Guest induced morphology transitions of star shaped pillar[5]arene trimer via endo host-guest and “exo-wall” electron-transfer interactions
2021-04-02WjhtAliWeitoGongMehdiHssnWeidongQuLuLiuGuilingNing
Wjht Ali,Weito Gong,*,Mehdi Hssn,Weidong Qu,Lu Liu,Guiling Ning,*
a State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, China
b Department of Chemistry University of Baltistan, Skardu, Pakistan
ABSTRACT Star shape bridged pillar[5]arene trimer (C3-PLT) based on benzene-1,3,5-tricarboxamide (BTAs) was successfully synthesized, which exhibited outstanding guest responsive morphology transition properties.The morphology tuning studies was efficiently achieved with the addition of competitive guest molecules G1 and G2 by various self-assembly mechanisms.C3-PLT itself displays nanofiber morphology through H-type π-π stacking, and this nanofiber morphology can be completely transformed into spherical vesicles by host-guest interaction G1, while upon addition of G2 into C3-PLT by means of“exo-wall”electron-transfer interactions,sheet superstructures can be observed.SEM,1H NMR, DOSY, fluorescence spectroscopy,and viscosity have verified the formation of supramolecular polymers and morphological transitions between C3-PLT with both guests.
Keywords:Pillar[5]arene Supramolecular polymers Host-guest interactions
Supramolecular self-assembly has gained tremendous attention because it offers adistinctive way to construct various functional supramolecular polymers with futuristic applications in the area of environmental sciences,biomedicalsciences,and nanodevices[1,2].The supramolecular self-assembly can be controlled or transformed by various parameters,including environmental stimuli such as pH,temperature,redox,ions,and light stimuli,polymer concentration,solvent, copolymer composition, hydrophilic fraction [3-8].Although several kinds of supramolecular polymers with different morphologies have been documented in past few decades, the controlled supramolecular architectures having divergent distinct morphologies such as micelles,vesicles,tubes,and lamellae always remain a prim focus and burning issue in the scientific communal due to the significant potential [9-15].These architectures exhibit unique and prospective applications in the field of material science,chemical science,biomedicine,biocatalysis,and electronic devices due to their variation in self-assembly and functional characteristics[16-18].However, in recent literature, most of the morphology transition research has been reported by using external stimuli,e.g.,by using different solvents, and introducing responsive moieties into the structure[19,20].Yao et al.reported vesicles and nanofiber morphological transformations through ultrasound reversible heat[21,22].UV irradiation based structural transformation was revealed by Kim and co-workers [23].Similarly, an enzyme triggered transformation from micelle to fiber was published by Ulijn’s group[24,25].Moreover,Meijer et al.have reported solvent dependent multifarious self-assemblies transition of benzene-1,3,5-tricarboxamide based derivatives from coil-to-globule [26].The first instanceof solvent-basedmorphological transformation in this field has provided by Zhao group’s based on bridged pillar[5]-arene trimer and biviologen guest through host-guest interaction.Further,different morphologies have been efficiently obtained,i.e.,vesicular structures (0D), tubular objects (1D), layers (2D), and stacked layers(3D)upon increasing the host-guest concentrations[27].In contrast, the guest-induced morphological transitions of supramolecular polymers are still unexplored [28-30].Therefore herein,we demonstrated morphology transitions of pillar[5]arene trimer C3-PLT before and after addition of guest molecules with different self-assembly approaches in CHCl3.Initially, a bridged pillar[5]arene trimer C3-PLT was synthesized by incorporating benzene-1,3,5-tricarboxamide(BTAs)intothe three identical pillar-[5]arene.However,we found that the pillar[5]arene trimer C3-PLT could directly self-assemble into well-defined nanofiber via H-type-stacking(Fig.1i).Interestingly,after addition of G1 guestinto host C3-PLT which showed an incredibly response by morphological transformation from nanofiber to spherical vesicles(Fig.1ii)due to the encapsulation of G1 into the cavity of pillar[5]arene trimer C3-PLT.This result revealed the formation of the hyperbranched supramolecular polymer through endo host-guest interaction like previously reported hyperbranched supramolecular polymer[31,32].
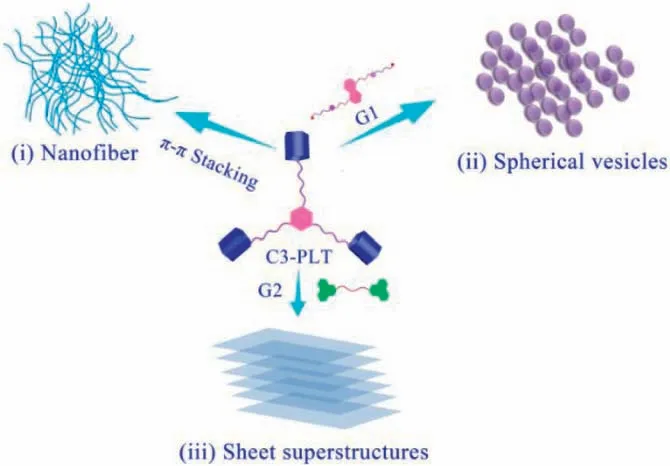
Fig.1.Graphical presentation of the formation of various morphologies of C3-PLT with different guests.
Furthermore, when the G2 guest was added into C3-PLT, the sheet superstructure morphology was observed(Fig.1iii)owing to the“exo-wall”interactions via π-π stacking between C3-PLT host and G2.Scanning electron microscopy (SEM) experiments,1H NMR, 2D DOSY, UV-vis spectroscopy, FL, and viscosity measurements assisted in visualizing the morphology transition from nanofiber to sheet superstructures and host-guest interactions in CHCl3.
Initially, to investigate the self-assembly behavior of C3-PLT host,the concentration-dependent UV-vis absorption spectra of C3-PLT was carried out in CHCl3solution shown in (Fig.2).The C3-PLT demonstrates an intensive absorption at 292 nm with 5 μmol/L solution.Upon increasing concentration of host from 5 μmol/L to 200 μmol/L enhancement in intensity, along with 17 nm, blue shift, was observed.This result confirmed the H-type(parallel plane-to-plane stacking) aggregation of C3-PLT [33].Moreover, in order to find more insights about the aggregation behavior of C3-PLT,we have examined fluorescence measurement.The fluorescence emission peak of host C3-PLT was recorded at 490 nm at 25°C.When the concentration of C3-PLT increases,the fluorescence intensity of C3-PLT displayed quenching behavior shown in (Fig.S9 in Supporting information).This fluorescence emission result demonstrated that the presence of π-π stacking in C3-PLT host molecules [34].
We reported in our previous work that, the host molecule C3-PLT could form super-gel upon irradiation with ultrasound at room temperature and precipitated in acetonitrile by a conventional heating-cooling process [34].

Fig.2.UV-vis absorption spectra of C3-PLT host in CHCl3 solution at different concentrations.
In addition, we acquired a distinct morphology in varying conditions and concentrations through SEM analysis.The acetonitrile-gel produced by sonication showed fibrillar network at 0.2 wt%, and the precipitate obtained from the heating-cooling method showed more rigid and straight rods morphology.Furthermore, concentration-dependent morphologies obtained by altering the concentration of acetonitrile at low concentration the gel self-assemble into pillar-like aggregates when the concentration increased the pillars start to fuse and form a rodlike structure.
Hence this work inspires us, and we envisioned that the host molecule has the ability to change its original morphology when adding appropriate guest molecules.
Therefore, we synthesized two different guests, G1 and G2, to further expand our work and to verify the morphology transition.Additionally, to get a more detailed understanding of the selfassembly process and the morphology transitions of C3-PLT,G1⊂C3-PLT, and G2⊂C3-PLT, SEM was carefully employed to visualize the changes in morphology.The morphology of C3-PLT host was studied before and after the addition of guest molecules G1 and G2 in CHCl3.The nanofiber morphology was observed for C3-PLT host due to the H-type π-π stacking of host molecule itself(Fig.3a).When G1 has been added to the host C3-PLT, these nanofibers have been completely transformed into vesicles which reveal the formation of hyperbranched supramolecular polymers by means of endo host-guest interaction(Fig.3b).As a contrast,the sheet superstructures morphology was observed when G2 was added,due to“exo-wall”interactions between C3-PLT and G2 due to the presence of donor-acceptor electron transfer effect in our system (Fig.3c).
The host-guest interactions can be readily monitored by fluorescence spectroscopy, so the formation of the host-guest complex between G1 with C3-PLT host in CHCl3solution was characterized by fluorescence titration (Fig.4).The fluorescence intensity was increased, and the emission wavelength showed a blue shift(~65 nm)owing to the encapsulation G1 into the cavity of the C3-PLT host.Similarly, fluorescence titration between C3-PLT host and G2 was also carried out in CHCl3to confirm the electron-transfer interactions.The highly quenching fluorescence spectra were obtained due to the presence of efficient donoracceptor effect among C3-PLT host and G2.
To provide further important insights into the host-guest complexation behavior between C3-PLT host and G1, the concentration-dependent1H NMR experiment was performed.These experiments were performed upon mixing G1 and C3-PLT at 1:2 ratio from 0.25 mmol/L to 80 mmol/L concentrations in CDCl3.As the concentration of the complex G1⊂C3-PLT increased, the relative intensity of 6 peaks of corresponding G1, Hi, Hii, Hiii, Hiv showed slight upfield shifting,even shifting towards the negative region indicating that the cyanoalkyl groups of G1, were encapsulated into the cavity of C3-PLT to form host-guest complexation (Fig.5).Moreover, all the signals gradually became broad with the increase in concentration which is the indication of the formation of supramolecular polymer.Host at different concentrations showed “exo-wall” interactions.

Fig.3.SEM Morphology transition studies of(a) C3-PLT Host,(b)G1⊂C3-PLT and(c) G2⊂C3-PLT in CHCl3.
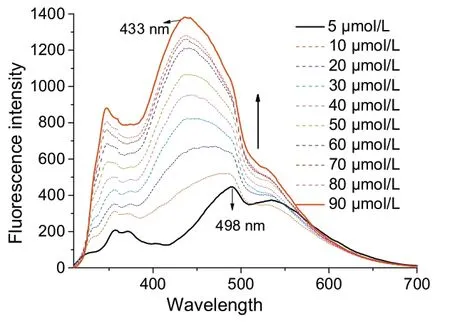
Fig.4.Emission spectra of G1⊂C3-PLT in CHCl3 solution at different concentrations.
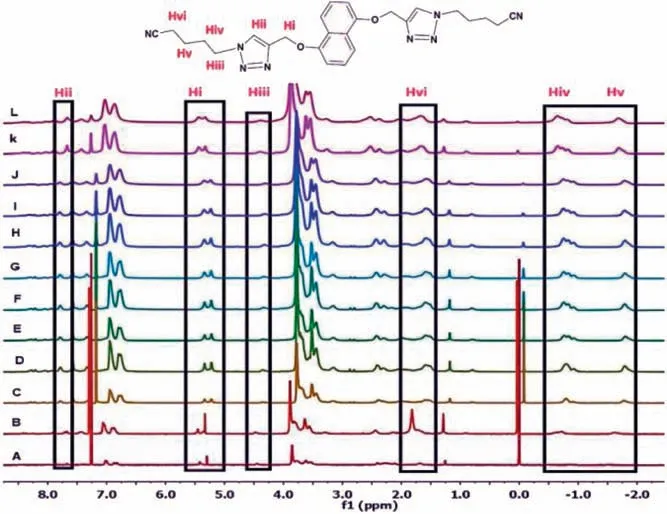
Fig.5.Concentration dependent 1H NMR spectra(400 MHz,CDCl3)of G1⊂C3-PLT Host at different concentrations: from (A) 2.5 mmol/L to (L) 80 mmol/L.
Similarly,the concentration-dependent1H NMR measurements between C3-PLT host and G2 were also recorded to confirm the presence of the “exo-wall” electron-transfer interactions (Fig. 6 and Fig.S10 in Supporting information).The protons signal of G2,Hi, Hii and Hiii showed obvious upfield shifts while the proton related to C3-PLT Ha, Hb, and Hc showed downfield shifts,suggesting that naphthalene diimide G2 was not threaded into the cavity of pillar[5]arene.This result verified the presence of “exowall” electron transfer interactions between electron-rich C3-PLT and electron-deficient G2.To further verify the donor-acceptor interactions between C3-PLT and G2, UV-vis titration was performed in CHCl3.We observed that a new and broad absorption band appeared with an absorption maxima at 420-435 nm in G2⊂C3-PLT complex, which was not found separately in the spectrum of either C3-PLT host or G2 (Fig.7).This outcome revealed the occurrence of the efficient charge-transfer interactions between C3-PLT and G2.All these results indicated that the presence of two kinds of noncovalent interactions in our system,endo host-guest interactions between C3-PLT and G1, and “exowall” interactions between C3-PLT and G2 by donor-acceptor interactions.
Viscosity is an important and direct index for supramolecular polymerization to provide direct physical evidence for the formation of a non-covalent supramolecular polymer.The micro-Ubbellohde viscometer was used to measure the specific viscosity of the equimolar mixture of G1⊂C3-PLT and G2⊂C3-PLT at 1:2 molar ratio in CHCl3solution (Fig.8).

Fig.6.Concentration dependent 1H NMR spectra (400 MHz, CDCl3, 298 K) of G2⊂C3-PLT.

Fig.7.UV-vis spectra showing CT adsorption band at(420 nm-450 nm)of 2 mmol/L at 1:1 mixture in CHCl3.
A double logarithmic representation of specific viscosity versus the concentration of G1⊂C3-PLT and G2⊂C3-PLT in CHCl3was obtained.In the low concentration region, the slope of the curve was 0.72, 0.70, respectively, which is a characteristic for noninteracting assemblies of constant size, indicating the predominance of cyclic oligomers in dilute solution.
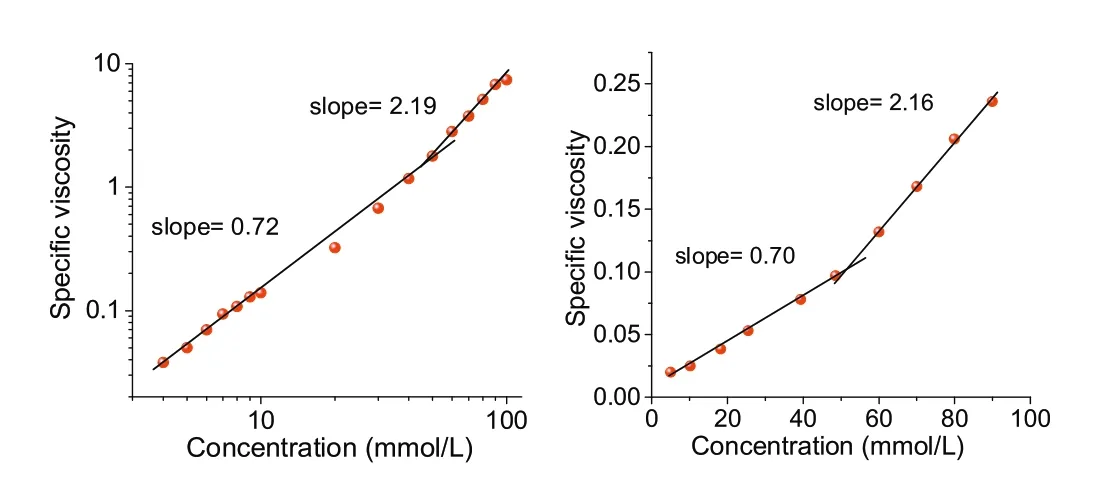
Fig.8.Specific viscosity measurements of G1⊂C3-PLT(left)and G2⊂C3-PLT(right).
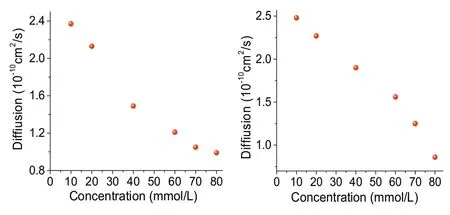
Fig.9.Concentration dependent DOSY (400 MHz, 298 K) plot of G1⊂C3-PLT (left)and G2⊂C3-PLT in CDCl3 (right).
When the concentration was increased above (48.90 mmol/L,50.91 mmol/L respectively), a sharp increase in the viscosity with remarkable increment in the slope value for both G1⊂C3-PLT(slope=2.19) and G2⊂C3-PLT (slope=2.16) was observed indicating a transition from small cyclic oligomers to more prominent supramolecular polymers.
In order to provide further evidence for the construction of supramolecular polymer with high-molecular weight aggregates two-dimensional diffusion-ordered1H NMR spectroscopy (DOSY)was conducted (Fig.9).A plot of diffusion coefficient against concentration shows that as the concentration of G1⊂C3-PLT and G2⊂C3-PLT increased from 2.4 mmol/L to 80 mmol/L, the measured weight-average diffusion coefficient (D) of G1⊂C3-PLT and G2⊂C3-PLT decreased remarkably from 2.3×10ˉ10m2/s,2.3×10ˉ10m2/s to 0.9×10ˉ10m2/s.These results showed that larger polymeric structures gradually formed from small oligomers to large-sized supramolecular polymers, which were inherently concentration-dependent.
In conclusion, a star-shape benzene-1,3,5-tricarboxamide(BTAs)bridged pillar[5]arene trimer C3-PLT host was successfully synthesized and shows outstanding guest responsive morphology transition properties.The C3-PLT host trimer shows nanofiber morphology via H-type π-π stacking and this nanofiber morphology was completely transformed into spherical vesicles via hostguest interaction with G1 and sheet superstructures by“exo wall”electron-transfer interactions with G2.Furthermore, both supramolecular self-assemblies can form hyperbranched supramolecular polymers, verified by1H NMR, DOSY, viscosity, FL, and SEM results.We envisage that this hyperbranched supramolecular with tunable morphologies will have potential applications in drug delivery and biomedical engineering.Further work is still ongoing in our lab.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China(No.21206016),the Fundamental Research Funds for the Central Universities(No.DUT-17LK07)and the Natural Science Foundation of Liaoning Province (No.2019-MS-046).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.044.
杂志排行
Chinese Chemical Letters的其它文章
- A biomass based photonic crystal made of “konjac tofu”
- Hydrothermal-assisted grinding route for WS2 quantum dots (QDs)from nanosheets with preferable tribological performance
- Superiority of poly(L-lactic acid) microspheres as dermal fillers
- Zwitterionic comb-like lipid polymers encapsulating linalool for increasing the fragrance retention time
- Construction of a nano-rectangular Zn-Nd complex with near-infrared luminescent response towards metal ions
- Synthesis and structure of Au19Ag4(S-Adm)15 nanocluster:Polymorphs and optical properties
