Tunable UCST thermoresponsive copolymers based on natural glycyrrhetinic acid
2021-04-02YirnZhoJingyoXiYuMinHuiLiJunHu
Yirn Zho,Jingyo M,Xi Yu,Min-Hui Li,b,*,Jun Hu,*
a Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
b Institut de Recherche de Chimie Paris, Chimie ParisTech, PSL Research University, CNRS, Paris 75005, France
ABSTRACT Water-soluble thermoresponsive polymers present either upper critical solution temperature(UCST)or lower critical solution temperature(LCST)depending on the location of their miscibility range with water at high temperatures or at low temperatures.Compared with LCST polymers, the water-soluble UCST polymers are still less explored until now.In this work three copolymers of P(AAm-co-GAA) were synthesized by copolymerizing two acrylamide monomers, acrylamide (AAm) and acrylamide functionalized with natural glycyrrhetinic acid (GAA), using reversible addition-fragmentation chain transfer (RAFT) polymerization.These copolymers exhibited the typical UCST thermoresponsive behavior, and their phase transition temperatures could be easily tuned to around 37 °C for potential biological applications.Moreover,the UCST of P(AAm-co-GAA)can be adjusted not only by the content of glycyrrhetinic acid(GA)and polymer concentrations,but also by the host-guest interactions between GA and cyclodextrins(β-and γ-CD).The suitable value of UCST and the biocompatible nature of GA and CDs may endow these copolymers with practical applications in biomedical chemistry
Keywords:Thermo-responsive polymer UCST Glycyrrhetinic acid Natural product Cyclodextrin
Water-soluble thermoresponsive polymers have been widely studied in polymer and material science due to the drastic hydrophilicity/hydrophobicity transformation upon changes of temperature[1,2].They can be divided into lower critical solution temperature (LCST)-type and upper critical solution temperature(UCST)-type,depending on the drop of miscibility at low temperatures or at high temperatures [3].After the discovery of LCST behavior of poly(N-isopropylacrylamide) by Heskins and coworkers in 1968[4],numerous water-soluble LCST polymers have been reported [5,6].Compared with LCST polymers, the UCST polymers are less explored since they were discovered later and the number of examples was still limited[7,8].The UCST behavior stems from either Coulomb interactions (the effect of amphoteric ions) or hydrogen bonding, namely C-UCST or HB-UCST, respectively [9].As C-UCST behavior depends heavily on ionic strength,type of ion and pH value, the potentials of C-UCST polymers are restricted because in most applications mild ionic strength is required [9,10].Thus, HB-UCST polymers which are less sensitive to electrolytes would be more suitable for preparing thermosensitive materials.
In 2012,Seuring and co-workers copolymerized acrylamide and acrylonitrile to afford poly(acrylamide-co-acrylonitrile) with the typical UCST behavior in water [11].Later, researchers used additional comonomers to copolymerize with acrylamide (AAm)and acrylonitrile(AN),and applied them in various fields[12-14].For example, Zhao and co-workers reported an ultrasensitive pHinduced water-soluble switch by incorporating either acrylic acid or 4-vinylpyridine comonomers in the random copolymer of acrylamide and acrylonitrile[12].Sukhishvili and co-workers have synthesized a HB-UCST block copolymer, poly(acrylamide-coacrylonitrile)-b-polyvinylpyrrolidone,and prepared its self-assembled films with thermoresponsive properties [13].Apparently,incorporating a suitable hydrophobic unit into the water-soluble polymer chain would be an effective strategy to obtain HB-UCST polymers.
Glycyrrhetinic acid (GA) is one of the physiologically natural triterpenoids in vivo and plays key roles in a variety of remarkable biological activities[15].In addition,GA has the rigid hydrophobic scaffold, great biocompatibility, multiple reactive sites and chiral centers.These characteristics can be used to make low molecular weight gels [16,17], supramolecular chiral structures [18],and functional polymers [19,20].In our previous works, GA has been incorporated into LCST-based polymers, “necklace”-like polypseudorotaxanes,and self-healable hydrogels[21-23].In this work,we proposed to take advantage of the hydrophobic backbone of GA to construct new biocompatible HB-UCST polymers.Three copolymers of P(AAm-co-GAA-x%) (x=2, 3, 4) were designed and synthesized (Scheme 1), where an acrylamide monomer bearing GA (GAA) copolymerized with acrylamide by reversible additionfragmentation chain transfer (RAFT) polymerization (synthetic details see Supporting Information).The results showed that P(AAm-co-GAA) exhibited the typical HB-UCST behavior with a phase transition temperature around 37°C.Moreover, their thermoresponsive behavior can be adjusted not only by the content of GA and polymer concentrations,but also the host-guest interactions between GA and β-/γ-cyclodextrin(CD).The suitable UCST and the biocompatible nature of GA and CDs may allow these copolymers to be useful in biomedical field.
The copolymers with different molar ratios of GAA and AAm were characterized by size exclusion chromatography(SEC)and1H NMR spectra (Figs.S1 and S2 in Supporting information).The1H NMR spectra of P(AAm-co-GAA-x%) (x=2, 3, 4) displayed the characteristic peaks corresponding to protons carried by GA (δ 5.49 ppm, assigned to Ha; 1.35, 1.05, 1.02, 0.92, 0.74, 0.71 ppm,assigned to methyl groups), and the content of GA unit in copolymers was calculated from the proton integration ratio between the peak at 5.49 ppm (Ha) versus backbone protons at 2.13 ppm(Hb,Hc)(Fig.S2).Meanwhile,in order to explore the UCST mechanism, two control copolymers, P(AAm-co-HAA-3%) and P(MMA-co-GAA-3%), were synthesized as shown in Scheme S1(synthetic details see Supporting Information).Compared with P(AAm-co-GAA-3%), the rigid hydrophobic backbone of GA was replaced with a flexible alkyl chain (15-hydroxypentadecanoic acid, HA) in P(AAm-co-HAA-3%), while the hydrophilic AAm was substituted by the H-bonding-free methyl methacrylate(MMA)in P(MMA-co-GAA-3%).All the information on compositions of these copolymers was illustrated in Table 1.
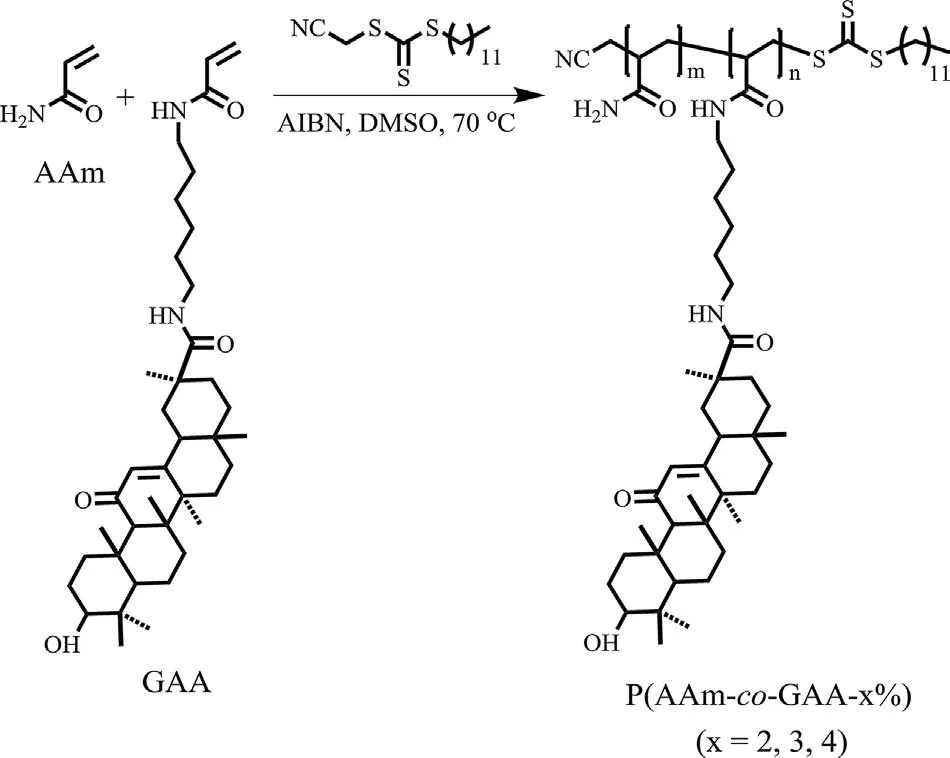
Scheme 1.Illustration of copolymerization of natural glycyrrhetinic acid-derived acrylamide (GAA) with acrylamide (AAm).
The thermal response of polymer aqueous solutions was recorded by UV-vis spectrometer.As shown in Fig.1A, the phase transition with a UCST of 32°C of P(AAm-co-GAA-3%) at the concentration of 15 mg/mL was observed upon cooling, strongly revealing the UCST behavior of P(AAm-co-GAA-3%) in the range from 10°C to 60°C.At a high temperature of 40°C,a clear solution formed, while at 20°C it appeared a cloudy suspension (inset in Fig.1A).Either increasing or decreasing the molar fraction of GAA to 4 mol%or 2 mol%led to the UCST higher than 60°C or lower than 10°C.It was because P(AAm-co-GAA-2%) was less hydrophobic in comparison with P(AAm-co-GAA-3%), while P(AAm-co-GAA-4%)was more hydrophobic.With increasing the GA content, the hydration shell of hydrophobic segments of copolymers became more ordered[24].In this case,a higher temperature was needed to dissolve the aggregates,i.e.,a high phase transition temperature.Apparently, the content of hydrophobic GA unit had a significant effect on UCST behavior.Notably, compared with the reported P(AAm-co-AN) which exhibited the UCST behavior only when AN content was more than 10 mol%[11],P(AAm-co-GAA)would show UCST behavior with the content of GAA as low as 3 mol%.Such difference was mainly due to GAA had a larger hydrophobic rigid backbone than AN moiety.
In addition, there was a significant concentration dependence of UCST.As shown in Fig.1B, the UCST of P(AAm-co-GAA-3%)decreased from 32°C, to 21°C, and to < 10°C when the concentration decreased from 15 mg/mL, to 10 mg/mL, and to 5 mg/mL.This observation can be explained by the strong interand intra-molecular H-bonding between polymer chains at the high concentration, as solubilizing copolymers required the disruption of polymer-polymer interactions by heat in favor of polymer-water interactions.
Moreover, this phase transition was reversible in multiple heating-cooling processes (3 cycles, Fig.1C), indicating that no chemical change happened in the system.It should be noted that there was a certain hysteresis which was attributed to the chain mobility of P(AAm-co-GAA-3%)below or above UCST.Above UCST,GA pendants was involved in the aggregates companied by the weakening of inter-and intra-molecular H-bonding, and these aggregates were stable enough to delay the generation of large clusters during the cooling process, thus causing the hysteresis[11].Besides, the thermoresponsive experiments of control polymers (P(AAm-co-HAA-3%) and P(MMA-co-GAA-3%) were performed.As shown in Fig.S3 (Supporting information), the transmittance of P(AAm-co-HAA-3%)aqueous solution maintained approximate 90%within the test temperature range from 10°C to 60°C, and did not exhibit any UCST property.It strongly revealed that only the rigid scaffold of GA can afford the suitable face-toface hydrophobic stacking effect in this UCST-type phase transition,since the better mobility of the flexible alkyl chain HA tended to form chain entanglements,thus restricting their supramolecular complexation and resulting in no phase transition[24].For the Hbonding-free P(MMA-co-GAA-3%) aqueous solution, its transmittance was less than 1% from 10°C to 60°C, and no UCST behavior was observed,which indicated that the H-bonding monomer AAm was necessary in this UCST system.Based on the results of control experiments, it was proposed that the synergistic effect of interand intra-molecular H-bonding of amide groups and the face-toface hydrophobic stacking of GA moieties promoted the appearance of UCST phenomenon.
More evidences for the UCST behavior of P(AAm-co-GAA-3%)were revealed by dynamic light scattering(DLS)measurements,1H NMR spectra,and transmission electron microscope(TEM)images.As shown in Fig.2A,the average particle size of P(AAm-co-GAA-3%)in water was approximately 30 nm when the temperature was above 40°C.Once cooling to 20°C, some large clusters around 1000 nm appeared rapidly.With the continuous decrease of temperature, more and more large clusters formed with the disappearance of small particles, eventually a turbid solution was obtained at 10°C.These observations can be further confirmed by TEM images, where the small particles around 40 nm were observed at 40°C (Fig.2B), while only irregular large precipitates were observed at 20°C (Fig.2C).The temperature-dependent1H NMR experiments showed that all proton peaks of P(AAm-co-GAA-3%) in D2O became broad with the decrease of temperature from 60°C to 25°C,especially the signals of methyl groups on GA(Fig.S4 in Supporting information).The explanation was that at hightemperature above UCST the GA side chain was exposed to D2O and presented good mobility,while at low temperature the intra-and inter-molecular H-bonding caused the GA side chain to be wrapped by polyacrylamide chains and to be less mobile.In conclusion, all results from the experiments of1H NMR, UV-vis,DLS and TEM were totally consistent.
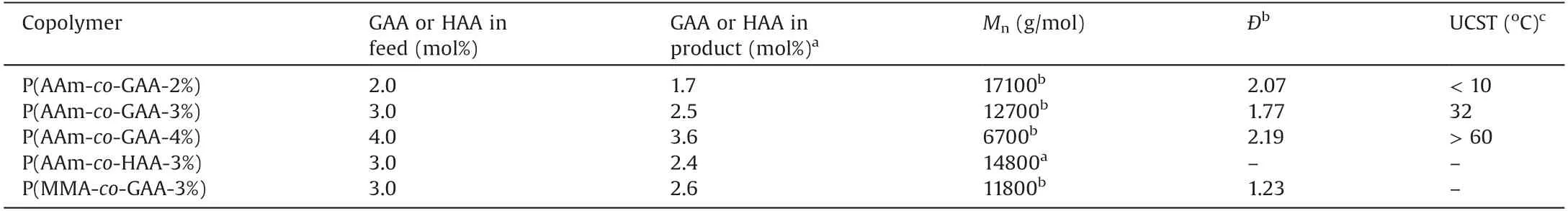
Table 1 Characteristics of these copolymers.
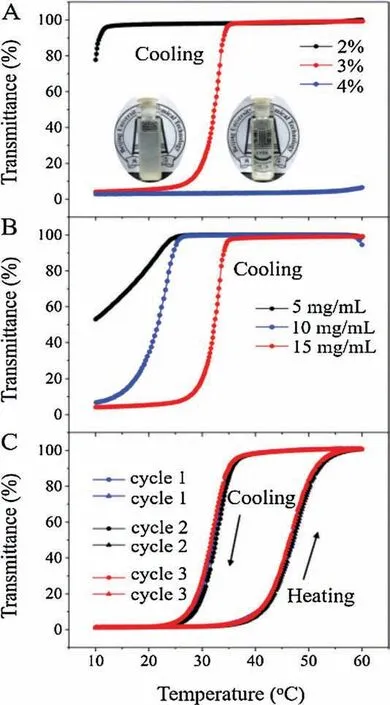
Fig.1.Transmittance of the copolymer aqueous solutions as a function of temperature at a wavelength of 450 nm: (A) with different GAA molar ratios of P(AAm-co-GAA-x%) (x=2, 3, 4) under the concentration of 15 mg/mL.Inset: digital photos of P(AAm-co-GAA-3%) at 20°C (left) and 40°C (right); (B) at different concentrations of P(AAm-co-GAA-3%);(C)the aqueous solution of P(AAm-co-GAA-3%)with three cooling-heating cycles at the concentration of 15 mg/mL.Cooling and heating rate is 1.0°C/min.
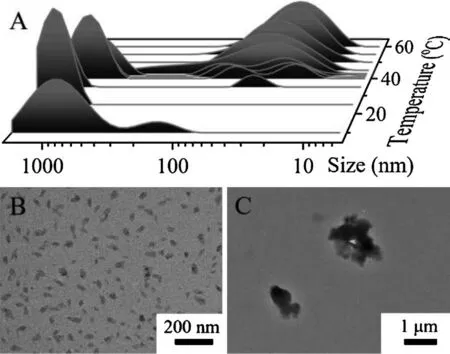
Fig.2.(A) Temperature-dependent size variation of P(AAm-co-GAA-3%) aqueous solution.TEM images of P(AAm-co-GAA-3%) aqueous solution at (B) 40°C and (C)20°C without stain.Concentration is 15 mg/mL.
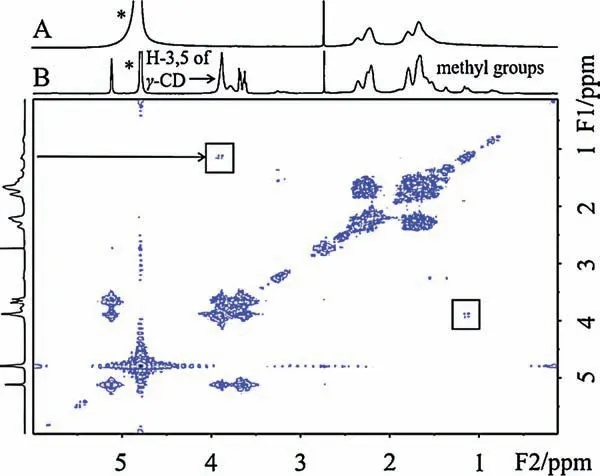
Fig.3.(A) 1H NMR spectrum of P(AAm-co-GAA-3%) in D2O (400 MHz, 25°C,concentration=15 mg/mL).(B)2D NOESY 1H NMR spectrum of P(AAm-co-GAA-3%)with γ-CD in D2O(600 MHz,25°C,[GA]/[γ-CD]=1:1,concentration=15 mg/mL).“*”means residual H signal in D2O.
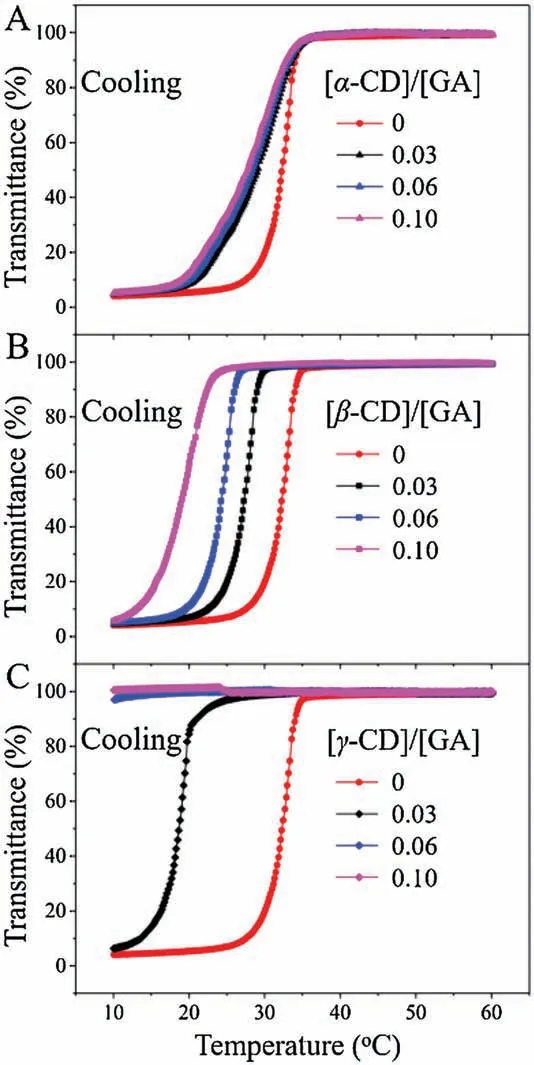
Fig.4.Transmittance of P(AAm-co-GAA-3%) aqueous solution as a function of temperature at a wavelength of 450 nm in the presence of(A)α-CD,(B)β-CD and(C)γ-CD.Cooling rate is 1.0°C/min.Concentration is 15 mg/mL.
Our previous work has revealed that GA (molecular size 0.58-0.60 nm)could form the host-guest inclusion complex with β-cyclodextrin (β-CD) (cavity diameter 0.60-0.65 nm, binding constant Ka=1.59×103L/mol) and with γ-cyclodextrin (γ-CD)(cavity diameter 0.70-0.83 nm,Ka=4.41×105L/mol),except α-CD(cavity diameter ~0.47 nm) [22,25].Inspired by the size-match effect between GA and CDs, we studied here the role of CDs in adjusting the USCT behavior of P(AAm-co-GAA-3%).The1H NMR spectrum was firstly used to confirm the complexation of CDs with GA units in P(AAm-co-GAA-3%).As shown in Fig.S5 (Supporting information), no signal of GA was observed in D2O at room temperature because of the poor solubility of P(AAm-co-GAA-3%).Upon addition of β-CD and γ-CD, respectively, the characteristic methyl peaks of GA units appeared,while no change was observed upon α-CD addition.Moreover, cross correlation signals were observed clearly in 2D NOESY1H NMR spectrum (rectangles in Fig.3 and Fig.S6 in Supporting information), indicating the close contact between GA methyl protons and β-/γ-CD protons(limit of detection for NOE signal:<5 Å).As in the aqueous environment GA and CD were phase-separated,these cross-correlation signals were attributed to the formation of inclusion complex by positioning GA moiety in the cavity of β-/γ-CD.
In the UV-vis absorption experiments, α-CD had no effect on the UCST behavior because of its small cavity diameter (Fig.4A)[25].The change in the curve shape was mainly due to the Hbonding between hydroxyl groups of α-CD and amide groups in polymers.In contrast,the significant changes were observed both for β-CD and γ-CD.In the presence of β-CD, P(AAm-co-GAA-3%)still exhibited the UCST property, while its UCST decreased gradually with the increase of molar ratio of [β-CD]/[GA](Fig.4B).When [β-CD]/[GA] increased from 0 to 0.03, 0.06 and 0.10,the UCST decreased from 32°C to 27°C,24°C,and 19°C.Such results were mainly due to the hydrophilic exterior of β-CD,which enhanced the hydrophilicity of polymers, thus resulting in the decrease of UCST.Note that the UCST was extremely sensitive to the molar ratio of[β-CD]/[GA].As shown in Fig.4B,a tiny increase of concentration ratio from 0 to 0.03 would result in a decrease of 5°C in UCST.This was totally different from the behavior of LCST polymers in the presence of β-CD as reported in our previous work,where an increase of[β-CD]/[GA]ratio from 0 to 5 only resulted in an increase of 13°C for the cloud point[21].As γ-CD had a stronger binding ability with GA and the better hydrophilicity compared with β-CD,it was more effective in regulating the USCT behavior of P(AAm-co-GAA-3%).As shown in Fig.4C, a decrease of 13°C for UCST was achieved upon addition of 0.03 equiv.γ-CD, and the UCST decreased further to lower than 10°C when 0.06 equiv.of γ-CD was added.Obviously, the host-guest interactions between GA and β/γ-CD would be an effective strategy to regulate the UCST behavior of polymers, and the UCST of P(AAm-co-GAA-3%) was more sensitive to γ-CD than to β-CD.
In summary, by incorporating acrylamide monomer functionalized by natural glycyrrhetinic acid into the polyacrylamide chains, three copolymers of P(AAm-co-GAA-x%) (x=2, 3, 4) were synthesized, which exhibited the typical HB-UCST behavior in water promoted by the synergistic effect of the intra-and intermolecular H-bonding of amide groups and the face-to-face hydrophobic stacking of GA units.Their phase transition temperatures could be easily adjusted to the temperature range from below 10°C to above 40°C by the GA content and polymer concentrations.Moreover, the UCST can be further tuned by β-CD or γ-CD effectively due to host-guest interactions between GA and CDs.The suitable UCST ranges and the biocompatible features of GA and CDs are particularly interesting for thermoresponsive polymers applied in biomedical field.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by National Key R&D Program of China(No.2017YFD0200302) and the National Natural Science Foundation of China (NSFC, No. 21604085).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.03.057.
杂志排行
Chinese Chemical Letters的其它文章
- A biomass based photonic crystal made of “konjac tofu”
- Hydrothermal-assisted grinding route for WS2 quantum dots (QDs)from nanosheets with preferable tribological performance
- Superiority of poly(L-lactic acid) microspheres as dermal fillers
- Zwitterionic comb-like lipid polymers encapsulating linalool for increasing the fragrance retention time
- Construction of a nano-rectangular Zn-Nd complex with near-infrared luminescent response towards metal ions
- Synthesis and structure of Au19Ag4(S-Adm)15 nanocluster:Polymorphs and optical properties
