Effect of acupuncture on cerebral hematoma volume and HO-1 expression in rats with acute cerebral hemorrhage
2021-04-15QiuXinChenTingTingYuYuZhangPengLiuXinZhangYingKongLuWenZhu
Qiu-Xin Chen,Ting-Ting Yu,Yu Zhang,Peng Liu,Xin Zhang,Ying Kong,Lu-Wen Zhu*
1The First Affiliated Hospital of Heilongjiang University of Chinese Medicine,Harbin,China; 2The Second Affiliated Hospital of Heilongjiang University of Chinese Medicine,Harbin,China; 3Heilongjiang University of Chinese Medicine,Harbin,China;4Shenzhen Baoan Hospital of traditional Chinese Medicine,Guangzhou,China.
Abstract Objective:To explore the effect of acupuncture on the expression of heme oxygenase-1 in rats with acute cerebral hemorrhage. Methods: 108 Wistar male rats were randomly divided into sham operation group, model group, and acupuncture combined with model group (referred to as acupuncture group).Each group was divided into 3 subgroups according to 1d, 3d and 7d, with 6 rats in each subgroup.The rat model of cerebral hemorrhage was established by autologous blood injection.Acupuncture was given at Baihui (GU20)and Qubin (GB7).Separately,at the 1st,3rd and 7th day,modified neurological severity score was used to evaluate the neurological function of rats,HE staining was used to measure the volume of cerebral hematoma and western blot was used to detect the expression of heme oxygenase-1 protein in cerebral hematoma tissue.Results:Compared with the model group,at each time point, the modified neurological severity score of the acupuncture group was significantly reduced (P <0.01); at the two time points of 3rd and 7th day, the cerebral hematoma volume of the acupuncture group was significantly reduced (P <0.05) and the expression of heme oxygenase-1 protein in brain tissue was significantly increased (P <0.05). Conclusion: Acupuncture may promote the expression of heme oxygenase-1 protein, reduce the volume of hematoma and the score of neurological deficit in rats with intracerebral hemorrhage, improve the performance of neurological deficit,and play a role in brain protection.
Key words: Acupuncture, Baihui acupoint, Qubin acupoint, Cerebral hemorrhage, Heme oxygenase-1, Modified neurological severity score
Background
Intracerebral hemorrhage (ICH) refers to nontraumatic hemorrhage in the brain parenchyma.The mortality rate for early ICH is 49.4%; most patients alive have a poor prognosis with kinds of neurological sequelae [1,2].Hemoglobin entering the brain tissue will cause secondary damage, such as disrupting the blood-brain barrier, causing focal edema, and inducing neuroglial apoptosis.Heme, the degradation product of hemoglobin, generates oxygen free radicals and oxidative stress responses, which can cause neuronal death [3].Heme oxygenase-1 (HO-1), which is heme-degradation enzyme and rate-limiting enzyme,can catalyzes the degradation of heme to carbon monoxide, catalytic iron and bilirubin.It is enhanced after ICH[4,5].
Acupuncture can improve the neurologic impairment symptoms of rats with acute ICH, alleviate pathological and ultrastructure damage, and promote restoration of neurological function [6−8].Electroacupuncture can promote HO-1 expression in the serum of patients with acute cerebral infarction and can alleviate neuronal damage after ischemia [9].However, there are few studies on the use of acupuncture as an intervention for ICH.In this study,autologous blood injection was used to create a rat model of ICH, and subsequently, a needle was used to stimulate the Qubin acupoint(GB7)passed through the Baihui acupoint(GU20).The effects of acupuncture on neurological function scores, hematoma absorption,and HO-1 expression in a rat model of ICH were observed to examine the protective mechanisms of acupuncture on hematoma absorption.
Materials and methods
Experimental materials
Experimentalanimals.Healthy 8-week-old SPF-grade male Wistar rats weighing 300 ± 20 g were obtained from the Experimental Animals Center of Norman Bethune Health Science Center of Jilin University(Animal Permit:SCXK(Jilin)2013−004,Animal Ethics Approval: 2017061001).The animals were housed in artificially controlled conditions within separate cages (five rats per cage) in the same room at a temperature of 22 ± 3℃, relative humidity of 60 ±5%, and light/dark cycle of 12 h.Pertinent regulations in the “Guidelines on Care for Experimental Animals”provided by the Ministry of Science and Technology were complied with during the study.
Primary reagents and equipments.HO-1 polyclonal antibody (WL02400) and internal reference antibody β-actin (WL01845) were purchased from Wanleibio Co.,Ltd (China).A stereotaxic instrument(ST-5ND-C)was purchased from Chengdu Instrument Factory(China).An electrophoresis apparatus (DYY-7C) and a dual-vertical protein electrophoresis tank(DYCZ-24DN) were purchased from Beijing Liuyi Biotechnology Co.,Ltd(China).
Experimental methods
Creation of models.The ICH rat model was created according to the literature [10].A solution of 1%sodium pentobarbital (50 mg/kg) was administered by intraperitoneal injection to the rats for anesthesia, and the rats were fixed on the stereotaxic instrument in a prone position.The rats’ skin was disinfected, and a midline incision was made.The periosteum was dissected using a periosteal dissector to expose the frontal suture and coronal suture.A dental drill was used to create a 1.00-mm hole 3.5 mm from the right and 0.2 mm from the back of the frontal suture(Bregma point)to reach the dural surface.Alcohol was used for disinfecting the rats’ tails, followed by excision of the tails 3 cm from the tip.A microsyringe was used to collect 50 µl blood.The microsyringe was then fixed on the stereotaxic instrument and was inserted at a depth of 6 mm along the hole,followed by injection of 50 µl nonheparinized blood into the caudate-putamen at a rate of 20 µl/minute.The needle was left in the site for 5 minutes before being slowly withdrawn.During this period, alcohol damped cotton balls were used to bandage the tail stump of the rats.Gentamicin was sprayed locally following surgery,and dental cement was used to seal the skull wound.The scalp was sutured, and iodophenol was used for local skin disinfection.Rats from the sham group underwent the same surgical procedures as the model group,albeit without blood injection.
The Bederson scale [11] was used for screening successfully created animal models after recovery from anesthesia.Rats with a score of 1–3 points were included in the experiment.The specific method of scoring was as follows: 0 point, no observable deficit;1 point, forepaw flexion (i.e., positive tail suspension test);2 points,decreased resistance to lateral push(i.e.,positive lateral push test) accompanied by forepaw flexion and inability to perform circling behavior; 3 points, decreased resistance to lateral push (i.e.,positive lateral push test) accompanied by forepaw flexion and presence of circling behavior.
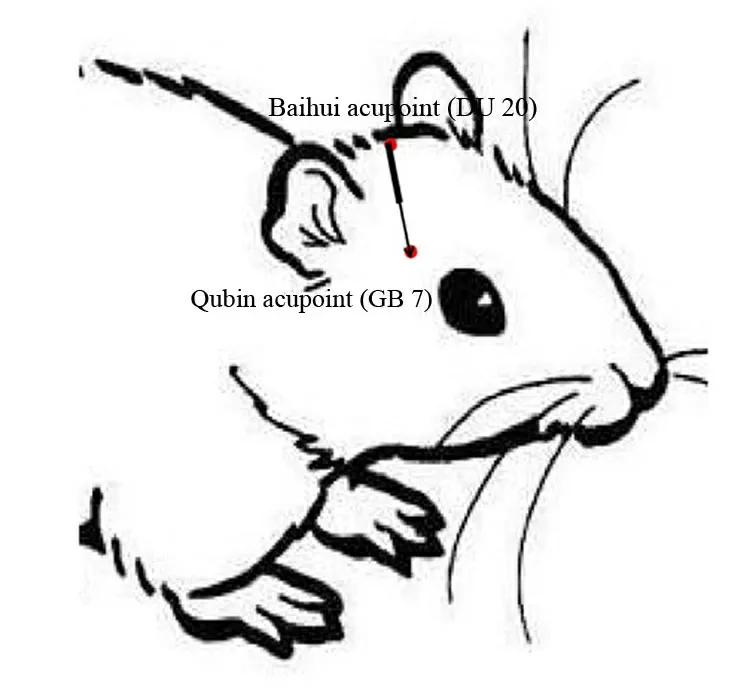
Figure 1 The Baihui(DU20)-penetrating-Qubin(GB7)acupuncture treatment method[12].
Grouping and intervention protocol.In total, 108 rats were randomized into sham, model, and acupuncture groups (n = 36, each).No intervention was performed on the sham or model groups.Acupuncture was performed 12 h after surgery in the acupuncture group.Rats were divided into three subgroups on days 1,3,and 7.
“Experimental Acupuncture” [13] was used as a reference for the acupuncture method to identify the GU20 and the GB7 (Figure 1) the affected side.The needle was passed from the GU20 to the GB7.The needle was 0.30 mm × 25 mm in size, inserted to a depth of 20 mm, and left in place for 30 minutes.The acupuncture procedure was performed once per day(Figure 2).
Observation markers and test methods
Neurological deficit score.Twelve rats were randomly selected from each group on days 1,3,and 7 after surgery.The modified neurological severity score(mNSS) [14, 15] was used to measure movement,sensation, and reflex functions in the rats.The score range was 0–18 points, with 1–6 points, 7–12 points,and 13–18 points representing mild, moderate, and severe damage,respectively.
Calculation of cerebral hematoma volume.Six rats were randomly selected from each group on days 1, 3,and 7 after surgery.After the rats were anesthetized,their brains were extracted and fixed.The longest transverse and longitudinal diameters of the largest hematoma slice perpendicular to each other were measured, followed by H&E staining.The corresponding hematoma volume was calculated based on the Coniglobus formula: hematoma volume = π/6 ×longest transverse diameter of the largest hematoma slice (mm) × longitudinal diameter (mm) × number of hematoma slices×slice thickness(mm).
Quantitation of HO-1 protein in brain tissues using western blot.Six rats were randomly selected from each group on days 1,3,and 7 after surgery.A solution of 2% sodium pentobarbital (0.4–0.5 ml/100 g) was injected intraperitoneally for deep anesthesia.The rats were rapidly decapitated on ice, and their brains were extracted.The olfactory bulb and brain tissues 4 mm from the front of the frontal pole were removed.Brain tissues at the injection site in the cerebrum’s right hemisphere were collected at the coronal plane and stored at −80℃.
Processed protein sample (30 µl) was electrophoresed on a 12% sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidene fluoride membrane.Standard protein markers were used as controls.The membranes were blocked with 5% skimmed milk, and HO-1 antibody (1:500) was added to the corresponding bands.The membranes were incubated at 4℃ overnight.After color development was completed, the nitrocellulose membranes were transferred to distilled water for terminating color development,dried using filter paper,and incubated in the dark for 20 minutes before scanning.Gel-Pro Analyzer software was used to analyze the optical density of the target band.
Statistical methods
SPSS22.0 was used for statistical processing.The final data are expressed as mean±standard deviation(¯X ±S).One-way ANOVA was used for a comparison between the groups at the different time points,and the LSD t-test was used for a pairwise comparison.The significance level was α = 0.05.A difference ofP<0.05 was considered statistically significant.
Results
mNSS scores
There was no significant neurological impairment in rats from the sham group after surgery.Rats from the model group exhibited symptoms of neurological impairment on day 1 after surgery.The symptoms of neurological defect were the most severe on the third day.Neurological deficit scores were significantly decreased on the third days and the seventh day after surgery in the acupuncture group compared with the model group(P<0.05)(Figure 3).
Figure 2 The flowchart of intervention protocol

Figure 3 Neurological impairment scores.*P <0.01 compared with the model group.
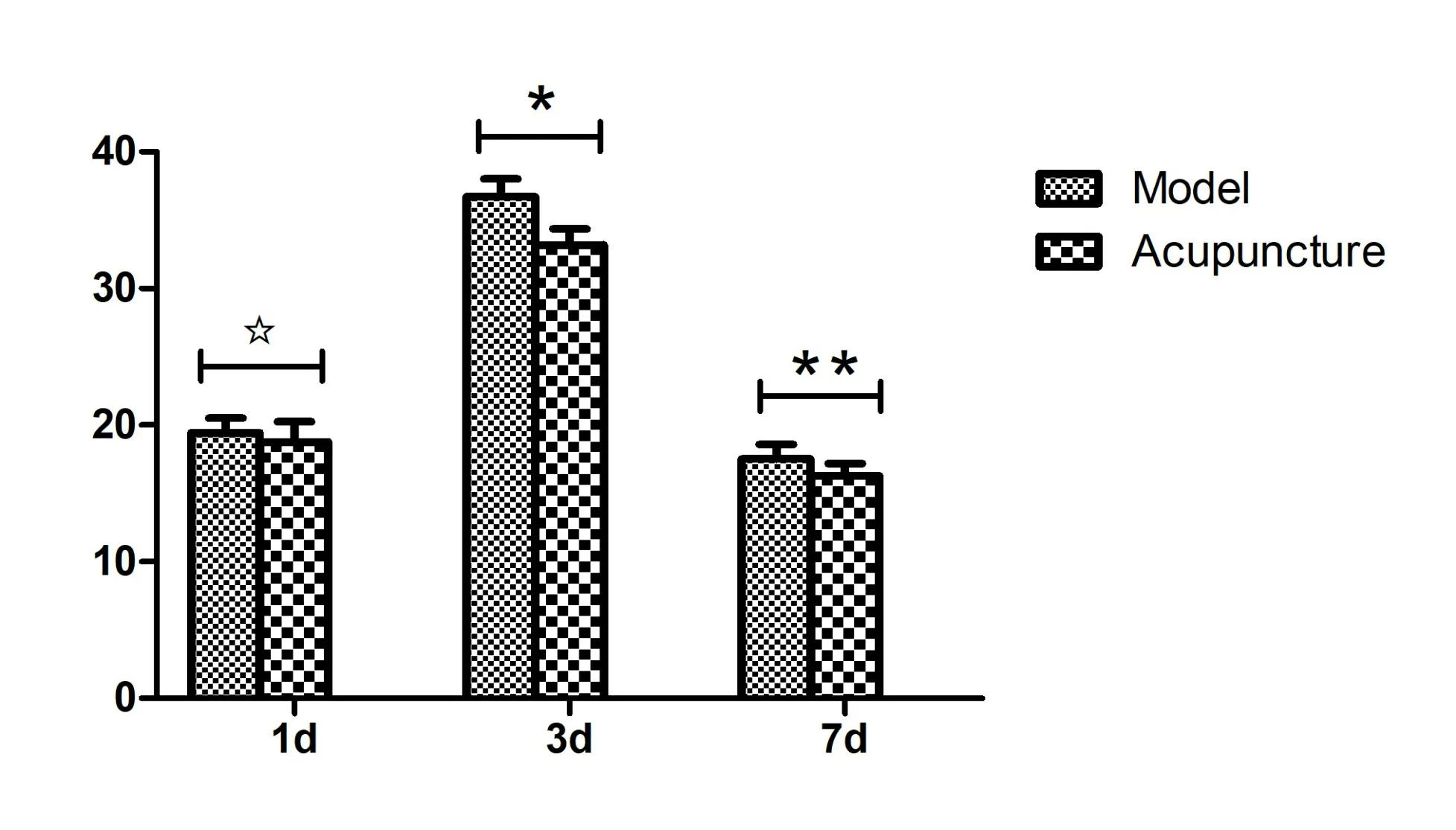
Figure 4 Cerebral hematoma volumes.*P <0.01,**P <0.05 compared with the model group.
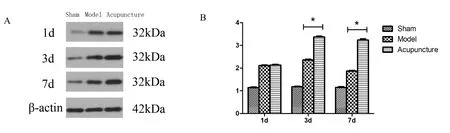
Figure 5 HO-1 protein expression level in the brain tissues.
Cerebral hematoma volumes
No cerebral hematomas occurred in the sham group.Significant cerebral hematomas were observed in the model group, wherein the cerebral hematoma volume was the highest on day 3 after surgery.Additionally,cerebral hematoma volume was significantly decreased in the acupuncture group compared with the model group on the third day and the seventh day (P<0.01 andP<0.05,respectively)(Figure 4).
Comparison of HO-1 protein expression
The brain tissues of rats from the sham group were weakly positive for HO-1 protein.HO-1 protein expression was increased in the brain tissues from the model group and reached a maximum on the 3rdday.HO-1 protein expression was significantly increased in the brain tissues from the acupuncture group the 3rdday and the 7thday compared with the model group (P<0.01 andP<0.05,respectively)(Figure 5).
Discussion
Secondary brain injury occurred after ICH for the hemoglobin toxicity, thrombin release, and reduced local blood flow in the brain tissues, which increases the mortality rate associated with ICH [16−18].HO-1 is the first rate-limiting enzyme in the heme metabolism pathway, which expresses in neurons,neuroglial cells, microglial cells, and astrocytes [19]Microglial HO-1 plays a vital role in alleviating neuronal death and promoting erythrocyte phagocytosis [20].The expression of HO-1 in brain tissues surrounding a hematoma is high after ICH,which increases with hemorrhage duration and negatively correlates with inflammation in tissues around the hematoma.HO-1 participates in cerebral edema formation, confers neuroprotective effects through hemoglobin metabolism, and reduces oxidative stress and inflammatory responses[21−23].
Acupuncture has a good safety profile and has been proven efficacious for ICH [24−26].In this study,ICH was treated by passing a needle from the GU20 to the GB7.The line connecting these two acupoints is known as the parietotemporal posterior diagonal line.It transverses the parietal, frontal, and temporal lobes,the Governor Vessel Meridian, the end point of the Bladder Meridian of Foot-Taiyang,and the Gallbladder Meridian of Foot-Shaoyang.The GU20 belongs to the Governor Vessel Meridian and is a site where various meridian Qi aggregate.This acupoint is connected to the Yin and Yang meridians, which could increase or decrease Qi.Therefore, GU20 is the acupoint for stroke treatment.The GB7 acupoint belongs to the Gallbladder Meridian of Foot-Shaoyang, where the Foot-Taiyang and Foot-Shaoyang meet.It could assist Governor Vessel meridian to regulate Qi and blood,thereby engendering therapeutic effects for the treatment of paralysis.
In this study,autologous blood injection was used to create a rat model of ICH.The use of this method ensured that the proportion of hematoma in the rat brain was consistent and that the hemorrhage site was precisely located.This method has the advantages of low infection and mortality rates for simulation of ICH in clinical practice.The mNSS scoring method was used for behavioral evaluation of neurological function for movement, sensation, and reflexes in the rats.This study was designed with the goal of improving neurological deficit after acute ICH using acupuncture.We selected 1, 3, and 7 days after surgery to observe the corresponding markers.Studies have also reported that behavior improvements occurred 14 days after acupuncture.Rats from the model group exhibited varying degrees of neurological impairment at the different time points, particularly in the movement and sensation functions, which had more pronounced presentations.The most severe neurological impairment were on the 3rdday after surgery, with the scores being highest on this day.The symptoms of neurological impairment were alleviated on the 7thday after surgery.The neurological deficit scores of the acupuncture group on the 3rdand 7thdays were significantly decreased compared with the model group, indicating that acupuncture can reduce neurological impairment.On the 3rdand 7thdays, the volume of the cerebral hematoma was significantly decreased in the acupuncture group compared with the model group,suggesting that acupuncture can decrease volume of the cerebral hematoma in rats and reduce compression of the surrounding brain tissues caused by the hematoma.HO-1 protein expression was increased in the model group.HO-1 protein expression in the acupuncture group was higher than that in the model group on the 3rdand 7thdays, indicating that acupuncture can promote HO-1 expression.
In summary,acupuncture of GB7 through the GU20 can promote HO-1 expression, resulting in protective effects of decreasing hematoma volume, alleviating hematoma compression, decreasing neurological deficit scores, and improving neurological deficit symptoms in rats with acute ICH.
