TLR3活化对人脂肪间充质干细胞成骨分化的影响
2021-04-12金兵男江鑫铭李霄霞赵春华


[摘要]"目的"探讨Poly(I:C)活化人脂肪间充质干细胞(hAMSCs)Toll样受体3(TLR3)后对hAMSCs成骨分化的影响。方法"胶原酶消化法分离提取hAMSCs,对其细胞形态、免疫学表型和成骨分化能力进行鉴定。将原代分离培养的hAMSCs随机分为对照组和TLR3组,对照组不做处理,TLR3组用含有20 mg/L Poly(I:C)的正常培养基处理6 h活化TLR3。CCK8法检测两组hAMSCs增殖能力变化,Real-time PCR方法检测hAMSCs成骨标志基因runt相关转录因子2(RUNX2)和骨钙素(OCN)mRNA表达,Western Blot方法检测RUNX2和OCN蛋白及NF-κB通路相关蛋白p-P65、P65表达。对照组和TLR3组均用间充质干细胞成骨诱导培养基诱导分化,12 d后用茜素红染色法检测两组hAMSCs成骨诱导后钙质沉积情况。结果"对照组和TLR3组hAMSCs的增殖能力差异无显著性(Pgt;0.05)。TLR3组hAMSCs成骨标志基因RUNX2及OCN的mRNA和蛋白表达均高于对照组(t=2.98~36.36,Plt;0.05),NF-κB通路相关蛋白P65及p-P65表达也高于对照组(t=3.52、13.85,Plt;0.05)。茜素红染色结果显示,成骨诱导12 d后TLR3组钙质沉积程度高于对照组。结论"TLR3活化能够通过激活NF-κB通路促进hAMSCs成骨分化。
[关键词]"间质干细胞;脂肪组织;Toll样受体3;成骨分化
[中图分类号]"R329.21
[文献标志码]"A
[文章编号]"2096-5532(2021)04-0475-06
间充质干细胞(MSCs)是目前国内外研究最多的一类成体干细胞[1-4],其来源广泛,在脂肪、骨髓、脐带、胎盘等多种成体组织中均广泛存在[5]。其中,人脂肪来源MSCs(hAMSCs)已成为再生医学领域中的理想种子细胞[6-10]。然而,在应用MSCs移植治疗骨质疏松等疾病的过程中常有炎症或细菌感染等情况发生,严重影响了MSCs移植的存活率和治疗效果。因此,寻找MSCs预处理方式,提高MSCs移植存活率,增强体内治疗效果,对MSCs的临床应用具有重要意义[11-13]。Toll样受体(TLRs)作为一类在固有免疫和适应性免疫中具有重要作用的模式识别受体,能够识别大量病原相关分子,介导机体免疫反应,维持机体的平衡状态,MSCs中也有部分表达TLRs[14]。已有研究显示,表达TLR3的MSCs在TLR3被Poly(I:C)活化后表现出抗炎作用,TLRs活化能够对MSCs的成骨分化产生影响[15]。使用Poly(I:C)活化MSCs的TLR3可以作为一种重要的MSCs预处理方法。本研究探讨TLR3活化对hAMSCs增殖及成骨分化的影响,为hAMSCs在骨质疏松等疾病治疗中应用提供理论依据。
1"材料与方法
1.1"细胞和试剂
实验所用hAMSCs为吸脂术病人废弃的脂肪组织原代分离扩增获得; DMEM高糖、DME/F12培养基购于美国Hyclone公司;胎牛血清购于Excell公司;青霉素和链霉素购于新赛美公司;胶原酶P购于美国Roche公司;碱性磷酸酶试剂盒、CCK8试剂盒、反转录试剂盒、SYBR Green Master Mix购于上海翊圣公司;Poly(I:C)、油红O、茜素红购于美国Sigma公司;SDS-PAGE凝胶试剂盒、磷酸酶抑制剂购于上海雅酶公司;D-hank’s液、PBS、组织裂解液购于北京索莱宝公司;小鼠抗人FITC-CD14、FITC-CD34、FITC-CD45、PE-CD73、FITC-CD90、PE-CD105、FITC-HLA-DR购于美国BD公司。
1.2"hAMSCs的分离培养
取成人吸脂术采集的脂肪组织,D-hank’s液清洗去除血细胞和麻醉药物。加入适量2 g/L胶原酶P,37 ℃恒温摇床消化30 min,100目筛网滤去未消化组织。加入足量D-hank’s液,1 500 r/min离心10 min,弃上清,重复2次。重悬细胞沉淀,接种于hAMSCs培养基中,于含CO2培养箱内37 ℃恒温培养,每3 d换液1次。当细胞生长达到80%融合时,进行传代培养。
1.3"流式细胞术检测免疫学表型
胰蛋白酶消化收集细胞,PBS清洗、重悬,加入相应直标抗体4 ℃孵育30 min,PBS清洗2次,重悬,流式细胞仪上检测MSCs免疫学表型。
1.4"实验分组及细胞增殖检测
取第6代hAMSCs以每孔1×104的密度接种于96孔板中,随机分为两组,对照组不作处理,TLR3组加入20 mg/L Poly(I:C)工作液处理6 h活化TLR3。分别在活化后的0、24和48 h时每孔加入 CCK8工作液10 μL,37 ℃孵育90 min,酶标仪检测450 nm波长处的吸光度(A)值,以其表示hAMSCs增殖能力。
1.5"hAMSCs体外成骨诱导分化及鉴定
取第6代hAMSCs以5×103/cm2的密度接种于24孔板中,待其生长至80%融合时加入成骨诱导培养基(含体积分数0.10"FBS、 2×10-4mol/L抗坏血酸和10 mmol/L β-甘油磷酸钠的DMEM高糖培养基)诱导成骨分化,3 d更换1次新鲜成骨诱导培养基。①碱性磷酸酶染色:成骨诱导3 d后,PBS冲洗2次,40 g/L多聚甲醛固定10 min,加入碱性磷酸酶染色工作液,37 ℃孵育20 min,PBS冲洗3次,光镜下观察对照组和TLR3组hAMSCs碱性磷酸酶活性变化情况。②茜素红染色:成骨诱导12 d后,PBS冲洗2次,用40 g/L多聚甲醛室温固定10 min,1 g/L茜素红染色工作液室温染色30 min;PBS冲洗3次后,在光镜下观察对照组和TLR3组hAMSCs钙质沉积情况。
1.6"hAMSCs体外成脂诱导分化及鉴定
取第6代hAMSCs,以5×103/cm2的密度接种于24孔板中,待其生长至90%融合时加入成脂诱导培养基(DMEM高糖培养基中含有体积分数0.10 FBS、1×10-6mol/L地塞米松、50 mg/L抗坏血酸和100 mg/L IBMX)诱导成脂分化,每3 d更换1次新鲜成脂诱导培养基。成脂诱导12 d后,PBS冲洗2次,40 g/L多聚甲醛室温固定10 min,油红O染色工作液室温染色30 min;PBS冲洗3次,光镜下观察hAMSCs脂滴形成情况。
1.7"Real-time PCR检测成骨标志基因runt相关转录因子2(RUNX2)和骨钙素(OCN)mRNA表达
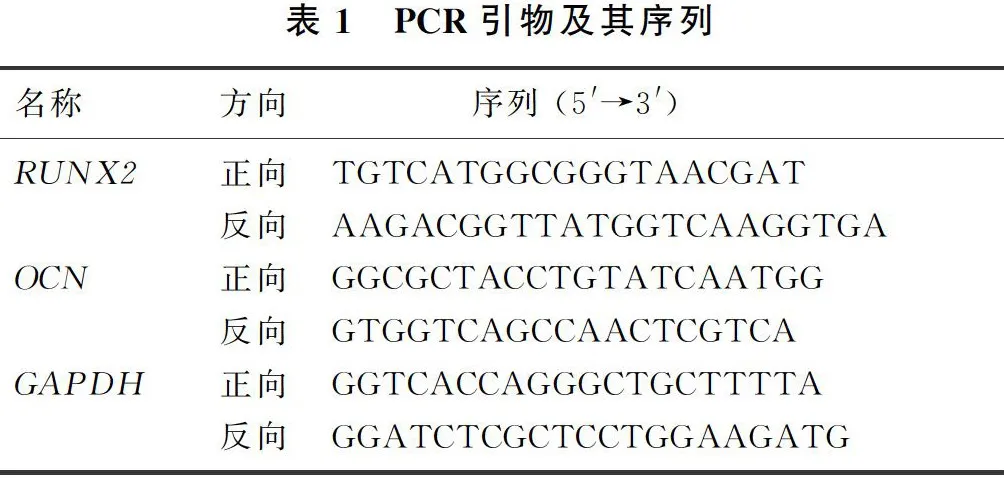
取第6代hAMSCs以5×103/cm2的密度接种于6孔板中,随机分为TLR3组和对照组,对照组不做处理,TLR3组用含有20 mg/L Poly(I:C)的正常培养基处理6 h,按照总RNA提取试剂说明书提取两组样本的细胞总RNA,应用反转录试剂盒进行反转录,反应条件:25 ℃、5 min,42 ℃、30 min,85 ℃、5 min。按照荧光定量试剂盒配制反应体系,反应条件:预变性95 ℃、30 s,变性95 ℃、10 s,退火/延伸60 ℃、30 s,共40个循环。以GAPDH为内参基因,2-ΔΔCt法计算hAMSCs成骨标志基因RUNX2和OCN mRNA表达情况。所用基因引物序列见表1。每个样本设置3个复孔,实验重复3次。
1.8"Western Blot方法检测相关蛋白表达
细胞按1.7方法分组及处理后,加入RIPA裂解液提取细胞总蛋白,蛋白样品应用SDS-PAGE进行电泳分离,然后转蛋白至硝酸纤维素膜上;50 g/L BSA室温封闭90 min,添加一抗(β-actin、GAPDH、RUNX2、OCN、P65、p-P65,1∶1 000稀释)4 ℃孵育过夜,添加羊抗兔二抗(1∶5 000稀释)室温孵育60 min,"使用超敏ECL发光液显影,Image J软件分析条带灰度值,以GAPDH或β-actin作为对照,计算目的蛋白RUNX2、OCN、P65和p-P65相对表达量。实验重复3次。
1.9"统计学分析
应用Graph Pad Prism 5软件进行统计学分析。计量资料数据以x2±s表示,两组间比较采用t检验。以Plt;0.05为有统计学意义。
2"结"果
2.1"hAMSCs形态、免疫学表型及成脂成骨分化能力鉴定
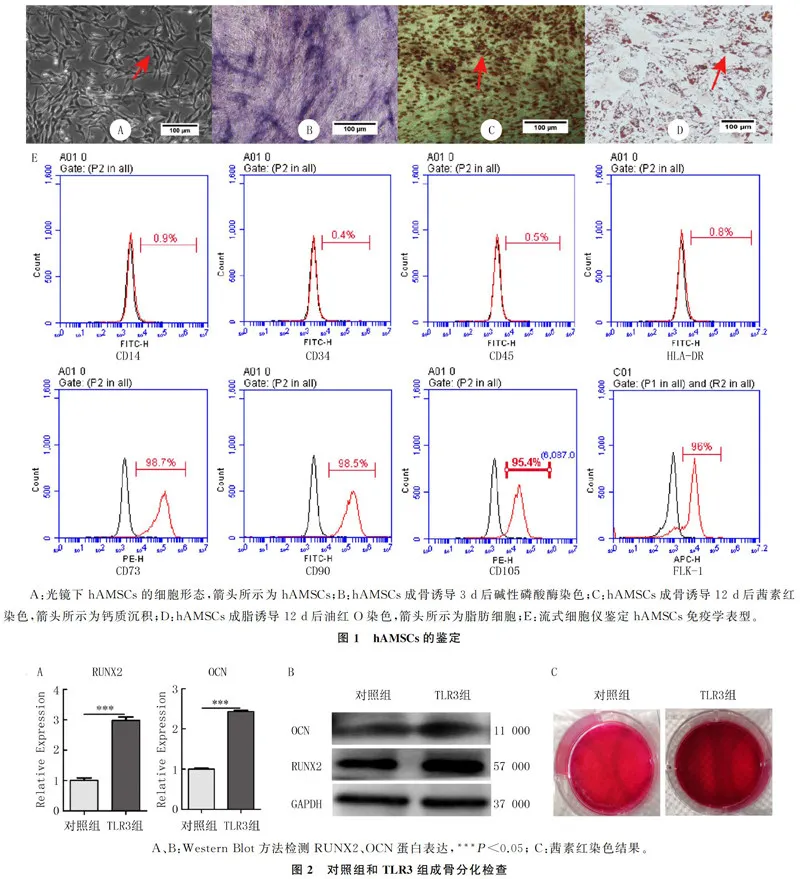
在光镜下观察,原代分离培养后的hAMSCs呈长梭形,旋涡状分布,贴壁生长(图1A);碱性磷酸酶染色后细胞可见蓝色沉淀,表明hAMSCs开始向成骨细胞分化(图1B);茜素红染色细胞可见染成红色的钙质沉淀,表明hAMSCs成骨诱导12 d后分化为成骨细胞,分泌了大量钙质沉积(图1C);油红O染色后细胞中可见红色脂滴,表明hAMSCs成脂诱导12 d后分化为脂肪细胞(图1D)。流式细胞术检测hAMSCs的免疫学表型结果显示,CD14阳性率为0.9%,CD34阳性率0.4%,CD45阳性率0.5%,HLA-DR阳性率0.8%,均lt;5%;CD73阳性率为98.7%,CD90阳性率98.5%,CD105阳性率95.4%,FLK-1阳性率96.0%,均gt;95%。符合国际细胞学会对于MSCs的定义(图1E)。证明从人脂肪组织中原代分离的细胞为hAMSCs。
2.2"各组hAMSCs增殖能力比较
CCK8法检测结果显示,对照组与TLR3组比较,hAMSCs活化后0、24和48 h时增殖能力差异无显著性(Pgt;0.05)。见表2。
2.3"各组RUNX2和OCN mRNA及蛋白表达的比较
Real-time PCR检测显示,TLR3组RUNX2和OCN的mRNA表达水平均较对照组显著增加(t=14.39、36.36,Plt;0.01)。Western Blot检测显示,TLR3组RUNX2和OCN蛋白表达均高于对照组(t=3.46、2.98,Plt;0.05)。见图2A、B和表3。茜素红染色结果显示,TLR3组红色钙质沉积明显多于对照组(图2C)。表明TLR3经Poly(I:C)活化后能够促进hAMSCs成骨分化,加快钙质沉积。
2.4"各组hAMSCs中NF-κB通路相关蛋白P65及p-P65表达比较
Western Blot结果显示,NF-κB通路相关蛋白p-P65和P65表达较对照组明显增加,差异有统计学意义(t=3.52、13.85,Plt;0.05)。见图3、表4。
3"讨"论
MSCs作为一类具有自我更新、多谱系分化、低免疫原性和免疫调节能力等多项优点的成体多能干细胞,目前在干细胞领域得到广泛的研究[15]。由于MSCs具有以上多项优点,目前MSCs移植已成为骨质疏松等疾病最佳治疗方法。但是在MSCs移植中经常有炎症或感染情况发生,降低了MSCs移植的成功率与治疗效果,而通过活化MSCs的TLR3介导hAMSCs免疫反应能够抑制炎症感染,提高MSCs移植的成功率和骨质疏松等疾病的治疗效果。但是活化的TLR3是否会影响hAMSCs成骨分化,目前没有定论。本实验结果表明,hAMSCs活化TLR3后,成骨标志基因RUNX2和OCN的mRNA和蛋白表达均明显增加,同时成骨诱导分化后钙质沉积程度更高,表明hAMSCs经Poly(I:C)处理活化TLR3后可能促进hAMSCs成骨分化。
MSCs在成骨分化过程中受到多种信号通路的调控,包括Wnt信号通路、Notch信号通路、BMP/TGF-β信号通路、MAPK信号通路、hedghog信号通路、FGF信号通路、PTH信号通路和IGF-1信号通路等[16-19],不同信号通路通过调控β-catenin、同源核蛋白MSX2和含有PDZ识别模体的转录辅助激活因子TAZ等因子的表达,上调成骨分化标志基因RUNX2和其下游基因OSX(osterix)的表达,提高碱性磷酸酶活性,调控成骨细胞特异性细胞外基质蛋白OCN表达和钙离子沉积,促进MSCs向成骨细胞分化[20-21]。大量的研究结果证明,许多因素都能够通过不同信号通路影响MSCs的成骨分化[22-28],其中TLRs活化对MSCs的成骨分化也有影响。例如使用LPS持续刺激骨髓来源MSCs活化TLR4后能够通过Wnt通路促进其增殖和成骨分化[29]。TLR2活化后也能够显著增强骨髓来源MSCs的成骨分化[30]。TLR9活化后能够促进脐带来源MSCs成骨分化而不影响其表型[31]。本实验结果显示,Poly(I:C)活化hAMSCs的TLR3后,NF-κB通路相关蛋白P65和p-P65表达明显增加,表明hAMSCs的TLR3活化后可能通过激活NF-κB通路来上调成骨标志基因RUNX2和OCN的表达,促进hAMSCs成骨分化。但是具体作用机制仍不明确,需要进一步探讨。
综上所述,Poly(I:C)活化hAMSCs的TLR3后,能够通过激活NF-κB通路上调hAMSCs成骨标志基因RUNX2和OCN mRNA和蛋白表达,加快钙质沉积,促进hAMSCs成骨分化。因此,活化hAMSCs的TLR3不仅能够抑制炎症,提高MSCs的移植存活率,还能够促进MSCs的成骨分化,加快钙质沉积,有利于增强骨质疏松病人的骨修复能力,为一种可行的MSC预处理方式。但是活化TLR3后促进MSCs成骨分化涉及的基因及信号通路需要进一步研究[32-33]。本文研究结果可为hAMSCs在骨质疏松等老年退行性疾病中的应用提供了一定的研究基础。
[参考文献]
[1]FRIEDENSTEIN A J,"PETRAKOVA K V,"KUROLESOVA A I,"et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues[J]."Transplantation,1968,6(2):230-247.
[2]GERSON S L. Mesenchymal stem cells: no longer second class marrow citizens[J]."Nature Medicine,"1999,5(3):262-264.
[3]SERGEANT E,"BUYSSE M,"DEVOS T,"et al. Multipotent mesenchymal stromal cells in kidney transplant recipients: the next big thing[J]? Blood Reviews,"2020:100718.
[4]GARCA-SNCHEZ D,"FERNNDEZ D,"RODRGUEZ-REY J C,"et al. Enhancing survival,"engraftment,"and osteogenic potential of mesenchymal stem cells[J]."World Journal of Stem Cells,"2019,11(10):748-763.
[5]PELEKANOS R A,"SARDESAI V S,"FUTREGA K,"et al. Isolation and expansion of mesenchymal stem/stromal cells derived from human placenta tissue[J]."Journal of Visualized Experiments: JoVE,"2016(112):54204.
[6]ZUK P A,"ZHU M,"MIZUNO H,"et al. Multilineage cells from human adipose tissue:implications for cell-based therapies[J]."Tissue Engineering,"2001,7(2):211-228.
[7]FENG N H,"HAN Q,"LI J,"et al. Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation[J]."Stem Cells and Development,"2014,23(5):515-529.
[8]LI J,"ZHU L,"QU X B,"et al. Stepwise differentiation of human adipose-derived mesenchymal stem cells toward definitive endoderm and pancreatic progenitor cells by mimicking pan-
creatic development in vivo[J]."Stem Cells and Development,"2013,22(10):1576-1587.
[9]WANG X,"AO J,"LU H P,"et al. Osteoimmune modulation and guided osteogenesis promoted by barrier membranes incorporated with S-Nitrosoglutathione (GSNO) and mesenchymal stem cell-derived exosomes[J]."International Journal of Nanomedicine,"2020,15:3483-3496.
[10]LEE D J,"KWON J,"CURRENT L,"et al. Osteogenic potential of mesenchymal stem cells from rat mandible to regenerate critical sized calvarial defect[J]."Journal of Tissue Enginee-ring,"2019,10:2041731419830427.
[11]HUANG Y,"TAN F B,"ZHUO Y,"et al. Hypoxia-preconditioned olfactory mucosa mesenchymal stem cells abolish cerebral ischemia/reperfusion-induced pyroptosis and apoptotic death of microglial cells by activating HIF-1α[J]."Aging,"2020,12(11):10931-10950.
[12]MORA-BOZA A,"GARCA-FERNNDEZ L,"BARBOSA F A,"et al. Glycerylphytate crosslinker as a potential osteoinductor of chitosan-based systems for guided bone regeneration[J]."Carbohydrate Polymers,"2020,241:116269.
[13]DING X L,"HUANG Y,"LI X M,"et al. Three-dimensional silk fibroin scaffolds incorporated with graphene for bone regeneration[J]."Journal of Biomedical Materials Research Part A,"2020:1-9.
[14]RAICEVIC G,"ROUAS R,"NAJAR M,"et al. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells[J]."Human Immunology,"2010,71(3):235-244.
[15]WU L X,"XIANG S Q,"HU X H,"et al. Prostate-specific anti-gen modulates the osteogenic differentiation of MSCs via the[CM(26*2]cadherin 11-Akt axis[J]."Clinical and Translational Medicine,2020,10(1):363-373.
[16]WANG J C,"LIU S Z,"LI J Y,"et al. Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells[J]."Stem Cell Research amp; Therapy,"2019,10(1):197.
[17]YANG M S,"LIU H X,"WANG Y H,"et al. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression[J]."Connective Tissue Research,"2019,60(6):583-596.
[18]JIANG T Y,"XIA C,"CHEN X T,"et al. Melatonin promotes the BMP9-induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/β-catenin signalling pathway[J]."Stem Cell Research amp; Therapy,"2019,10(1):408.
[19]ZHAO X E,"YANG Z S,"ZHANG H,"et al. Resveratrol promotes osteogenic differentiation of canine bone marrow mesenchymal stem cells through wnt/beta-catenin signaling pathway[J]."Cellular Reprogramming,"2018,20(6):371-381.
[20]ZHANG L T,"LIU R M,"LUO Y,"et al. Hyaluronic acid promotes osteogenic differentiation of human amniotic mesenchymal stem cells via the TGF-β/Smad signalling pathway[J]."Life Sciences,"2019,232:116669.
[21]XIA P,"GU R,"ZHANG W,"et al. MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/β-Catenin signaling pathway via downregulating Myd88[J]."Journal of Cellular Physiology,"2019,234(12):22675-22686.
[22]LEE J,"CHA H,"PARK T H,"et al."Enhanced osteogenic differentiation of human mesenchymal stem cells by direct delivery of Cbfβ protein[J]."Biotechnology and Bioengineering,"2020:1-14.
[23]MORTADA I,"MORTADA R. Epigenetic changes in mesenchymal stem cells differentiation[J]."European Journal of
Medical Genetics,"2018,61(2):114-118.
[24]ZHANG Q H,"CHANG B,"ZHENG G Z,"et al. Quercetin stimulates osteogenic differentiation of bone marrow stromal cells through miRNA-206/connexin 43 pathway[J]."American Journal of Translational Research,"2020,12(5):2062-2070.
[25]LIAO J Y,"XIAO H Z,"DAI G M,"et al. Recombinant adenovirus (AdEasy system) mediated exogenous expression of long non-coding RNA H19 (lncRNA H19) biphasic regulating osteogenic differentiation of mesenchymal stem cells (MSCs)[J]."American Journal of Translational Research,"2020,12(5):1700-1713.
[26]DA W,"TAO L,"WEN K C,"et al. Protective role of melatonin against postmenopausal bone loss via enhancement of citrate secretion from osteoblasts[J]."Frontiers in Pharmacology,"2020,11:667.
[27]KIM D H,"KIM D H,"HECK B E,"et al. A natural supplement formula reduces anti-oxidative stress and enhances osteo-chondrogenic differentiation potential in mesenchymal stem cells[J]."Journal of Clinical Biochemistry and Nutrition,"2020,66(3):206-212.
[28]LI D W,"HE J,"HE F L,"et al. Silk fibroin/chitosan thin film promotes osteogenic and adipogenic differentiation of rat bone marrow-derived mesenchymal stem cells[J]."Journal of Biomaterials Applications,"2018,32(9):1164-1173.
[29]HE X Q,"WANG H,"JIN T,"et al. TLR4 activation promotes bone marrow MSC proliferation and osteogenic differentiation via Wnt3a and Wnt5a signaling[J]."PLoS One,"2016,11(3):e0149876.
[30]ZHOU Q,"GU X Y,"DONG J C,"et al. The use of TLR2 mo-
dified BMSCs for enhanced bone regeneration in the inflammatory micro-environment[J]."Artificial Cells,"Nanomedicine,"and Biotechnology,"2019,47(1):3329-3337.
[31]YANG Y F,"WANG Y W,"LI L,"et al. Tolllike receptor 9 agonist stimulation enables osteogenic differentiation without altering the immune status of umbilical cord mesenchymal stem cells[J]."Molecular Medicine Reports,"2015,12(6):8077-8084.
[32]WU L,"SONG J,"XUE J C,"et al. MircoRNA-143-3p regulating ARL6 is involved in the cadmium-induced inhibition of osteogenic differentiation in human bone marrow mesenchymal stem cells[J]."Toxicology Letters,"2020,331:159-166.
[33]董晨露,刘笑涵,吴琳. 骨髓间充质干细胞成骨分化的研究与进展[J]."中国骨伤,"2019,32(3):288-292.
(本文编辑"黄建乡)