Friable Callus Induction of Hedera nepalensis var. sinensis
2021-04-08DingfanXUYanjunLIUYawenLIUJunxuanHUANGChunxiaWUJiankeLI
Dingfan XU, Yanjun LIU, Yawen LIU, Junxuan HUANG, Chunxia WU, Jianke LI
College of Horticulture and Landscape Architecture, Tianjin Agricultural University, Tianjin 300384, China
Abstract [Objectives] The purpose was to establish an induction system for friable callus of Hedera nepalensis var. sinensis with different parts. [Methods] By screening the most suitable explant and adjusting the hormone ratio of medium, friable calli of H. nepalensis var. sinensis were induced. [Results] The calli could be induced from leaves, petioles and stem segments, but the ideal explant was stem segments, with induction rate reaching 98%. The optimal medium for callus proliferation was MS+0.5 mg/L KT+1.0 mg/L 2,4-D+30.0 g/L sucrose. After 3-4 generations of subculture on MS+0.5 mg/L BA+1.0 mg/L 2,4-D+30.0 g/L sucrose, favorable friable calli of H. nepalensis var. sinensis were obtained. [Conclusions] The friable calli induced in this experiment can lay a foundation for in-vitro regeneration and cellular secondary metabolite production of H. nepalensis var. sinensis.
Key words Hedera nepalensis var. sinensis, Stem segment, Friable callus, Hormone
1 Introduction
Hedera
nepalensis
var. sinensis (Araliaceae:Hedera
) is an evergreen climbing vine, with woody and creeping stems and aerial roots. It is an important vertical greening material and has extremely high medicinal value, with broad development prospects. In recent years, reports onH
.nepalensis
var. sinensis mainly focus on the analysis and extraction of medicinal active ingredients, cultivation, and physiological and biochemical properties. However, there are relatively few studies on its tissue culture, and the limited existing studies focus on rapid propagation. There are few reports on the induction of callus. In this study, different parts of asepticH
.nepalensis
var. sinensis seedlings were used for callus induction. By adjusting the ratio and dosage of hormones in the medium, the friable callus ofH
.nepalensis
var. sinensis could be induced, so as to lay the foundation for the research of somatic cell regeneration, virus-free seedling culture and secondary metabolite production ofH
.nepalensis
var. sinensis. In addition, callus can also be used for studies on metabolism physiology, gene expression and regulation, phylogeny and genetic evolution of plant, and is an indispensable test material for plant production and scientific research.2 Materials and methods
2.1 Materials
H
.nepalensis
var. sinensis tissue culture seedlings subcultured for 2 years were used as the experimental material. They were provided by the landscape plant laboratory of Tianjin Agricultural University.2.2 Methods
2.2.1
Selection of explant. Leaves, petioles and stem segments ofH
.nepalensis
var. sinensis tissue culture seedlings were used as explants, respectively. The edge of the leaves was cut off, and the retaining part was cut into small pieces of about 2-3 cm. The petioles were separated from the junctions with the leaves and the stems and were no longer cut. The growth point of the stem segment was cut, and the length of stem segment is about 0.5 cm. The explants were placed flat on the surface of the callus induction medium (MS+1.0 mg/L 2,4-D+30.0 g/L sucrose, agar 7.0 g/L, pH 5.8, sterilized under 121 ℃, 0.1 MPa for 20 min). The medium was placed in 100-mL wide-mouthed Erlenmeyer flasks, 30 mL for each Erlenmeyer flask. For each treatment, 10 Erlenmeyer flasks, five explants per Erlenmeyer flask, were prepared. The culture conditions were as follows: 24 h light, 1 000-1 500 lx, (25±1) ℃. The inoculated materials were observed and recorded every day. After 30 d, the number of explants forming callus were counted, and the callus induction rate was calculated.Callus induction rate (%) = Number of explants forming callus/Number of explants inoculated ×100%.
2.2.2
Proliferation and culture of callus. The calli induced from stem segments was cut into small pieces of 2-3 mmand then placed flat on the surface of the callus proliferation medium, not letting them sink into the medium with artificial pressure. The callus proliferation medium was prepared by adding different concentrations of KT and 2,4-D, as well as 30.0 g/L sucrose and 7.0 g/L agar, into the MS basic medium. After adjusting the pH to 5.8, the proliferation medium was sterilized at 121 ℃ for 20 min. A total of 10 Erlenmeyer flasks were arranged for each treatment, and in each Erlenmeyer flask, there were 5 calli. The culture conditions were the same as Section2.2.1
. The growth of the calli was observed and recorded every day. After 30 d, the results were analyzed.2.2.3
Induction of friable callus. The calli induced in the previous step were subcultured on the selected medium to obtain a large number of well-growing calli. The calli were divided into small pieces of 2-3 mm in a sterile petri dish, and those with a loose structure were selected for subculture. Various friable callus induction media were used, and the inoculation method and the medium preparation method were the same as above-mentioned. Subculture was carried out once every 14 d. The medium used was the same. Loosely structured calli were selected. After 3 generations of subculture, the results were analyzed. The culture conditions were as follows: 24 h light, 1 000-1 500 lx, (23±1) ℃.3 Results and analysis
3.1 Effect of different explants on callus induction (Table 1)
Different explants were used for callus induction ofH
.nepalensis
var. sinensis. After 10 d, callus appeared in the wound of some explants. After about 30 d, callus appeared on most of the explants. However, the growth of the calli was obviously different. The specific results are shown in Table 1.Most explants can induce explants, showing thatH
.nepalensis
var. sinensis is easier to induce callus. At the beginning of inoculation, the wound of some explants grew white soft callus. With the extension of the culture, most of the explants grew callus. After 40 d of culture, the callus induction rate of the stem segments was up to 98%. In terms of growth rate, the calli induced from stem segments showed the highest growth rate. Soft white calli appeared on both the ends. Differently, only one end of the petioles grew callus, and the structure was tight. On leaves, calli appeared only in the position with vein, with tight structure and extremely slow growth rate. In short, stem segment is ideal explant for inducing callus.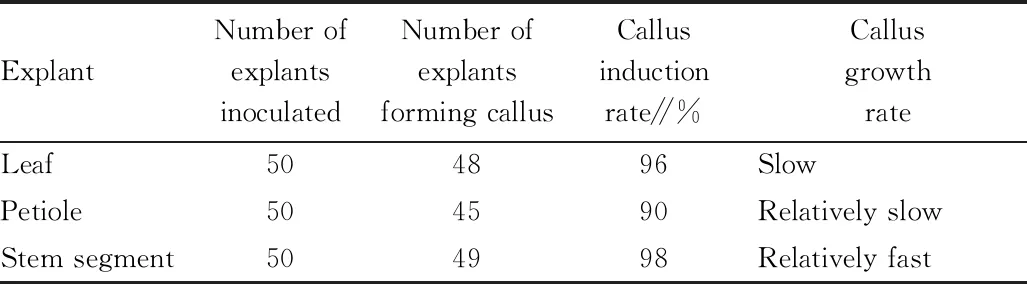
Table 1 Effect of different explants on callus induction of Hedera nepalensis var. sinensis
3.2 Effect of different hormone combinations on growth of callus (Table 2)
The calli induced in the previous step were mostly white soft. In order to promote the growth and improve the structure of calli, in this experiment, the combination of cytokinin KT and 2,4-D was used for callus culture. After 30 d, the growth rate and structure of calli on different media showed different changes.Table 2 shows that different combinations of KT and 2,4-D had a greater impact on the growth rate and morphological structure of callus. When 2,4-D was used alone, the calli grew faster. As the concentration of 2,4-D increased, the growth rate of calli increased, but the texture of calli became softer and softer. When the concentration of 2,4-D reached 2.0 mg/L, the calli were extremely soft, and with the extension of the culture time, the calli became transparent and gradually died. This shows that the high concentration of 2,4-D had a toxic effect on the calli. When KT was added to the medium, the growth rate of calli became slower, but increased when the concentration of 2,4-D was 2.0 mg/L, showing that the addition of KT can alleviate the effect of 2,4-D and can also reduce the toxic effect of high concentration of 2,4-D. A higher ratio of KT to 2,4-D inhibited the growth rate of calli, but increased the density of calli, making their texture hard; on the contrary, the growth rate of calli increased and their texture became softer. Considering growth rate and callus texture comprehensively, MS+0.5 mg/L KT+1.0 mg/L 2,4-D could be selected to obtain the ideal callus induction effect forH
.nepalensis
var. sinensis.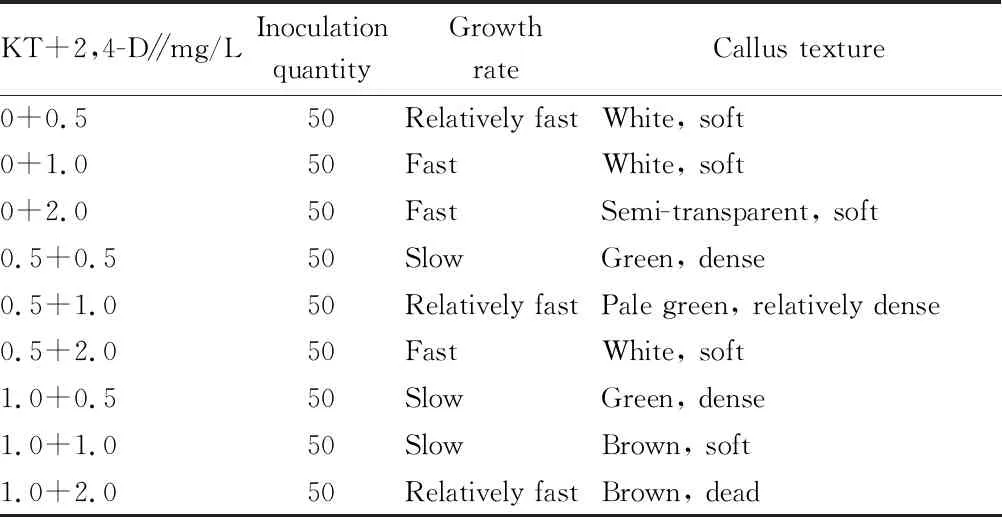
Table 2 Effect of different hormone combinations on callus growth of Hedera nepalensis var. sinensis
3.3 Effect of media with different hormone combinations on induction of friable callus (Table 3)
In order to obtain friableH
.nepalensis
var. sinensis calli, different concentrations of KT or BA were used in combination with 2,4-D. Combined with the selection of callus during subculture, after 4 generations of subculture, ideal calli ofH
.nepalensis
var. sinensis were obtained.The effects of different cytokinins on the growth ofH
.nepalensis
var. sinensis calli were quite different. They not only affected the growth rate but also had a greater impact on the texture and structure of the calli. When KT or BA was used alone, the growth rate of calli was slower, their texture became harder, and they were easy to differentiate. When KT was used in combination with 2,4-D, their ratio showed different effect on calli. When the ratio was higher, the texture of calli became hard, and the growth rate slew down. On the contrary, when the ratio was low, the growth rate of calli increased, and their texture became softer. The combined use of BA and 2,4-D also showed the similar results. But compared with KT, it had a different effect on the structure of calli. When using KT, the structure of calli was tighter. In contrast, when BA was used in combination with an appropriate concentration of 2,4-D, a loosely structured callus type appeared. The callus clusters were easy to disperse and fragile, and this kind of calli was exactly the type people need. Therefore, ideal calli ofH
.nepalensis
var. sinensis could be induced after 3-4 generations of subculture using MS+0.5 mg/L BA+1.0 mg/L 2,4-D as the medium.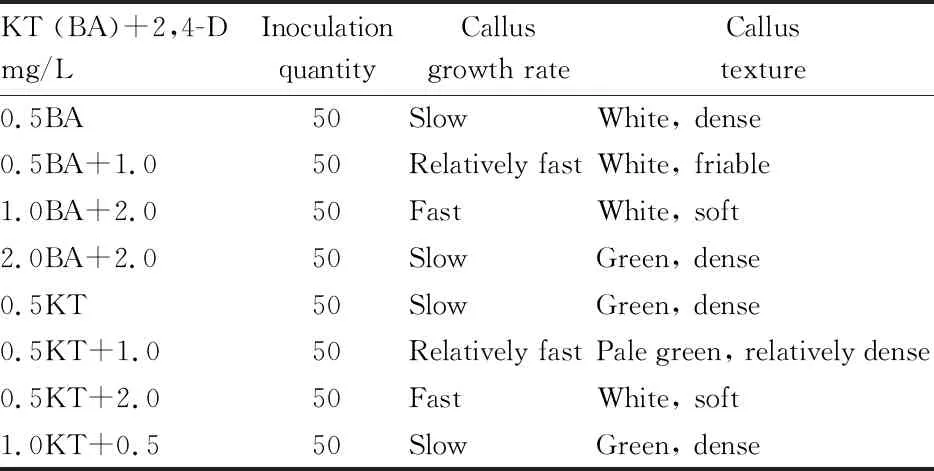
Table 3 Effect of different hormone combinations on friable callus induction of Hedera nepalensis var. sinensis
4 Discussion and conclusions
The position of explants and different developmental stages have a great influence on the induction of callus. In the experiment, callus induction was conducted on the explants of different parts. The results show that the stem segment was the best explant to induce callus, with higher callus induction rate and growth rate, similar to the results of Shi Yanyuet
al
.on cauliflower callus induction. This is due to differences in the physiological state and detachment ability of explants. It is generally believed that young and tender explants are easier to induce callus, and it is difficult for aging tissues to form callus. This is because the young and tender explants are in an active physiological state and contain more meristematic cells, and some meristematic cells can change the direction of differentiation under the action of exogenous hormones, then transform into parenchyma cells and grow and divide rapidly to form callus. Therefore, the number of meristems contained in the explant determines the difficulty of callus induction.Plant growth regulators play an important role in the process of callus induction. In most instances, medium containing auxin 2,4-D can successfully induce explants to produce callus. However, the calli growing on the induction medium containing only 2,4-D are relatively soft. They are mucinous, vesicular, and generally difficult to differentiate and have a low frequency of regeneration. In this study, the calli induced from stem segments as explants with 1.0 mg/L 2,4-D were mostly soft calli. However, when 1.0 mg/L 2,4-D was used in combination with 0.5 mg/L KT, the quality of calli was obviously improved. Proper cytokinin and auxin concentration ratio can induce ideal callus type. Generally, a certain amount of auxin and cytokinin are used in conjunction with callus culture. For example, Li Xianget
al
.used 5.0 mg/L 2,4-D and 0.1 mg/L 6-BA to induce the ideal calli ofAkebia
quinata
(Houtt.) Decne. This is because cytokinin promotes cell division and differentiation, and it makes individual cells smaller, delaying the aging of calli to a certain extent, thereby increasing the concentration of callus cytoplasm. As a result, the texture of calli becomes hard. However, in the process of callus induction, the concentration of cytokinin should not be too high. If the concentration of cytokinin is too high, calli may change to the direction of organization.In order to obtain friable calli, this study used two kinds of cytokinins in combination with 2,4-D, and the results show that when BA was used in combination with 2,4-D, ideal friable calli ofH
.nepalensis
var. sinensis were induced, showing that different hormones have different effects on callus. This is mainly determined by the plant’s tolerance to hormones. When the concentration of cytokinin is increased, it can change the way of cell differentiation, but it will also increase the toxic effect on cells. When the KT concentration in this experiment increased, the calli grew normally in the early stage, but the toxic effect began to appear in the later stage. The calli slew down in growth rate, became darker, and finally vitrified, browned and died. It shows thatH
.nepalensis
var. sinensis has poor tolerance to KT. Therefore, the type of cytokinin should be adjusted in time according to the actual situation, in order to obtain the ideal induction effect.In this experiment, ideal friable calli ofH
.nepalensis
var. sinensis were induced by adjusting the explants used and medium formula. The specific method is summarized as follows. Firstly, a certain number of asepticH
.nepalensis
var. sinensis seedlings were cultivated. Stem segments, about 0.5 cm in length, were used as the explants. They were inoculated on the medium of MS+0.5 mg/L KT+1.0 mg/L 2,4-D+30.0 g/L sucrose to induce soft calli. Then, the soft calli obtained were inoculated to the medium of MS+0.5 mg/L BA+1.0 mg/L 2,4-D. After 3-4 generations of subculture, the ideal friable calli ofH
.nepalensis
var. sinensis were obtained. The friable calli induced in this study can lay the foundation for thein
vitro
regeneration and secondary metabolite production ofH
.nepalensis
var. sinensis, and can also be further used for inducing friable embryogenic calli.杂志排行
Asian Agricultural Research的其它文章
- Ecological Investigation and Ecological Security Assessment of Xizhijiang River Basin in Huizhou City of Guangdong Province
- Molecular Cloning and Bioinformatics Analysis of araC Gene of Vibrio alginolyticus
- Occurrence Regularity and Integrated Control Technology of Canker in Citrus
- Activation Effect of Hydrochemical Energy in Regenerative Agriculture on Nutrients of Arsenic Sandstone
- IS-LM Model Based Analysis on the Role of China’s Financial Policy for Supporting Agriculture
- Prediction and Protection of Cultivated Land in China
