Synthesis and Catalytic Performance of a New V-doped CeO2-supported Alkali-activated-steel-slag-based Photocatalyst
2021-04-16KANGLeDUHuilingDENGJunJINGXinruiZHANGSenZNANGYaojun
KANG Le, DU Huiling, DENG Jun, JING Xinrui, ZHANG Sen, ZNANG Yaojun
(1. College of Materials Science and Engineering, Xi’an University of Science and Technology, Xi’an 710054, China; 2. College of Safety Science and Engineering, Xi’an University of Science and Technology, Xi’an 710054, China; 3. College of Materials and Mineral Resources,Xi’an University of Architecture and Technology, Xi’an 710055, China)
Abstract: A novel V-doped CeO2-supported alkali-activated-steel-slag-based catalyst (V-CeO2/AC) for photocatalytic decomposition of water to hydrogen was prepared via co-impregnation method. The chemical composition, mineral phase, morphology, and optical performances of the synthesized catalyst samples were characterized by XRF, XRD, SEM, UV-Vis DRS, and so on. XRD and SEM results show that calcium silicate hydrate (Ca1.5SiO3.5·xH2O) mineral phase is formed in the carrier sample, and the prepared catalyst specimens are made up of approximately 50 nm particles. After 6 hours of xenon lamp irradiation, the catalyst supported on V-doped 8wt% CeO2 exhibits the highest photocatalytic hydrogen production activity (8 292 μmol/g), which is attributed to the interaction between the V-doped CeO2 active components and FeO existed in catalyst carrier.A possible photocatalytic decomposition of water for hydrogen production mechanism over the V-8CeO2/AC catalyst was proposed.
Key words: V-doped CeO2; alkali-activated-steel-slag-based catalyst; photocatalysis; water splitting;hydrogen production
1 Introduction
In recent years, the storage of industrial solid waste and energy shortage has become one of the main environmental problems in the world. Steel slag is the main solid waste discharged from steelmaking process,which belongs to typical multi-component complex silicate slag system[1-5]. With the increasing demand for steel products in the national economic development,the discharge of steel slag in steelmaking enterprises has increased sharply year by year[6]. In 2016, the total output of crude steel was 808 million tons, accounting for 49.6% of the world’s total output of crude steel.According to the estimation that the discharge of steel slag is 10% of the output of crude steel, the discharge of steel slag in China in 2016 was about 100 million tons[7-10]. The accumulation of steel slag not only occupies a large amount of land resources, but also causes serious environmental pollution. At present, the comprehensive utilization of steel slag is mostly confined to low value-added areas such as cement clinker[11,12], road building material[13-16], concrete admixture[17,18], soil improver and adsorbent[19,20]in wastewater treatment.However, due to its complex composition, high basicity and hardness, such as converter slag containing more than 20% iron oxide, more than 60% calcium silicate and composite calcium aluminate[2], its poor activity and stability, the utilization rate has been low, which limits the scale and extensiveness of its engineering application. Therefore, converting steel slag into new materials with high value-added will bring enormous environmental, social and economic benefits.
In this paper, a new V-doped CeO2-supported alkali-activated-steel-slag-based catalyst (V-CeO2/AC)was synthesized by incipient wetness co-impregnation method, and was applied to photocatalytic decomposition of water for hydrogen production research. It is a new exploration to solve the environmental problems caused by energy shortage and solid waste accumulation. It opens up a new way for the utilization of steel slag-based cementitious materials and realizes the dual purposes of hydrogen energy preparation and solid waste recycling.
2 Experimental
2.1 Materials
Sodium hydroxide, cerium nitrate hexahydrate,ammonium metavanadate, ammonium acetate, sodium sulfide nonahydrate, and anhydrous sodium sulfate were provided by Sinopharm Chemical Reagent Co.,Ltd. and they were all analytical reagent. Steel slag (SS)was obtained from Hancheng Longmen Iron and Steel Co. Ltd. Toughening agent of silica fume was provided by Xi’an Linyuan Company.
2.2 Catalyst synthesis
The process flow is shown in Fig.1, and then the V-doped CeO2-supported alkali-activated-steel-slag-based catalyst was prepared. Steel slag and toughening agent were mixed for 10 minutes to uniformity according to the predetermined mass ratio.The content of sodium hydroxide and acrylic resin emulsion accounts for 4wt% and 0.1wt% of the mixture respectively. Water-slag ratio was 0.32. Experiment was in progress according to GB/T 17671-1999 standard for“Test method for strength of cement mortar”. The prepared test pieces were cured in a constant temperature drying box at 80 ℃ for 6 hours, then, demoulded and maintained for 3 days. The test pieces were calcined for 6 hours at 450 ℃. Subsequently, a new type of alkali-activated-steel-slag-based catalyst carrier (ACC) with 20 MPa was prepared and its chemical components are shown in Table 1.
The carrier particles were put into NH4Ac aqueous solution for ion-exchange and 2.5 mol/L of NH4Ac aqueous solution was replaced every 2 hours and repeated many times. The specimen was washed and filtrated using deionized water repeatedly and dried at 85 ℃ for 10 h. 1.0091 g Ce(NO3)3·6H2O (1wt% CeO2,relative to the quality of catalyst carrier) and 0.0018 g NH4VO3(0.2wt% V, relative to the quality of CeO2)were dissolved in 25 mL distilled water and impregnated in a beaker containing 40 g ACC specimen for 20 h.After drying the mixed specimen, then it was calcined at 400 ℃ for 4 h to get V-doped 1wt% CeO2-supported alkali-activated-steel-slag-based catalyst (V-1CeO2/AC). Meanwhile, we prepared the same proportion of V-doped 8wt% and 16wt% CeO2supported alkali-activated-steel-slag-based catalysts, which were named V-8CeO2/AC, and V-16CeO2/AC. The chemical components are shown in Table 1.
2.3 Catalyst characterization

Fig.1 The technical route of the catalyst preparation

Table 1 Chemical components of samples/wt%
The compressive strength of alkali-activated-steel-slag-based catalyst carrier was tested on a YAW-300 automatic pressure testing machine at a loading speed of 2.4 kN/s. Elemental analysis of the series catalyst samples was carried out using a Bruker S4 pioneer X-ray fluorescence (XRF) analyzer. The mineral phase information of samples was detected by a D/MAX-2200 X-ray diffraction (XRD) analyzer with the following conditions: 40 kV voltage, 40 mA current,Cu Ka radiation (λ=1.5406 Å). The structure and morphology of prepared materials were detected by a Hitachi high-resolution field emission scanning electron microscope S-4800 (FESEM). The UV-visible diffuse reflectance spectrum (UV-Vis DRS) of the catalysts was characterized by using a Perkin-Elmer Lambd 950 spectrophotometer. The photoluminescence spectrum(PL) of synthesized catalysts was carried out using an F-4500 fluorescence spectrophotometer.
2.4 Performance evaluation of the synthesized catalysts
Catalytic activity test for photocatalytic decomposition of water for hydrogen production was carried out using a gas-closed system. The light source of solar simulation was a CHF-XM-300 W xenon lamp. The distance between the lamp and the quartz reactor was 15 cm. The catalyst (0.1 g) was put into 100 mL aqueous solution containing Na2S·9H2O (6.25 g) and Na2SO3(4.36 g). The gas-closed system was vacuumed. The hydrogen yield and rate were tested on a GC-7900 gas chromatograph (molecular sieve 5A, TCD) using N2as a carrier gas after simulated solar irradiation for 6 hours.
3 Results and discussion
3.1 Composition of the prepared catalyst samples
The main chemical compositions of the synthesized materials are exhibited in Table 1. Because of the action of sodium hydroxide as an alkali activator, the content of Na2O in the ACC sample increased rapidly to the maximum value (4.21wt%). After ammonium ion exchange reaction, the content of Na2O in the catalyst samples decreased to about 0.20wt%. As shown in the Table 1, contents of V2O5and CeO2in the catalyst samples increased significantly with the increase of impregnation amount. Kanget al[11]reported that Na+ions in the alkali-activated steel slag-based binding material could be exchanged by NH4+ions in NH4Ac aqueous solution after a certain time at room temperature. Sazamaet al[21]introduced that the Na+ions in the geopolymer network could be exchanged by NH4+, Cu2+, and Co2+ions after 24 h.

Fig.2 X-ray diffraction patterns of the synthesized samples

Fig.3 SEM images of specimens: (a) ACC sample; (b) V-8CeO2/AC sample
Fig.2 represents the mineral phases information of samples. The steel slag (SS) powders mainly exhibit the following mineral phases, for example, Ca(OH)2(2θ=18.11°, 34.35°, 46.88°, JCPDS No. 44-1481), Ca2SiO4(2θ= 22.89°, 32.57°, 33.04°, JCPDS No. 86-0399),Ca2Fe2O5(2θ= 24.21°, 33.52°, JCPDS No. 19-0222),Ca3SiO5(2θ= 29.46°, 31.26°, 33.02°, 33.60°, 42.95°,49.63°, JCPDS No. 84-0594), CaO (2θ= 35.42°,37.81°, JCPDS No. 74-1226), FeO(2θ= 42.47°, 54.28°,60.98°, JCPDS No. 77-2367). The intensities of some diffraction peaks clearly decreased and some peaks disappeared in the pattern of ACC sample compared with the pattern of SS specimen in Fig.2, indicating that some mineral phases in SS specimen could react with sodium hydroxide solution to produce new kind of hydrated products, calcium silicate hydrate (CSH)(Ca1.5SiO3.5·xH2O, JCPDS No. 33-0306) and its peak position was at 2θ= 29.46°, 32.56°. Zhanget al[22]introduced that it could generate new CSH gel in the Na,-Ca-cementitious material system. As shown as Fig.2,when the content of CeO2is less, it can be seen that the pattern of V-CeO2/AC and V-8CeO2/AC specimens was similar to that of ACC sample except for the diminution of peak intensities of Ca(OH)2. Further increase the load of CeO2content, there are three distinct diffraction peaks of CeO2in the V-16CeO2/AC specimen. Its peak position located at 2θ= 28.52°, 47.74°, and 56.44° and its JCPDS number is 34-0394. Perhaps due to the V content is so small that no V crystalline compound is found in Fig.2. The average crystallite size of CeO2particles in the V-16CeO2/AC sample is about 10 nm,which was calculated by Eq. (1) according to the Debye-Scherrer formula[23]:

where,Kis the Scherrer constant of the order of unity(0.943),θhklis the angle of diffraction for (111) plane andβhklis the full width at half maximum (FWHM) excluding the instrumental broadening.
Fig.3 displays the structure and morphology of the synthesized catalyst samples. The synthesized catalyst samples were composed of irregular particles. The ACC sample consists of particles with an average size of about 50 nm in Fig.3(a). The V-8CeO2/AC sample shows microstructure with an average size of 25 nm in Fig.3(b).
3.2 Semiconductor properties of the catalyst samples
Fig.4 represents the UV-Vis diffuse reflectance spectra of the prepared catalysts in the range of 244-800 nm. It’s reported in the literature that CeO2has an excellent UV absorption property due to its wider optical bandgap value (Eg= 2.90 eV)[10]. Compared with CeO2semiconductor, all the V-doped CeO2catalyst samples caused red-shift of the UV-Vis spectra. Owing to V-doped CeO2semiconductor was coupled with narrow bandgap FeO semiconductor in catalyst carrier,which enhanced ability to absorb visible-light. The maximum absorption wavelength of ACC, V-CeO2/AC,V-8CeO2/AC, and V-16CeO2/AC samples are 564, 548,484, and 523 nm, the bandgap (Eg) values corresponds to 2.19, 2.26, 2.56, and 2.37 eV, respectively.
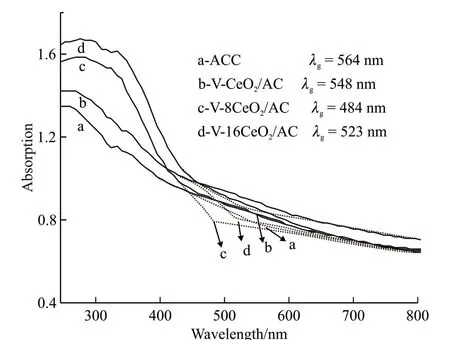
Fig.4 UV-Vis diffuse reflectance spectra of catalysts
Fig.5 displays the fluorescence emission spectra of the prepared samples. It can be observed that the peak intensity at 440-490 nm is different, which is attributed to the fluorescence emitted by the recombination of electrons and holes after excited by the bandgap.The fluorescence intensity of the supported samples was decreased, indicating that the rate of recombination of photogenerated electrons and holes decreased after loaded. In the catalyst system, thep-njunction has formed between V-doped CeO2active components and the FeO semiconductor in the carrier, which could prevent the recombination of electron-hole pairs.Therefore, the fluorescence intensity of the loaded samples would be inhibited. The fluorescence intensity of V-8CeO2/AC specimen was the lowest. However, with the further increase of the content of V-doped CeO2semiconductors, we found that the fluorescence intensity of the sample will increase slightly. The reason may be that the active component as a recombination center promotes the recombination of photogenerated electron-hole pairs.

Fig.5 Photoluminescence spectra of the catalysts
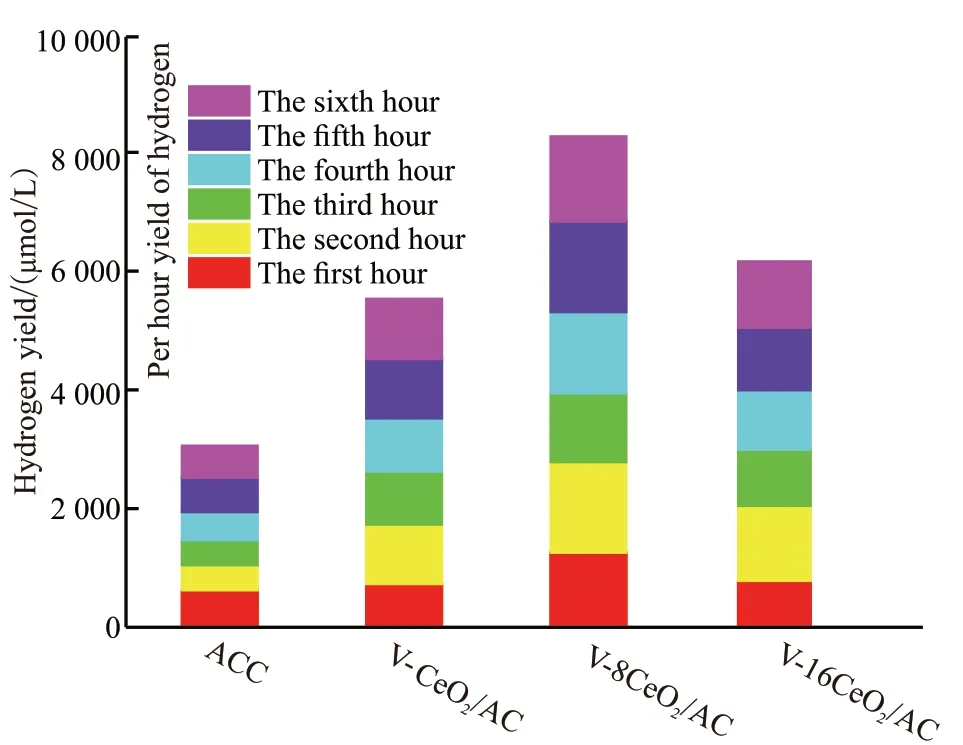
Fig.6 Activities of hydrogen production for the catalysts
The catalytic activity was evaluated by photocatalytic decomposition of water to produce hydrogen.Fig.6 displays the hydrogen production properties of the prepared samples. The results show that the hydrogen yield of the supported catalysts is higher than that of other samples, and the order of hydrogen yield is:V-8CeO2/AC > V-16CeO2/AC > V-CeO2/AC > ACC sample. V-8CeO2/AC sample exhibited the highest photocatalytic activity according to the rate of hydrogen production. Its hydrogen generation rate was 1 382 μmol/(g×h) and the highest hydrogen yield was up to 8 292 μmol/g under the irradiation xenon lamp for 6 h.The excellent performance of hydrogen production for V-8CeO2/AC specimen may be attributed to the interaction between the V-doped CeO2active components and FeO existed in catalyst carrier.

Fig.7 Schematic diagram of the photocatalytic reaction mechanism of V-8CeO2/AC sample
A schematic diagram of the photocatalytic hydrogen production reaction mechanism from water-splitting of catalyst proposed is shown in Fig.7. V belongs to the donor impurity and the V doping provides CeO2semiconductor particles with donor level near the conduction band. When the simulated solar irradiation energy is higher than the bandgap energy, the electrons in V-doped CeO2and FeO coupled semiconductors could absorb photon energy and transfer it from the valence band to conduction band. Photogenerated electrons rapidly transitions from conduction band of V-doped CeO2semiconductor to the conduction band of FeO semiconductor. The photo-electrons in the CSH matrix could combine with water molecules to form H2immediately.The mechanism of photocatalytic hydrogen production reaction by water-splitting under simulated sunlight irradiation can be described as follows in Eqs.(2)-(5):hydrogen is produced when water molecules are reduced by photogenerated electrons. At the same time,the sacrificial reagents (sodium sulfide nonahydrate or anhydrous sodium sulfate) are oxidized by photogenerated holes to form SO42-or S2O32-. Hence, the excellent activity of hydrogen production should probably be ascribed to the high separation efficiency of photogenerated electron-hole pairs:

4 Conclusions
A new V-doped CeO2-supported alkali-activated-steel-slag-based catalyst for hydrogen production by water-splitting was synthesized via co-impregnation method. XRD and FESEM results show that some amorphous aluminosilicate phases in steel slag react with alkali activator to form alkali-activated-steel-slag-based catalyst carrier with compact structure and excellent mechanical properties with an average particle size of about 50 nm. V-8CeO2/AC specimen exhibit the highest photocatalytic hydrogen production activity and hydrogen generation rate. It should probably be attributed to the coupled semiconductors formed by V-doped CeO2and FeO existed in catalyst carrier could promote the high efficiency separation of the photogenerated electron-hole pairs.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Comparative Case Study on Adhesion of Three Common Sizing Agents to Cotton and Polyester Yarns
- Effect of Outer Carbon Layer Thickness of Carboncovered N-doped Hollow Carbon Nanospheres on Its Electrocatalytic Performance
- Ceramification of Composites of MgO-Al2O3-SiO2/Boron Phenolic Resin with Different Calcine Time
- Natural Fresh Proteins Directed Hierarchically Porous Nitrogen-doped TiO2 as with High Performance as Photocatalyts and Electrode Materials
- Dynamic Adsorption of Toluene on Hierarchical Porous Carbons with Varying Pore Structure
- Self-propagating High-temperature Synthesis of Sm and Zr Co-doped Gd2Ti2O7 Pyrochlore Ceramics as Nuclear Waste Forms
