Chemical proteomics reveals ligustilide targets SMAD3, inhibiting collagen synthesis in aortic endothelial cells
2021-04-02WeiLeiFukuiShenNinweiChngLinNiuYunyunHouBoliZhngMinJingGngBi
Wei Lei,Fukui Shen,Ninwei Chng,Lin Niu,Yunyun Hou,Boli Zhng,Min Jing,*,Gng Bi,*
a State Key Laboratory of Medicinal Chemical Biology, College of Pharmacy and Tianjin Key Laboratory of Molecular Drug Research, Nankai University, Tianjin 300353, China
b Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
ABSTRACTAtherosclerosis is a persistent inflammatory state, while vascular endothelial fibrosis is one of the primary causes of atherosclerosis development.Although ligustilide (Lig) was shown to exert obvious antiatherogenic effects in previous studies,its precise mechanism has not been deeply discussed.In this paper, we designed a Lig-derived photoaffinity labelling (PAL) probe to identify potential therapeutic targets of Lig via chemical proteomics approach.Mothers against decapentaplegic homologue 3(SMAD3),a signal transmitter of transforming growth factor-β(TGF-β)which promotes the development of vascular fibrosis, was identified as a potential target of Lig.Lig suppressed the phosphorylation and nuclear translocation of SMAD3 by blocking the interaction between SMAD3 and TGF-β receptor 1,thereby inhibiting the collagen synthesis process.Hence, developing a novel SMAD3 inhibitor may present a promising therapeutic option for preventing vascular fibrosis.
Keywords:Ligustilide SMAD3 Collagen synthesis Chemical proteomics Photoaffinity labelling
Atherosclerosis is a persistent inflammatory state accompanied by lipid overload and is the cause of most cardiovascular diseases,such as stroke and coronary heart diseases [1].During the development of atherosclerosis, vascular sclerosis occurs, which is primarily due to the continuous fibrosis caused by vascular inflammation[2].Therefore,vascular fibrosis,characterized by the excessive deposition of extracellular matrix, including collagens,proteoglycans and inflammatory cytokines, is one of the vital causes of vascular function loss in the development of atherosclerosis [3].Antiplatelet agents, lipid-lowering medicines and thrombolytics are the most widely used clinical antiatherogenic drugs and aim to inhibit lipid production,block plaque formation or promote plaque digestion.Some of these medications, such as statins,act to decrease the amount of collagen by regulating small guanosine triphosphate (GTP)-binding proteins, exerting beneficial effects by preventing vascular fibrosis[4-6].However,there is still no clinically efficient medicine available to address vascular fibrosis.Therefore, it is urgently needed to identify novel medicines that can effectively prevent vascular fibrosis.
Ligustilide(Lig),characterized by α, β-unsaturated γ lactone,is a multi-active compound with many beneficial effects, such as anti-inflammation, antioxidation [7], nephroprotection [8] and vasodilatation [9].Recent research has indicated that Lig rescues atherosclerosis by promoting the expression of genes that are downstream of nuclear factor-erythroid 2-related factor 2 (NRF2)and inhibits vascular inflammation [10,11].In addition, Lig is a primary component in herbal medicines, such as Ligusticum Chuanxiong Hort. and Angelica Sinensis(Oliv.)Diels. It was reported that Radix Angelica Sinensis exerts obvious inhibition effects on interstitial pulmonary fibrosis, epidural fibrosis and myocardial fibrosis [12-14].This information can help to identify novel mechanisms for preventing atherosclerosis and vascular fibrosis by applying traditional medicine experience.
Chemical proteomics, and in particular photoaffinity labelling(PAL), is a potent strategy for generating information regarding protein-ligand interactions [15,16].PAL enables us to study noncovalent kinetic intermediates and heterogeneous mixtures and has been successfully implemented to identify the targets of many bioactive small molecules[17,18].In this study,we investigated the potential mechanism of Lig in vascular endothelial fibrosis.A chemical proteomics approach with an active ingredient ligustilide-derived photoaffinity labelling (Lig-PAL) probe was used to bridge the protein targets and anti-fibrosis functions.Furthermore,mothers against decapentaplegic homologue 3(SMAD3)(as one of the most likely targets)was identified by LC-MS/MS analysis and Western blot.Lig was found to suppress the phosphorylation and nuclear translocation of SMAD3 by blocking the mutual interaction of SMAD3 and TGF-β receptor 1, thereby preventing the collagen synthesis process in the development of atherosclerosis.
The material information and detailed experimental procedures were described in Supporting information.
To explore the effective target of Lig in alleviating vascular endothelial fibrosis, a Lig-PAL probe was designed to fish the potential targets using chemical proteomics(Fig.1A).The detailed synthesis and evaluation data are shown(Figs.S1,S2,S3A and B in Supporting information).The MTT assay results indicated that Lig and Lig-PAL probe had a similar cell viability effect in mouse aortic endothelial cells(MAECs)(Fig.S3C in Supporting information).The anti-inflammation activity of the Lig-PAL probe was evaluated by monitoring the suppression of interleukin-6(IL-6)expression and transforming growth factor-β 1 (TGF-β1) secretion in MAECs stimulated by tumor necrosis factor-α (TNF-α).The results revealed that the Lig-PAL probe maintained the same effects on IL-6 and TGF-β 1 inhibition with Lig at 10-4-10-5mol/L (Fig.1B).Thus, the Lig-PAL probe can be used for the target fishing process.
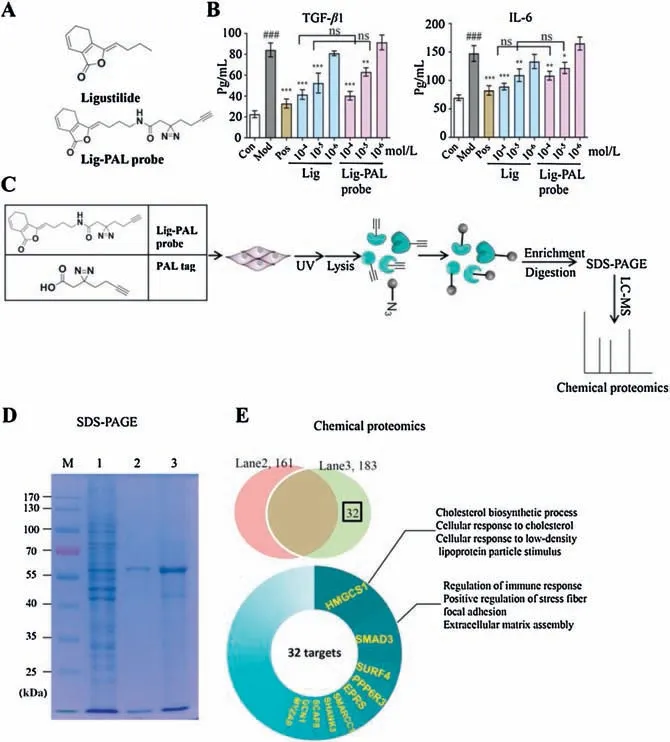
Fig.1.Target profiling via Lig-PAL probe in MAECs.(A)Molecular structures of Lig and Lig-PAL probe.(B)Evaluation of the activities of Lig and Lig-PAL probe via the detection of IL-6 expression and TGF-β1 secretion in MAECs stimulated with TNF-α.The data represent the mean± SD (n=3); *P <0.05, **P <0.01, ***P <0.001 compared to the model group; ###P <0.001 compared to the control group;ns:no significant difference (one-way ANOVA with a Bonferroni correction).(C) Overall scheme of the PAL experiments to identify the targets of Lig.(D) Evaluating the enrichment efficiency of the Lig-PAL probe by SDS-PAGE.Lane 1,total protein from MAEC lysates;1/30 of each lysate was loaded; Lane 2 and Lane 3 contain proteins enriched from per MAEC lysate by unloaded PAL tag-modified MMs and Lig-PAL probe-modified MMs.(E) Protein identification by LC-MS.Venn diagram showing the number of proteins identified from Lane 2 and Lane 3.The relative weights of the top 10 proteins in the 32 deduced proteins are represented in the pie graph.The biological functions of the top two proteins, HMGCS1 and SMAD3, are annotated.
To identify potential targets, the Lig-PAL probe was used to incubate TNF-α-stimulated MAECs for 8 h.These MAECs were then irradiated at 365 nm to induce an optical crosslinking.Finally,the proteins were extracted and enriched by azide-modified magnetic microspheres (MMs) and identified by LC-MS analysis (Fig.1C).The enrichment efficiency was evaluated by SDS-PAGE, and the proteins captured by the Lig-PAL probes are apparent in lane 3 in Fig.1D.The nonspecific proteins in lane 2 from the PAL tag group,which were labelled only with the photoaffinity tag,were analysed by the same procedure.The results of LC-MS spectrometry identified 161 proteins in lane 2 and 183 proteins in lane 3.Thirtytwo proteins related only to Lig-PAL probe were identified, and their biological functions were analysed using KEGG (Fig.1E).Among these 32 proteins, hydroxymethylglutaryl-CoA synthase 1(HMGCS1)and SMAD3 had the highest proteomics scores(Fig.S4 in Supporting information).These proteins play important roles in lipid production,the immune response,cell adhesion and fibrosis.
To validate that SMAD3 is a potential target of Lig,a competitive capture assay was carried out.MAECs were first separated into 4 groups in which cells were stimulated by regular medium,2×10-5mol/L PAL tag,1×10-4mol/L Lig with 2×10-5mol/L Lig-PAL probe or 2×10-5mol/L Lig-PAL probe alone.TNF-α (20 ng/mL) was added simultaneously.The protein supernatants were analysed by Western blot.During these processes,free Lig as a competitor was used to compete for SMAD3 that was captured by Lig-PAL probe,and the effect was detected by Western blot analysis.The Lig-PAL probe (2×10-5mol/L) was first used to label SMAD3 in MAECs stimulated by TNF-α (lane 4 in Fig.2A).As shown in lane 3 of Fig.2A, free Lig (1×10-4mol/L) could reduce the binding of Lig-PAL probe in the process of capturing SMAD3.This finding indicates that SMAD3 may be a specific target of Lig.
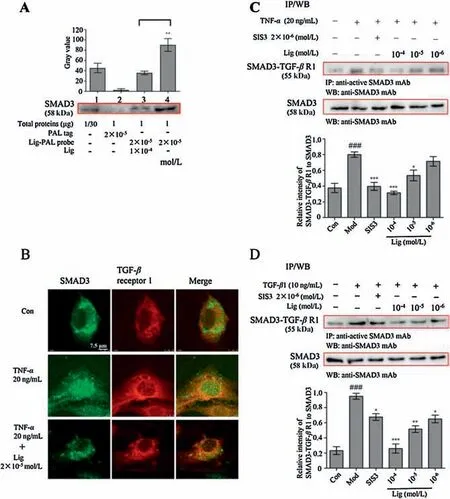
Fig.2.Lig inhibits the combination of SMAD3 and TGF-β receptor 1(TGF-β R1).(A)Lig-PAL probe binding SMAD3 in the presence of free Lig was detected by Western blot.Control group(Lane 1):total protein from MAEC lysates(loading quantity was 1/30 of the lysate); proteins enriched from the MAEC lysates in the PAL tag group(Lane 2)and Lig-PAL probe-modified MM group(Lane 4);competition group(Lane 3): proteins enriched from the MAEC lysates by 2×10-5 mol/L Lig-PAL probemodified MMs in the presence of 1×10-4 mol/L Lig.##P <0.01 compared to the competition group,(n=3).(B)The colocalization of SMAD3 and TGF-β receptor 1 in MAECs treated(or not)with 2×10-5 mol/L Lig.Scale bar 7.5 μm.(C)Western blot of TGF-β receptor 1 Co-IP with SMAD3 from MAECs after TNF-α stimulation for 8 h.(D)Western blot of TGF-β receptor 1 Co-IP with SMAD3 from MAECs after TGF-β1 stimulation for 30 min.The data represents the mean± SD(n=3);*P <0.05, **P <0.01, ***P <0.001 compared to the model group; ###P <0.001 compared to the control group.Two-tailed Student’s t-test for A and one-way ANOVA with a Bonferroni correction for C and D.
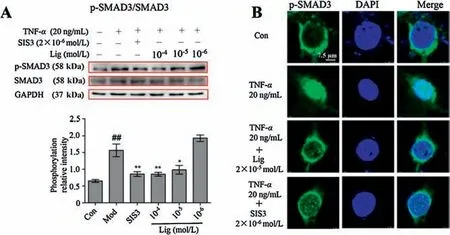
Fig.3.Lig suppresses the phosphorylation and unclear translocation of SMAD3.(A)Lig decreased the TNF-α-induced rise of p-SMAD3/SMAD3 in MAECs.(B) Lig attenuated the TNF-α-induced nuclear translocation of p-SMAD3 in MAECs.The pseudo green represents p-SMAD3,while the blue fluorescence represents nuclei.Scale bar, 7.5 μm.The data represent the mean± SD (n=3); *P <0.05, **P <0.01 compared to the model group; ##P <0.01 compared to the control group(one-way ANOVA with a Bonferroni correction).
In this activation, SMAD3 is recruited to the TGF-β receptor kinase 1 for phosphorylation.In MAECs stimulated with TNF-α, abundant colocalization of SMAD3 and TGF-β receptor 1 was observed(Fig.2B and Fig.S5 in Supporting information).However,once Lig was trafficked to SMAD3, the colocalization of SMAD3 with TGF-β receptor 1 was disrupted(Fig. 2B and Fig.S5).The Co-IP assay results also demonstrated that SMAD3 combined with TGF-β receptor 1 was increased after TNF-α stimulation.However, Lig significantly decreased the amount of SMAD3 combined with TGFβ receptor 1 in a concentration-dependent manner, as did the positive SMAD3 inhibitor SIS3 (Fig.2C).Besides, the Lig exerted similar inhibition effect during TGF-β 1 stimulation for 30 min(Fig.2D).Then, the effects of Lig on the TGF-β /SMAD3 signaling cascade were also investigated.
The phosphorylation of SMAD3 by TGF-β receptor 1 induces the aggregation of SMAD complexes and initiates the nuclear translocation of SMAD3.As shown in Fig.3A, the p-SMAD3/SMAD3 ratio increased following TNF-α stimulation, while SIS3 and Lig could effectively reduce the high phosphorylation ratio.Next, we investigated the inhibitory effect on the Lig-mediated nuclear translocation of p-SMAD3.As shown in Fig.3B, TNF-α stimulation induced the translocation of p-SMAD3 from the cytosol to the nucleus.However,this translocation was effectively reduced upon Lig and SIS3 treatment.These results reveal that Lig inhibits the mutual interaction of SMAD3 and receptor kinase 1 and inhibits the phosphorylation and nuclear translocation of SMAD3.
MAECs stimulated by TNF-α highly express collagen I (COL I)and COL III,the key components of vascular fibre[19].However,Lig and SIS3 could effectively downregulate the expression of these two fibrous proteins(Fig.4A).Once SMAD3 was silenced by siRNA,TNF-α could not upregulate the expression of COL I and COL III,and Lig and SIS3 also did not exert effective regulation on COL I and COL III expression(Fig. 4B and Fig.S6 in Supporting information).These results indicate that Lig suppresses the expression of COL I and COL III through inhibiting the activity of SMAD3.
The photo-crosslinking progress is initiated by UV irradiation to produce carbene groups.Diazirine,the absorption of which is 340-380 nm far from the 280 nm absorption of proteins, was widely used as a kind of photo-crosslinker [20].When the transient protein-small molecule structure exists, the active carbene can attack the protein to generate protein-probe complex for further enrichment and proteomics analysis [21].In this study, we designed a novel alkyne and diazirine-containing probe, which retains the anti-IL-6 and anti-TGF-β activities of Lig,and this probe efficiently helps us identify the targets of Lig improving vascular endothelial fibrosis and atherosclerosis.
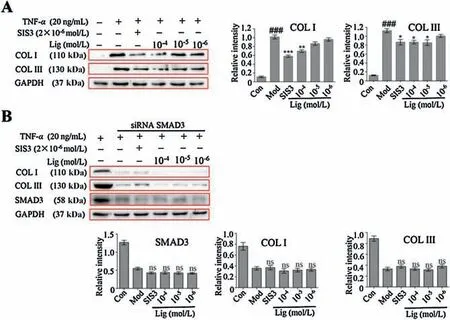
Fig.4.Lig inhibits the expression of collagens in MAECs through targeting on SMAD3.(A)Lig suppressed the TNF-α-induced high expression of COL I and COL III in MAECs.(B) siRNA interference test; Lig lost its suppression effect on COL I and COL III expression once SMAD3 was silenced by siRNA.The data represent the mean± SD(n=3);*P <0.05, **P <0.01,***P <0.001 compared to the model group;###P <0.001 compared to the control group;ns:no significant difference,compared to the model group (one-way ANOVA with a Bonferroni correction).
Atherosclerosis is one of the most important catalysts of cardiovascular disease, causing a huge financial burden for patients worldwide.In the development of atherosclerosis,the accumulation of oxidized low-density lipoprotein (oxLDL) and cholesterol in vascular lesions stimulates the overlying endothelial cells (ECs) to produce collagens and adhesion molecules, acting as a source of myofibroblasts to instigate vascular fibrosis [22,23].The primary cytokines and biological pathways during vascular fibrosis are TGF-β, peroxisome proliferator-activated receptor gamma, connective tissue growth factors, matrix metalloproteinases and the renin-angiotensinaldosterone system[24].SMAD3,as the signal transducer of TGF-β, participates in the synthesis of proteoglycans and collagen[25,26],and acts as a key promoter in SMC and EC fibrosis[27].Therefore,targeting SMAD3 to block the TGF-β /SMAD3 pathway may be an ideal approach for preventing vascular fibrosis and the development of atherosclerosis.In the current study, our results proved that Lig can effectively target SMAD3 to prevent fibrosis.Previous studies indicated that Lig ameliorated vascular and circulatory inflammation by indirectly slowing the expression of AP-1 and NFkappa B in atherosclerotic mice [10,11], which is helpful for interfering vascular fibrosis through the crosstalk of antiinflammatory signaling.However, Lig obstructing TGF-β /SMAD3 pathway is a direct and efficient approach to blocking vascular fibrosis.
In this study, a Lig-PAL probe showed a powerful capacity for target capture,and SMAD3 was identified as a putative target.Our results suggested that Lig suppresses the phosphorylation and nuclear translocation of SMAD3 by blocking the binding of SMAD3 to TGF-β receptor 1.Our results also showed that Lig effectively inhibits the expression of COL I and COL III.Therefore,our findings may point to another avenue for preventing atherosclerosis.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was financially supported by National Key Research and Development Program of China (Nos.2018YFC1704800,2018YFC1704805), National Natural Science Foundation of China(No.81673637) and the Key R&D Program of Tianjin (No.18YFYZCG00060).
Appendix A.Supplementary data
Supplementarymaterialrelatedtothisarticlecanbefound,inthe online version,at doi:https://doi.org/10.1016/j.cclet.2020.10.049.
References
[1] P.Libby, P.M.Ridker, G.K.Hansson, Nature 473 (2011) 317-325.
[2] C.P.Denton, Semin.Arthritis Rheum.49 (2019) S3-S7.
[3] N.A.Shirwany, M.H.Zou, Acta Pharmacol.Sin.31 (2010) 1267-1276.
[4] E.Agabiti-Rosei, M.Heagerty, D.Rizzoni, J.Hypertens.27 (2009) 1107-1114.
[5] L.F.Fried, Kidney Int.74 (2008) 571-576.
[6] F.Simko, Eur.J.Clin.Invest.37 (2007) 681-691.
[7] J.Y.Yang, H.H.Chen, J.Wu, et al., Chin.Herb.Med.1 (2012) 26-32.
[8] V.Bunel, M.H.Antoine, J.Nortier, et al., Toxicol.In Vitro 29 (2015) 458-467.
[9] W.Lei, J.N.Ni, X.T.Xia, et al., J.Chromatogr.B 15 (2018) 220-227.
[10] Y.Zhu, Y.Zhang, X.Huang, et al., Atherosclerosis 284 (2019) 110-120.
[11] W.Lei, Y.F.Deng, X.Y.Hu, et al., Biomed.Pharmacother.117 (2019) 109074.
[12] L.Wang, Y.Sun, C.Ruan, et al., Biotechnol.Equip.28 (2014) 923-928.
[13] C.Zhang,X.Kong,H.Zhou,et al.,Evid.Complement.Alternat.Med.2013(2013)291814.
[14] C.Ma, Z.Fu, H.Guo, et al., Biomed.Pharmacother.112 (2019) 108596.
[15] Y.Ren, Q.S.Sun, Z.G.Yuan, et al., Chin.Chem.Lett.30 (2019) 1233-1236.
[16] J.T.Du, J.Guo, D.W.Kang, et al., Chin.Chem.Lett.31 (2020) 1695-1708.
[17] G.D.Hu, H.Y.Jia, L.N.Zhao, et al., Chin.Chem.Lett.30 (2019) 1704-1716.
[18] S.Pan, H.Zhang, C.Wang, et al., Nat.Prod.Rep.5 (2016) 612-620.
[19] Y.Zhang, Z.Wang, X.Ma, et al., Acta Pharm.Sin.B 9 (2019) 294-303.
[20] M.Matthias, Matrix Biol.28-29 (2018) 106-121.
[21] R.A.Smith, J.R.Knowles, J.Am.Chem.Soc.95 (1973) 5072-5073.
[22] A.J.Lusis, Nature 407 (2000) 233-241.
[23] X.Sun,B.Nkennor,O.Mastikhina,et al.,Semin.Cell Dev.Biol.101(2020)78-86.
[24] T.H.Lan, X.Q.Huang, H.M.Tan, et al., Cardiovasc.Pathol.22 (2013) 401-407.
[25] Y.Zhu, H.Tao, C.Jin, et al., Mol.Med.Rep.12 (2015) 5573-5579.
[26] K.Jin, Z.M.Luo, B.Zhang, et al., Acta Pharm.Sin.B 1 (2018) 23-33.
[27] L.Montorfano, A.Becerra, R.Cerro, et al., Lab.Invest.94 (2014) 1068-1082.
杂志排行
Chinese Chemical Letters的其它文章
- A biomass based photonic crystal made of “konjac tofu”
- Hydrothermal-assisted grinding route for WS2 quantum dots (QDs)from nanosheets with preferable tribological performance
- Superiority of poly(L-lactic acid) microspheres as dermal fillers
- Zwitterionic comb-like lipid polymers encapsulating linalool for increasing the fragrance retention time
- Construction of a nano-rectangular Zn-Nd complex with near-infrared luminescent response towards metal ions
- Synthesis and structure of Au19Ag4(S-Adm)15 nanocluster:Polymorphs and optical properties
