solution(100 mg·L-1) was adjusted to 2, 4, 6, 8, and 10 with either 1 M NaOH solution or 1 M HCl solution. 50 mg of graphene aerogel was added to 20 mL methyl blue solutions with different pH and left for 30 min of adsorption. After adsorption, the concentration of the adsorbed methyl blue solution was measured with the UVVis tests and the percentage of adsorbed methyl blue by graphene aerogel (Q) was calculated. The pH of methyl blue solution had a slight effect on theQof graphene aerogel. At a pH above 8,Qreached over about 99%.When the pH decreased,Qslightly decreased. TheQvalue of graphene aerogel was 98.2%, 98.1%, and 97.1% when the pH of the methyl blue solution was 2,4, and 6, respectively. Such a pH dependent adsorption behavior can be explained by the different charge states of graphene aerogel at different aqueous environment.Graphene aerogel could be partially positively charged under acidic environment, which may result in electrostatic repulsion of the positively charged methyl blue molecules. On the contrary, graphene aerogel may be partially negatively charged under alkaline environment, which is beneficial for its absorbance of methyl blue. The current results suggest that tuning pH of the dyeing waste water may have important implication for the efficient water treatment process. In practical waste water treatment, the pH of the waste water can be adjusted to achieve cost-effective treatment. However,the pH of the waste water should be finely adjusted to avoid secondary pollution and subsequent treatment.
Next, the effect of solution temperature on theQvalue of graphene aerogel was investigated. 50 mg of graphene aerogel was added to 20 mL methyl blue solutions with temperature of 20, 30, 40, 50, and 60 ℃,respectively. TheQvalue of graphene aerogel obtained from these individual experiments was recorded and plotted in Fig.3. The results revealed that temperature had a minor effect on the adsorption performance of graphene aerogel. With the increase of temperature from 20 to 60 ℃, theQvalue of graphene aerogel increased from 98.1% to 99.1% because of facilitated diffusion process in the solution. The current results indicate that the dye removal with graphene aerogel could proceed without heating the solution in practical applications.
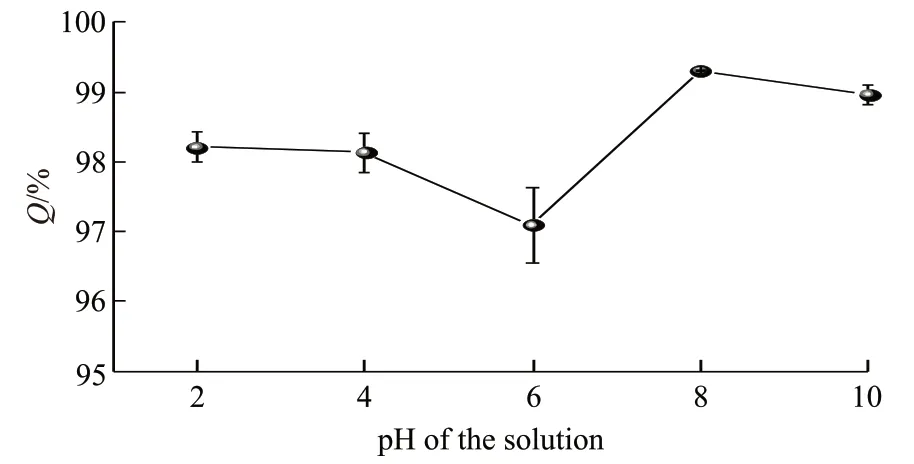
Fig.2 The effect of pH of methyl blue solution on the Q value of graphene aerogel
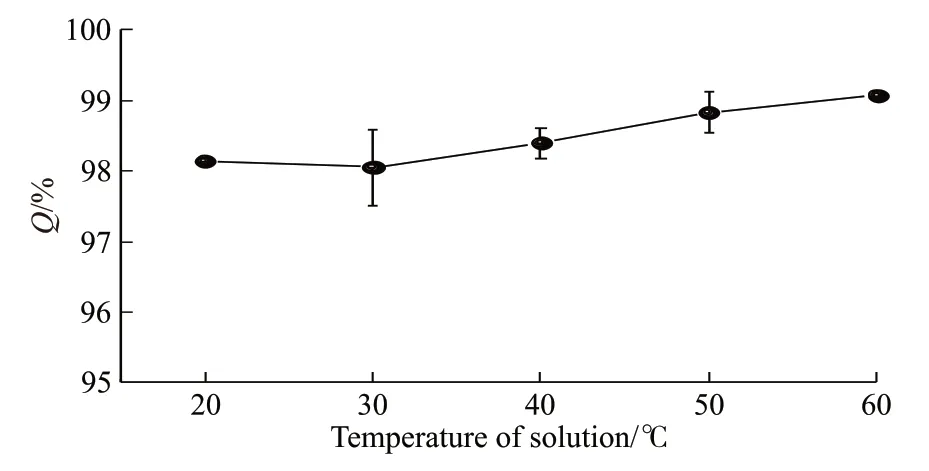
Fig.3 The effect of temperature of methyl blue solution on the Q value of graphene aerogel
A cost-effective dye removal process not only requires high removal percentage but also a rapid removal process. To study if the adsorption of methyl blue could proceed rapidly on graphene aerogel, a timelapse adsorption experiment was conducted. 20 mg of graphene aerogel was doped into methyl blue solution with a concentration of 100 mg·L-1(pH=6). The methyl blue concentration of the adsorbed solution was analyzed after different adsorption time (5, 10, 20, 30, and 40 min). It can be seen from Fig.4(a) that theQvalue generally increased with the prolongation of the adsorption time. TheQvalue was measured to be 94.4%,98.2%, 97.2%, 99.l%, and 99.2% after adsorption time of 5, 10, 20, 30, and 40 min, respectively. After 10 min of adsorption, theQvalue increased slowly with the further increasing of adsorption time. From the results above, an adsorption time of 10 min was suggested to balance the removal percentage and time cost. Furthermore, the kinetics of the adsorption behavior was investigated to gain deeper insight on the mechanism of the adsorption process. Considering the absence of functional groups on the graphene aerogel, the dominant adsorption process of methyl blue on graphene aerogel should be physical adsorption. Therefore, a quasi-first order kinetic model, in which the adsorption process is controlled by the mass transport, is used to analyze the kinetic characteristics of the adsorption process. The adsorption behavior was fitted with the following equation:

where,qtis the adsorption amount of graphene adsorbent att,qeis the adsorption amount at equilibrium state,kis the rate constant for adsorption, and e is the natural constant. Theqeandkwas calculated to be 2.4524 and 0.4185, respectively. The fitting results showed that the experimental data can be well fitted to the equation (R2=0.972), which validate our hypothesis that the adsorption is primarily dominated by the mass transport process.
Finally, the adsorption isotherm of graphene aerogel was investigated to further understand the adsorption behavior of the adsorbent. 50 mg of graphene aerogel was added into methyl blue solution (pH=6) with different concentrations (100, 125, 150, 175, 200, 225,and 250 mg·L-1). The concentration of the adsorbed solution was analyzed with UV-Vis measurement (with proper dilution) after reaching adsorption saturation.Both Langmuir and Freundlich model was applied for the analysis to distinguish if the adsorption proceeded through the monolayer adsorption or multilayer adsorption. For the Langmuir model, the following equation was applied:
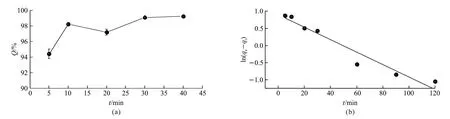
Fig.4 (a) The change of Q value with the adsorption time; (b) Kinetic analysis of the adsorption behavior of graphene aerogel with the quasifirst order kinetic model
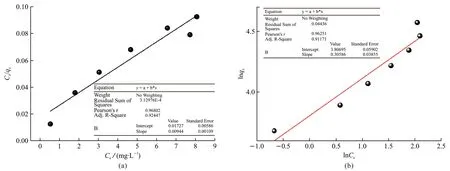
Fig.5 Fitting of the experimental results with (a) Langmuir and (b) Freundlich equation

where,KLis the Langmuir constant (related to the affinity between the adsorbent and adsorption site), andqmaxis the maximum adsorption capacity. For the Freundlich model, the following equation was applied:

where,KFis the Freundlich constant andnis the constant related to the difficulty of adsorption. The fitting of the experimental data with the Langmuir and Freundlich equation is shown in Fig.5. The experimental data fitted better with the Langmuir model, suggesting that the adsorption of methyl blue on graphene aerogel is a mono-layer adsorption.
4 Conclusions
Graphene aerogel was synthesized and used for the adsorption of methyl blue from aqueous solution.The graphene aerogel exhibits a pH dependent adsorption performance with a high adsorption capacity at a higher pH. On the contrary, the adsorption capacity of graphene aerogel is less sensitive to the temperature of the aqueous solution. In addition, our mechanism study suggests that physical adsorption of methyl blue is the dominant adsorption mode on graphene aerogel and the adsorption is most probably mono-layer adsorption. Our results suggest that the graphene aerogel is a promising adsorbent for treatment of waste water.Furthermore, the mechanism outlined in this study may provide guidance for the design for the development of efficient adsorbent. A prominent advantage of the graphene aerogel adsorbent is its capability of regeneration. In future studies, we will attempt to investigate the recycling of used graphene aerogel and study the performance of the recycled graphene aerogel in waste water treatment.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
