Noncentrosymmetric K2Mn3(SO4)3F2·4H2O and Rb2Mn3(SO4)3F2·2H2O with pseudo-KTP structures
2021-04-02YngZhouYnqingLiQingrnDingYouchoLiuYngxinChenXitoLiuXioyingHungLinLiSngenZhoJunhuLuo
Yng Zhou,Ynqing Li,Qingrn Ding,Youcho Liu,Yngxin Chen,Xito Liu,c,Xioying Hung,Lin Li,c,Sngen Zho,c,*,Junhu Luo,c,*
a College of Chemistry and Materials Science, Fujian Normal University, Fuzhou 350007, China
b State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China
c Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350108, China
ABSTRACT Two fluoride sulfates, K2Mn3(SO4)3F2·4H2O (I) and Rb2Mn3(SO4)3F2·2H2O (∏) are obtained by water solution method.Single-crystal X-ray diffraction analysis indicated that they crystallize in space groups of Cmc21.Their structures feature a pseudo-KTP structure consisting of interconnecting[Mn3(SO4)3F2(H2O)2]∞layers, which are further packing along the a axis with alkali metal cations balancing the charges.The structure relationships between the two compounds are discussed.Secondharmonic generation measurements manifest that I and ∏have similar second-harmonic generation responses of about 0.2 and 0.25 times that of KH2PO4.
Keywords:Sulfate Second harmonic generation Crystal structure X-ray diffraction Thermogravimetric analysis
Nonlinear optical(NLO)materials are technically importance in producing coherent light sources[1-8].On the one hand,the NLO materials must meet the crystallographically non-centrosymmtric(NCS).On the other hand, the crystal must satisfy several fundamental requirements such as a wide transparent region,relatively large SHG response,and sufficient birefringence from the viewpoint of optics[9].Over the past decades, a series of notable NLO crystals have been discovered and used, including β-BaB2O4[10], LiB3O5[11], CsLiB6O10[12,13], KBe2B2O6F2[14] and KTiOPO4(KTP)[15].With the rapid development of scientific and technical applications, the search for new NLO materials is still of current academic and commercial interests.
In recent years,several chemical systems of NLO materials have been reported.In terms of borates, notable examples include NaSr3Be3B3O9F4[16], Na2CsBe6B5O15[17], Cd4BiO(BO3)3[18],Pb2Ba3(BO3)3Cl [19], as well as Li4Sr(BO3)2[20], Rb3Al3B3O10F[21], and K3Ba3Li2Al4B6O20F [22] recently reported by our group.Moreover, there are many carbonates (e.g., ABCO3F (A=K, Rb, Cs;B=Ca, Sr, Ba) [23], KMgCO3F [24], CsPbCO3F [25]), nitrates (e.g.,Bi3TeO6OH(NO3)2[26], Pb2(BO3)(NO3) [27], Sr2(OH)3NO3[28]),phosphates (e.g., Ba3P3O10X (X=Cl, Br) [29], RbBa2(PO3)5[30],RbNaMgP2O7[31]) have been obtained.However, there are fewer reports on sulfate-based NLO materials.One of the main reasons is that sulfates tend to decomposing and releasing SO3gas at high temperature, making it difficult to grow bulk crystals by the popular high-temperature molten techniques.Recently,two deep-UV NLO sulfates NH4NaLi2(SO4)2and (NH4)2Na3Li9(SO4)7were reported by our group [32], more importantly, they are facile for the crystal growth by a water solution method.Both sulfates are phase-matchable and revealed evident second-harmonic generation (SHG) (1.1×KH2PO4(KDP) and 0.5×KDP), respectively.Furthermore, due to the unsatisfactory arrangement of the two sulfates,there is much room for improving the SHG responses.In addition, Zou and his colleague recently reported a new sulfate CsSbF2SO4, which exhibits a strong SHG response.CsSbF2SO4was designed by simultaneously substituting [SbO4F2]7-and [SO4]2-units for[TiO6]8-and[PO4]3-functional groups in KTP,respectively[33].In this work,two NCS fluoride sulfates,K2Mn3(SO4)3F2·4H2O(I) and Rb2Mn3(SO4)3F2·2H2O (∏) were successfully synthesized by a facile water solution method.The two compounds feature pseudo-KTP structures and are NLO-active.
Single crystals of I and ∏were obtained via water solution.I and ∏were prepared through the three steps, which are as follows: (i) The KF or RbF and MnSO4·H2O of stoichiometric ratio were dissolved in deionized water, respectively, and finally the completely dissolved solution were obtained, (ii) heating the mixture at about 343 K under stirring for 1-2 h, (iii) evaporating the mixture at 333 K.Light pink single crystals were obtained.Their crystal structures were determined by single-crystal X-ray diffraction (XRD) (detailed descriptions are listed in Tables S1-S4 in Supporting information).Their powder XRD patterns were consistent with the simulated ones determined from the singlecrystal XRD analysis (Fig.S1 in Supporting information).The Energy Disperse Spectroscopy results (Fig.S2 in Supporting information) confirmed the existence of F element in the two compounds.
I and ∏crystallize in the same NCS space group Cmc21with similar crystal structures.Hence, the structure of I will be illuminated in detail as a representative.There are two crystallographically unique manganese atoms, i.e., Mn1 and Mn2 and two crystallographically unique sulfur atoms, i.e., S1 and S2 in the structure of I.The Mn1 atom is attached to two fluorine atoms and four oxygen atoms to form Mn1F2O4octahedron with Mn1-F distances of 2.103 Å and 2.143 Å and Mn1-O distances ranging from 2.137(3)Å to 2.266(4)Å.The Mn2 atom is attached to two fluorine atoms, three oxygen atoms and one water molecule to form Mn2F2O3(H2O)octahedron with the Mn2-F distances of 2.125(2)Å and 2.199(2) Å and Mn2-O distances ranging from 2.127(3) Å to 2.185(3)Å.The sulfur atoms coordinate with four oxygen atoms to form a SO4tetrahedron with the S1-O distances ranging from 1.456(4)Å to 1.503(4)Å and S2-O distances ranging from 1.456(3)Å to 1.482(3) Å.Mn1O4F2and Mn2O3F2(H2O) octahedra connected with each other via sharing edge and sharing corner to form infinite chains along c axis (Fig.1a), which are further connected with SO4tetrahedra to form[Mn3(SO4)3F2(H2O)2]∞layers in the bc plane (Fig.1b and Fig.S3 in Supporting information for ∏).The charge balancing K+cations and the rest water molecules reside between the layers.
From the viewpoint of structural evolution,I can be regarded as a derivative of KTP,in which the TiO6octahedra and PO4tetrahedra are substituted by Mn1O4F2and Mn2F2O3(H2O)octahedra and SO4tetrahedra,respectively (Figs.1c and d).Different from the threedimensional framework composed of PO4and TiO6in KTP, I exhibits a layered structure with [Mn3(SO4)3F2(H2O)2]∞layers.Meanwhile, the connection of octahedra is different.In the structure of KTP, the distorted TiO6octahedra share their corners through axial oxygen atoms to form infinite chains.In comparison,each Mn1O4F2octahedron is linked to four Mn2O3F2(H2O)octahedra via corner-sharing and two neighboring Mn2O3F2(H2O)octahedra are connected via edge-sharing in the infinite chains of[Mn3O10F2(H2O)2] (Fig.S4 in Supporting information).
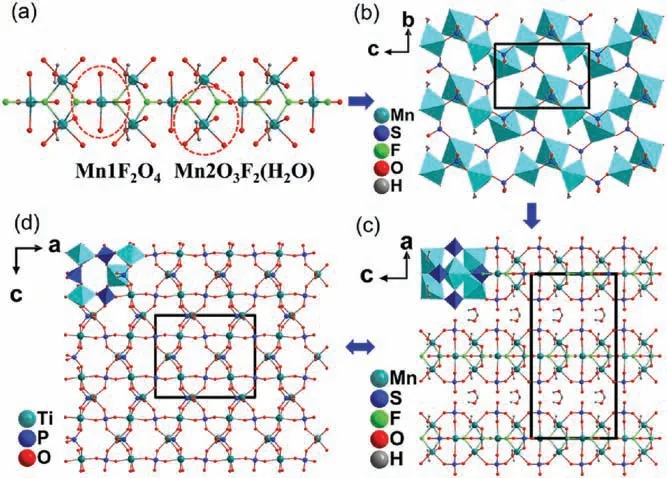
Fig.1.(a) [Mn3O10F2(H2O)2] chain.(b) [Mn3(SO4)3F2(H2O)2]∞layer.Structural comparison of (c)I and(d) KTiOPO4.Dark blue tetrahedra represent SO4 and light blue octahedra represent Mn1O4F2 and Mn2O3F2(H2O).
Despite I and ∏ have different molecular formula, they crystallize in the same space group and have similar layered structures composed of Mn1O4F2and Mn2F2O3(H2O) octahedra and SO4tetrahedra.As shown in Fig.2a, H2O molecular and K+cations occupy the interlayer spacing of I,whereas only Rb+cations reside in the interlayer spacing of ∏.This structural difference is responsible to the different formula.As shown in Fig.2b, each K atom is coordinated to six oxygen atoms and two water molecular;each Rb atom is coordinated to eight oxygen atoms and only one water molecular.Clearly, the additional water molecular coordinated to the K atoms can provide a larger steric hindrance than oxygen atom, thereby stopping the crystal structure of I from collapse,since the K-O distances are evidently shorter than that of Rb-O (Fig.2b).
The infrared spectra of I and ∏ are presented in Fig.S5(Supporting information).The moderate absorption peaks of the two compounds observed at 3280/1653 cm-1and 3462/1642 cm-1are attributable to O--H stretching and bending vibrations.Due to the interconnection of the distorted Mn1O4F2and Mn2O3F2(H2O)octahedral units,The SO42-tetrahedra are very slightly asymmetric,these differential bond lengths in SO42-tetrahedra trigger the degeneration of all stretching and bending vibrational peaks[34].The signature peaks at lower wavenumbers corresponds to the SO42-building blocks.The peaks at 1143/1081 cm-1and 1199/1106 cm-1correspond to SO42-asymmetric stretching vibrations.The peaks at 979 cm-1and 1000 cm-1correspond to SO42-symmetric stretching vibrations and the peaks at 653/612 cm-1and 697/616 cm-1correspond to SO42-asymmetric bending vibrations [35].
Fig.3 shows the thermogravimetric analysis curve of I and ∏.The result of the test indicated that I and ∏gradually lost weight starting from about 377 K and 473 K,the weight losses of 11.2%for I and 5.4% for ∏, respectively.Obviously, more crystal water in I is the reason for the obvious difference in weight loss between the two compounds.After heating to 873 K in the programmable furnace, the residues of I and ∏ were K2Mn2(SO4)3and Rb2Mn2(SO4)3, respectively.They were confirmed by the powder XRD analysis(Fig.S6 in Supporting information).The powder SHG measurements(Fig.4a)reveal that I and ∏exhibit SHG intensities of about 0.2 and 0.25×KDP respectively.The SHG tests further confirm the NCS structures of the titled compounds.Based on the anionic group theory, the traditional studies on NLO materials mainly focus on the π-conjugated system,such as[BO3]3-,[CO3]3-and [NO3]3-, which feature triangular planar geometry.In comparison,sulfates are analogous to phosphates,the microscopic second-order nonlinear susceptibility of the SO4and PO4units are one order of magnitude smaller than that of the planar B--O groups,BO3and B3O6.As such,the SHG coefficients of sulfates and phosphates are relatively small [36].Theoretical calculations on the relationships between the crystal structures and SHG responses will be carried out in the future.The UV-vis nearinfrared diffuse reflectance spectrum of I and ∏are shown in Fig.4b.The powders measurements indicate that I and ∏display the optical band gap of 5.54 eV and 5.55 eV, respectively.It suggesting that I and ∏are broad-band gap materials.(Fig.4b).
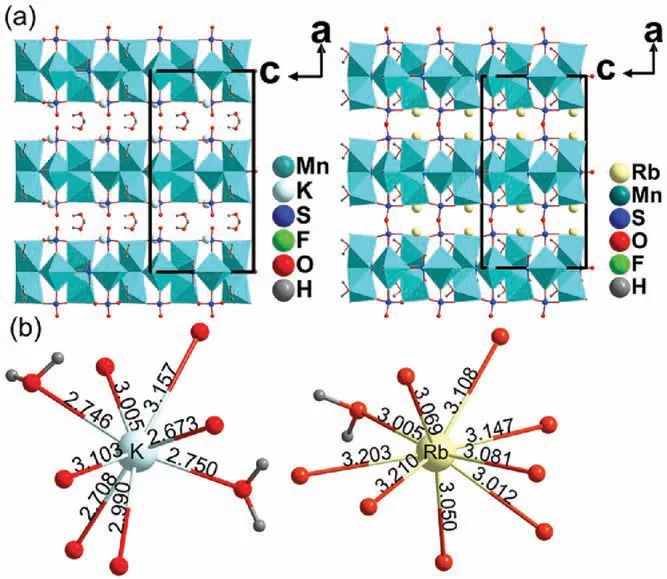
Fig.2.(a) Structural comparison of I and ∏.(b) The coordination environment of the alkali atoms.
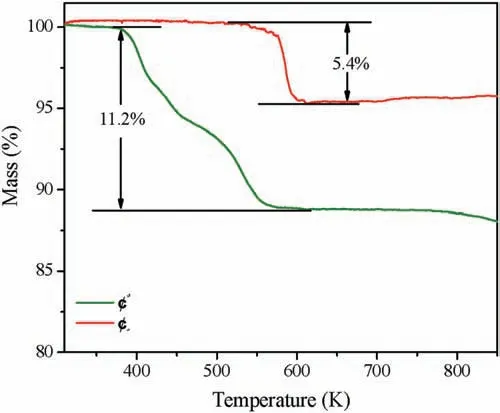
Fig.3.Thermogravimetric analysis of I and ∏.
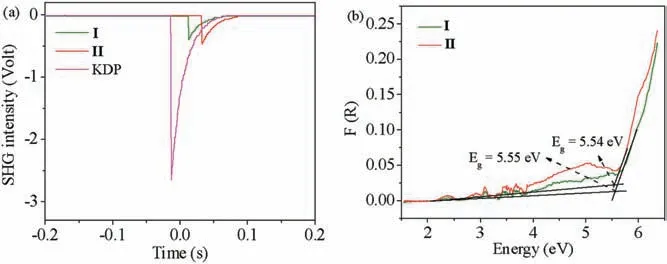
Fig.4.(a) Comparison on SHG signals for I, ∏and KDP.(b) Diffuse reflectance absorption cure of I and ∏.
In summary, we have successfully synthesized two new NCS fluoride sulfates by the water solution method, I and ∏.Despite the two sulfates have different molecular formula,they crystallize in the same space group and have similar pseudo-KTP layered structures.It can be speculated that the steric hindrance of the additional water molecular in comparison with oxygen atom coordinated to the K atoms can prevent the crystal structure of I from collapse.In addition, they exhibit relatively weak SHG responses of about 0.2 and 0.25×KDP,respectively,which further confirmed that they are NCS.This work enriches the structure chemistry of sulfates.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China(Nos.21833010,21525104,21971238,61975207, 21921001), the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (No.ZDBS-LYSLH024), Key Laboratory of Functional Crystals and Laser Technology, TIPC, CAS (No.FCLT 202003), as well as Key Laboratory of New Processing Technology for Nonferrous Metal & Materials,Ministry of Education/Guangxi Key Laboratory of Optical and Electronic Materials and Devices (No.20KF-11).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.014.
杂志排行
Chinese Chemical Letters的其它文章
- A biomass based photonic crystal made of “konjac tofu”
- Hydrothermal-assisted grinding route for WS2 quantum dots (QDs)from nanosheets with preferable tribological performance
- Superiority of poly(L-lactic acid) microspheres as dermal fillers
- Zwitterionic comb-like lipid polymers encapsulating linalool for increasing the fragrance retention time
- Construction of a nano-rectangular Zn-Nd complex with near-infrared luminescent response towards metal ions
- Synthesis and structure of Au19Ag4(S-Adm)15 nanocluster:Polymorphs and optical properties
