GOECP/SEOR radiotherapy guidelines for small-cell lung cancer
2021-04-02FelipeCouagoCarolinadelaPintaSusanaGonzaloCastaliaFernndezPiedadAlmendrosPatriciaCalvoBegoTaboadaAntoniomezCaamaJosLuispezGuerraMarisaChustJosAntonioGonzlezFerreiraAnalvarezGonzlezFrancescCasas
Felipe Couñago, Carolina de la Pinta, Susana Gonzalo, Castalia Fernández, Piedad Almendros, Patricia Calvo,Begoña Taboada, Antonio Gómez-Caamaño, José Luis López Guerra, Marisa Chust, José Antonio González Ferreira, Ana Álvarez González, Francesc Casas
Felipe Couñago, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Hospital La Luz, Universidad Europea de Madrid, Madrid 28223, Madrid, Spain
Carolina de la Pinta, Department of Radiation Oncology, Hospital Universitario Ramón y Cajal,Madrid 28034, Spain
Susana Gonzalo, Department of Radiation Oncology, Hospital Universitario La Princesa,Madrid 28006, Spain
Castalia Fernández, Department of Radiation Oncology, GenesisCare Madrid, Madrid 28043,Spain
Piedad Almendros, Department of Radiation Oncology, Hospital General Universitario,Valencia 46014, Spain
Patricia Calvo, Begoña Taboada, Antonio Gómez-Caamaño, Department of Radiation Oncology,Hospital Clínico Universitario Santiago de Compostela, Santiago de Compostela 15706, Spain
José Luis López Guerra, Department of Radiation Oncology, Hospital Universitario Virgen del Rocío, Sevilla 41013, Spain
Marisa Chust, Department of Radiation Oncology, Fundación Instituto Valenciano de Oncología, Valencia 46009, Spain
José Antonio González Ferreira, Department of Radiation Oncology, GenesisCare-Spain, Sevilla 41092, Spain
Ana Álvarez González, Department of Radiation Oncology, HGU Gregorio Marañón, Madrid 28007, Spain
Francesc Casas, Department of Radiation Oncology, Thoracic Unit, Hospital Clinic, Barcelona 08036, Spain
Abstract Small cell lung cancer (SCLC) accounts for approximately 20% of all lung cancers.The main treatment is chemotherapy (Ch). However, the addition of radiotherapy significantly improves overall survival (OS) in patients with non-metastatic SCLC and in those with metastatic SCLC who respond to Ch. Prophylactic cranial irradiation reduces the risk of brain metastases and improves OS in both metastatic and non-metastatic patients. The 5-year OS rate in patients with limited-stage disease (non-metastatic) is slightly higher than 30%, but less than 5%in patients with extensive-stage disease (metastatic). The present clinical guidelines were developed by Spanish radiation oncologists on behalf of the Oncologic Group for the Study of Lung Cancer/Spanish Society of Radiation Oncology to provide a current review of the diagnosis, planning, and treatment of SCLC. These guidelines emphasise treatment fields, radiation techniques,fractionation, concomitant treatment, and the optimal timing of Ch and radiotherapy. Finally, we discuss the main indications for reirradiation in local recurrence.
Key Words: Small cell lung cancer; Chemotherapy; Hyperfractionated radiation therapy;Prophylactic brain irradiation; Brain metastases; Reirradiation
INTRODUCTION
Evolution of the multimodal treatment of small-cell lung cancer
Small cell lung cancer (SCLC) was first described by Barnard[1]in 1926. Prior to that time, SCLC was considered a mediastinal lymphosarcoma and later it was known as“oat cell carcinoma of the bronchus”[2]. The treatment of SCLC has evolved over time and this evolution is reflected in published randomised controlled trials (RCT). Table 1 summarizes the main clinical trials carried out to date to evaluate the treatment of SCLC. Based on advances in thoracic surgery during World War II, surgery became the treatment of choice in SCLC until 1960[3].
The first RCT comparing surgery to radiotherapy (RT) in SCLC was performed in the 1960s[4,5]. After that, the Veterans Administration Lung Study Group (VALSG)defined SCLC as either limited-stage (LS) or extensive-stage (ES) disease depending on whether or not the disease could be encompassed within a single RT field[6].
Later, a RCT comparing cyclophosphamide (CP) to placebo found that CP significantly improved overall survival (OS)[7]. In a subsequent clinical trial, combined therapy (RT + CP) increased survivalvsRT alone[8]. Other RCTs later confirmed those findings[9,10], while the findings of a subsequent RCT conducted by Perezet al[11]suggested that prophylactic cranial irradiation (PCI) could further prolong survival.
Polychemotherapy (PCh) regimens were evaluated in various clinical trials[12-14], one of which concluded that PCh was superior to CP[15]. As a result, RT was relegated to being used as consolidation therapy[16,17]. Other studies found that combiningchemotherapy (Ch) and RT (chemoradiotherapy; CRT) resulted in median survival outcomes of 12-15 mo and 5-year OS rates ranging from 10%-15%. While CRT seemed to yield comparable OS outcomes, treatment-related toxicity was substantially greater when the two treatment modalities were combined[18,19]. Two meta-analyses (MTA)found that adding RT to Ch improved 3-year OS[20-22].

Table 1 Chronological changes in the combined treatment of small cell lung cancer
Based on previous experience with PCh regimens, a treatment protocol involving RT to the brain and thorax combined with high dose Ch [CP, Adriamycin and Vincristine (CAV)] was developed, achieving greater local control (LC) than either treatment alone[23]. However, that approach was abandoned due to high levels of treatment-related toxicity[24]. Cisplatin-etoposide Ch combined with RT has shown to be effective in the treatment of SCLC with a manageable toxicity profile[25].
The next advance in treatment protocols for LS-SCLC came from a RCT comparing early thoracic RT (TRT) to late TRT, both groups received concurrent PCh, showing a significant benefit in 5-year OS for early TRT[26]. A phase II trial compared once-daily TRT to twice-daily (1.5 Gy/session) accelerated hyperfractionated RT (AHF-RT) (45 Gy). All patients received concurrent cisplatin-etoposide PCh[27]. The median survival in the AHF-RT group was 23 mo, with a 3-year OS of 30%[28].
Another RCT[29]was performed to compare initial (weeks 1 to 4) to delayed (weeks 6-9) AHF-RT (54 Gy), with all patients receiving concurrent low-dose PCh. Median survival in the early group was 34 movs26 mo in the delayed group; 5-year OS was 30%vs15%, respectively (P= 0.027). Another RCT[30]compared AHF-RT combined with cisplatin-etoposide administered concurrently or sequentially. The results of that trial showed that concurrent CRT was associated with significantly better OS outcomes(P< 0.05) than sequential treatment. Later, another phase III trial[31]compared concurrent AHF-RT to normofractionated TRT, with both groups also receiving adjuvant cisplatin-etoposide PCh. OS was significantly better in the AHF-RT group (P= 0.04). Nevertheless, this OS benefit was not confirmed in a subsequent RCT comparing normofractionated RT to AHF-RT[32]. A MTA found that patients with LSSCLC who underwent PCI after achieving complete remission had a 6% improvement in 3-year OS[33].
The so-called “Turrisi scheme” (AHF-RT) became controversial when critics rejected this treatment approach for the following reasons: (1) The TRT doses in the accelerated and normofractionated regimens are not biologically-equivalent; (2) This scheme implies higher treatment-related costs for the health care system; (3) Twice-daily RT means important organizational challenges; and (4) AHF-RT is associated with higher rates of esophageal toxicity. Furthermore, high-dose, normofractionated regimens seem to show equivalent results in terms of OS[34]and some MTA have found contradictory results[35,36].
Advances in SCLC staging, PCI in stage IV disease
Two important studies were published in 2007: The first proposed staging SCLC according to the AJCC (American Joint Committee on Cancer) TNM (tumor-nodemetastasis) criteria[37]. The second study reported improved OS outcomes in patients with ES-SCLC who had a confirmed response to PCh and subsequently received PCI[38]. Based on those findings, PCI became the standard treatment for patients with LS-SCLC who showed a partial response (PR) to treatment.
Regarding diagnostic techniques, 18F-fluorodeoxyglucose (FDG) positron-emission tomography-computed tomography (PET/CT) was incorporated into clinical practice because it provides more accurate staging of supraclavicular and distant disease, thus reducing the need for prophylactic mediastinal irradiation[39,40].
Phase III trials: CREST for stage IV disease and CONVERT for stages I-III
In recent years, the results of two landmark RCT were published: CREST and CONVERT[41-43]. CREST was performed to evaluate consolidative TRT in patients with ES-SCLC who respond to Ch. Median survival was significantly longer in the treatment group with better 5-year OS rates. In the CREST trial[41,42], patients were randomised to consolidative TRT (30 Gy in 10 fractions) or no TRT (control group). All patients received PCI. Thoracic recurrences were significantly reduced in the intervention groupvscontrol (80%vs44%). The 2-year OS was 13%vs3%. A post hoc analysis revealed a greater survival benefit in patients who underwent thoracic RT after PCh[44]and in patients with ≤ 3 extrapulmonary metastases who received consolidative TRT[45].
In the CONVERT trial[43], patients were randomised to twice-daily AHF-RT (45 Gy)vsonce-daily RT (66 Gy). Both groups received PCI. There were no significant differences between the groups in OS, progression-free survival (PFS), LC, or metastatic progression. Based on these findings, the Turrisi scheme remained the treatment of choice due to higher compliance rates (98%vs83%) with the TRT dose. A subsequent MTA showed that OS was better in patients treated with earlyvslate TRT in patients who complied with the prescribed dose intensity of the Ch regimen[46].
Consolidation RT for metastatic lesions in stage IV SCLC
Consolidation immunotherapy in ES-SCLC:In a RCT, patients with stage IV ESSCLC and ≤ 4 extracranial metastases after complete or partial response to PCh were randomised to PCI alone or PCI plus consolidative TRT for intrathoracic disease and extracranial metastases. The trial was closed at the interim analysis due to slow recruitment and the absence of significant differences in OS[47]. The risk of first thoracic recurrence was lower in the consolidative TRT group and in patients whose metastases were irradiated.
Recently, several phase III trials have reported better survival outcomes in patients with ES-SCLC when combining carboplatin-etoposide with atezolizumab[48]or durvalumab[49,50]. The results of two RCTs comparing dose escalation with the normofractionated schemevsthe “Turrisi” scheme are pending: CALGB 30610(normofractionation to 70 Gy) and a Norwegian study involving high-dose (60 Gy)AHF-RT in the experimental arm[51].
DIAGNOSIS, EVALUATION OF DISEASE SPREAD AND STAGING OF SCLC
SCLC is commonly diagnosed in advanced stages. Early detection of SCLC with computed tomography has not shown to provide any benefit. Given the aggressive nature of advanced SCLC, the diagnosis should be made in a few weeks, but treatment should not be initiated until histological confirmation[52]is done.
In patients with SCLC, the following key variables should be registered: Age,comorbidities, tobacco use, and ECOG status. Patients should undergo a complete physical examination with comprehensive blood tests. Molecular markers provide little value, although pro-gastrin-releasing peptide has shown specificity in the diagnosis and follow-up of some patients with SCLC[53].
Paraneoplastic syndromes can occur due to the production of polypeptides(syndrome of inappropriate antidiuretic hormone secretion or Cushing’s syndrome),or autoimmune phenomena (Lambert-Eaton syndrome, anti-Hu-associated encephalomyelitis, among others).
Staging is performedviathoracoabdominal computed tomography with intravenous contrast (IVC), unless contraindicated. Preferably, magnetic resonance imaging (MRI) should be used to perform the brain study due to its greater sensitivity and better definition of brain structures. Brain metastases (BM) are present in more than 12% of patients previously classified with LS-SCLC.
18F-FDG PET/CT imaging is recommended in LS-SCLC, but considered optional in ES-SCLC if it implies a delay in treatment. This imaging modality provides the best mediastinal staging and distant localization of metastases (adrenal and bone)[54]. Due to its high sensitivity (close to 100%) and specificity (90%), 18F-FDG PET/CT imaging changes the therapeutic approach in 28% of patients[55], including re-staging in 15% of cases [95% confidence interval (CI): 9-21][56].
18F-FDG PET/CT images should be acquired under the same positioning and immobilization conditions of the planned treatment. This imaging modality can help to optimize radiation doses and treatment volumes[57]. One study reported a higher risk of supraclavicular lymph node (LN) recurrence when CT-based nodal irradiation was omitted[58]. It has been suggested that better definition of volumes could influence both LC and OS[59]. Although this improvement in OS has not been demonstrated in prospective studies[60], some have reported lower rates of esophagitis[57]. In patients who undergo sequential treatment, Ch can reduce glucose consumption; consequently,restaging with 18F-FDG PET/CT may underestimate mediastinal involvement, which is why this imaging modality is not appropriate for planning RT[61,62].
Bony metastases are often present in these patients. A bone scan is indicated if 18FFDG PET/CT is not available[63]. If there is suspected bone marrow involvement, a biopsy is indicated (level of evidence V, grade of recommendation C)[64]. Abdominal MRI with IVC may be necessary to identify hepatic or adrenal lesions (level of evidence V, grade of recommendation C).
Histologic confirmation is generally obtained through bronchoscopy, endobronchial ultrasound (EBUS), or transesophageal endoscopic ultrasound (level of evidence III,grade of recommendation C)[65]. EBUS-guided needle aspiration is highly effective,with a sensitivity of 97.4%, specificity of 100%, negative predictive value of 60%, and positive predictive value of 100%[66]. Peripheral nodal biopsy, mediastinoscopy, or diagnostic thoracoscopy will rarely be necessary. In patients with ES-SCLC, it is recommended to biopsy the soft tissue lesions that offer the highest yield with the least risk (e.g., skin, liver). Prior to surgery or RT, all patients should undergo respiratory function testing. Table 2 summarizes the imaging tests recommended for staging SCLC.
According to the World Health Organization[67], SCLC is an epithelial neoplasm of neuroendocrine origin, with small cells on Alcian blue staining, scant cytoplasm,dispersed chromatin, inconspicuous nucleoli, numerous mitoses (> 10/2 mm2) and abundant necrosis. These lesions may be found as well in adenocarcinoma cells or squamous cell carcinoma. Immunohistochemistry will clarify diagnostic uncertainty based on expression of chromogranin, synaptophysin, and CD56, although these markers may be absent in 5%-10% of cases. CK7 is present in 25% and TTF-1 in 85%-90% of cases. Ki-67 Levels are elevated in most patients, thus facilitating differential diagnosis with other neuroendocrine tumours. Programmed death ligand 1 expression is currently being studied to determine if immunotherapy can be added to current treatment approaches. To identify new therapeutic targets, other molecular profiles can be considered in nonsmokers with ES-SCLC.
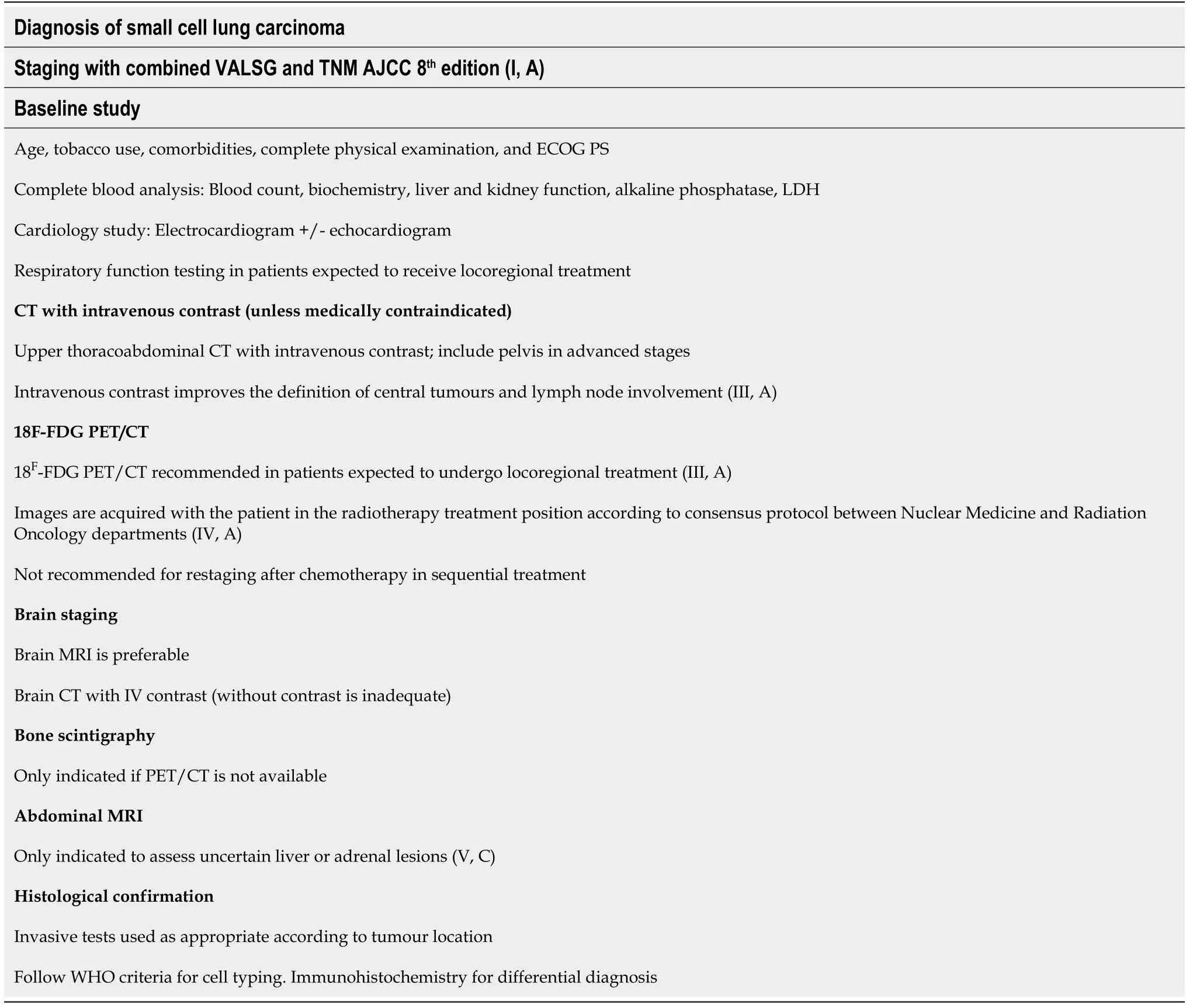
Table 2 Diagnostic staging recommendations for small cell lung cancer
TNM staging
Staging for SCLC is based on the VALSG classification system. However, the TNM staging described in the AJCC 8thedition (level of evidence I, grade of recommendation A) should also be utilized[68,69]. Tables 3 and 4 summarize TNM staging for SCLC.
In patient with LS-SCLC, the tumour is confined to the hemithorax (stages I-III). All other cases of SCLC are considered ES-SCLC (stage IV). However, there are discrepancies between the various guidelines (NCCN/CSCO, ESMO) with regard to the presence of pleural effusion, large primary tumour, or contralateral supraclavicular LNs. If the only evidence of metastasis is pleural or pericardial effusion without the presence of malignant cells, these cases should be considered non-metastatic (M0)(level of evidence V, grade of recommendation B).
In patients with a single metastatic site, initiation of PCh should not be delayed. The patient should be re-evaluated after two cycles of PCh to determine the status of the target lesion (level of evidence V, grade of recommendation C).
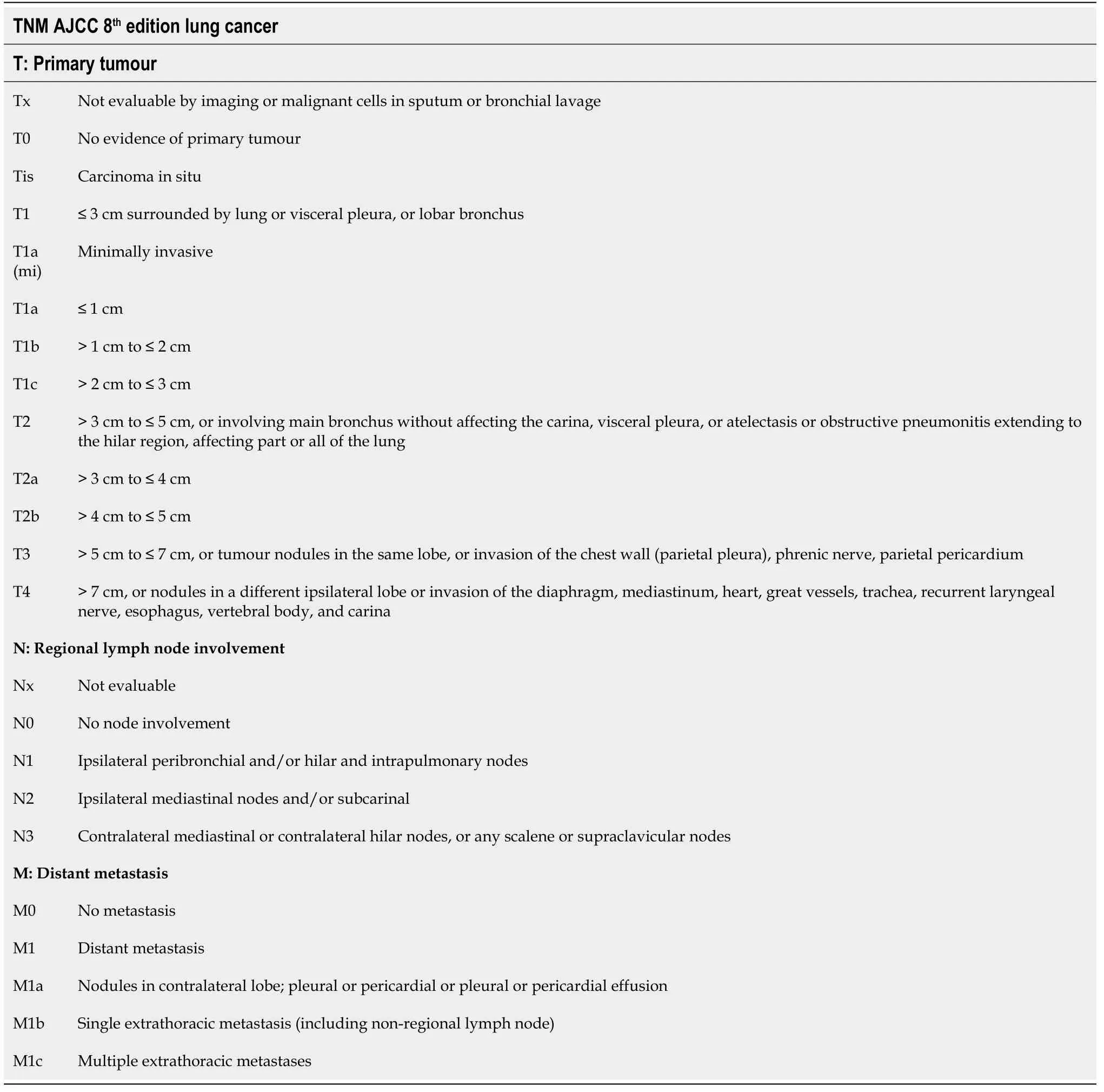
Table 3 Tumor-node-metastasis American Joint Committee on Cancer 8th edition lung cancer
CLINICAL TREATMENT INDICATIONS FOR EACH SCLC STAGE
LS disease: early stages I-IIA (T1-2N0M0)
Surgery:Patients with stage I (T1-2N0) disease may benefit from surgery[70]. These patients should undergo invasive mediastinal staging with 18F-FDG PET/CT imaging[71]. Surgery consists of lobectomy with mediastinal lymphadenectomy (level of evidence II, grade of recommendation A). Based on data from retrospective studies, 5-year OS is approximately 57%[70].
Stereotactic body radiation therapy:CRT are indicated in patients with inoperable stage cT1-2N0M0 SCLC. Data from the National Cancer Data Base showed that there are no differences in OS between patients treated with stereotactic body radiation therapy (SBRT) followed by PCh and those treated with concurrent CRT[72]. As a result of those findings, the proportion of patients treated with SBRT increased sharply from 2004 (0.4%) to 2013 (6.4%). A multicenter study reported that SBRT (50 Gy, 5 fractions)yielded a LC rate of 97.4% at 1 year and 96.1% at 3 years, with a median PFS at 1 and 3 years of 58.3% and 53.2%, respectively[73]. The median survival was 17.8 mo (69.9% at 1year and 34% at 3 years), with only minimal toxicity (grade 2 pneumonitis: 5.2%).Thus, SBRT achieves a high LC rate in patients with early, inoperable SCLC; however,sequential treatment with PCh is essential[74].
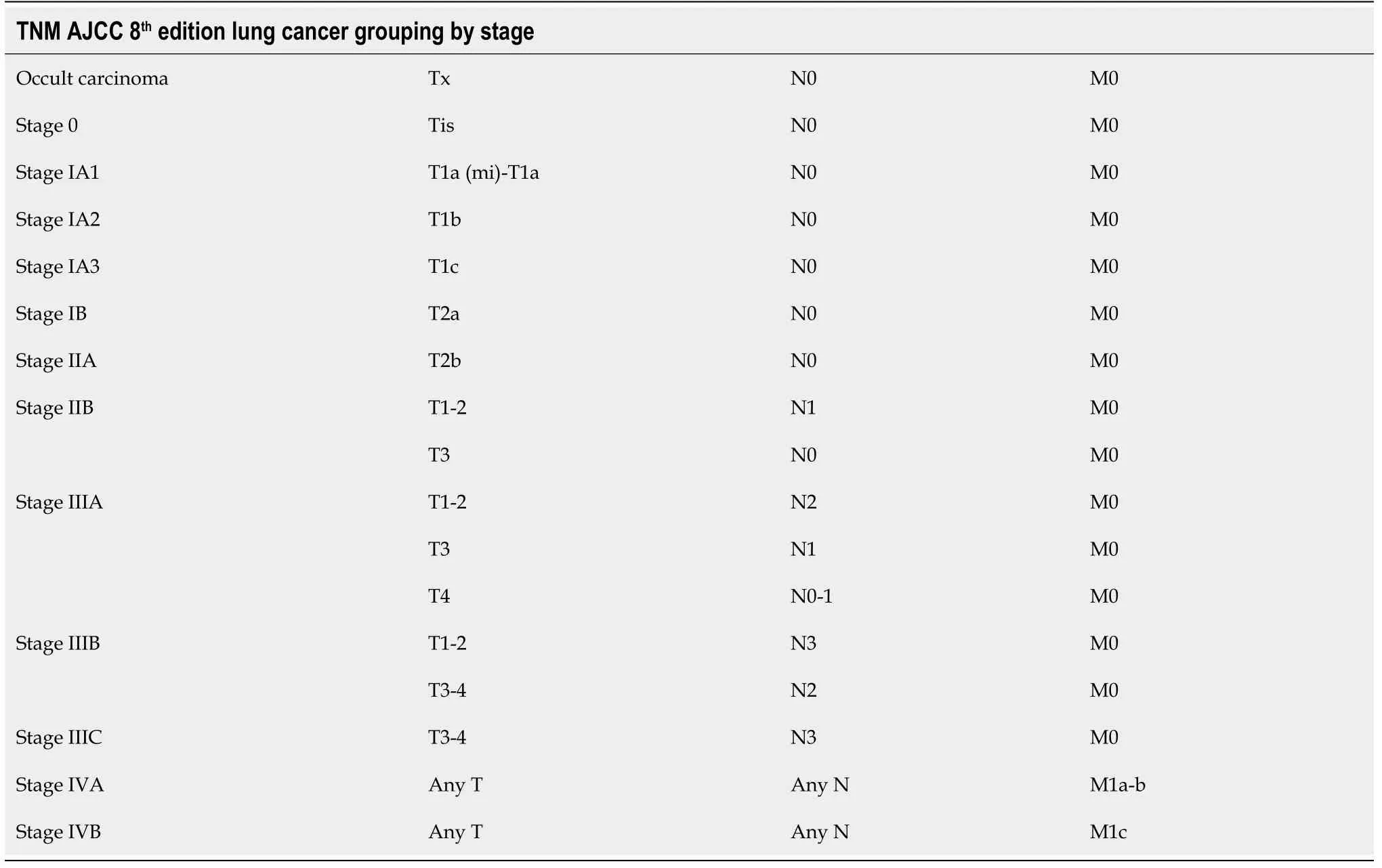
Table 4 Grouping by tumor-node-metastasis stage: American Joint Committee on Cancer 8th edition lung cancer
Adjuvant treatment:Adjuvant PCh should be administered after surgical resection.RT should be included in patients with mediastinal nodal involvement. Figure 1 shows the evaluation algorithm for adjuvant TRT.
A review of studies that included adjuvant TRT found that the addition of this treatment modality improved median OS in pN1 and pN2 disease[75]. Adjuvant TRT was associated with a lower risk of death in both pN1 [hazard ratio (HR): 0.79; 95%CI:0.63-1.00,P= 0.05] and pN2 disease (HR: 0.60; 95%CI: 0.48-0.75,P< 0.0001), but not with better OS in patients without nodal involvement. OS was better (HR 0.72, 95%CI:0.57-0.90,P= 0.004) in patients who underwent sublobar resection.
LS disease: IIB-IIIC (T3-4, N0, M0; T 1-4, N1-3, M0)
The standard treatment for stage IIB and IIIC is concomitant CRT (level of evidence I,grade of recommendation A).
Two MTA concluded that PCh plus TRT improve OS[20,21]in LS-SCLC. Another MTA[36]demonstrated that early TRT is superior to late TRT in patients receiving concomitant CRT. Ideally, TRT should start with the first or second cycle of PCh (level of evidence I, grade of recommendation A). However, PCh should not be delayed in order to initiate concomitant TRT. Another study[76]found that a shorter interval between initiation of PCh and completion of TRT is associated with a better OS (level of evidence I, grade of recommendation A).
A recent MTA[77]compared the efficacy and toxicity of twice-daily to once-daily CRT, finding significantly better overall survival with the twice-daily regimen and no differences in toxicity.
AHF-RT (45 Gy) with an interfraction interval of ≥ 6 h is recommended for normal tissue repair. Alternatively, higher dose (66 Gy) normofractionated RT can be considered as an individual option for each patient. AHF-RT is preferable in patients with a good performance status (PS) and baseline lung function.
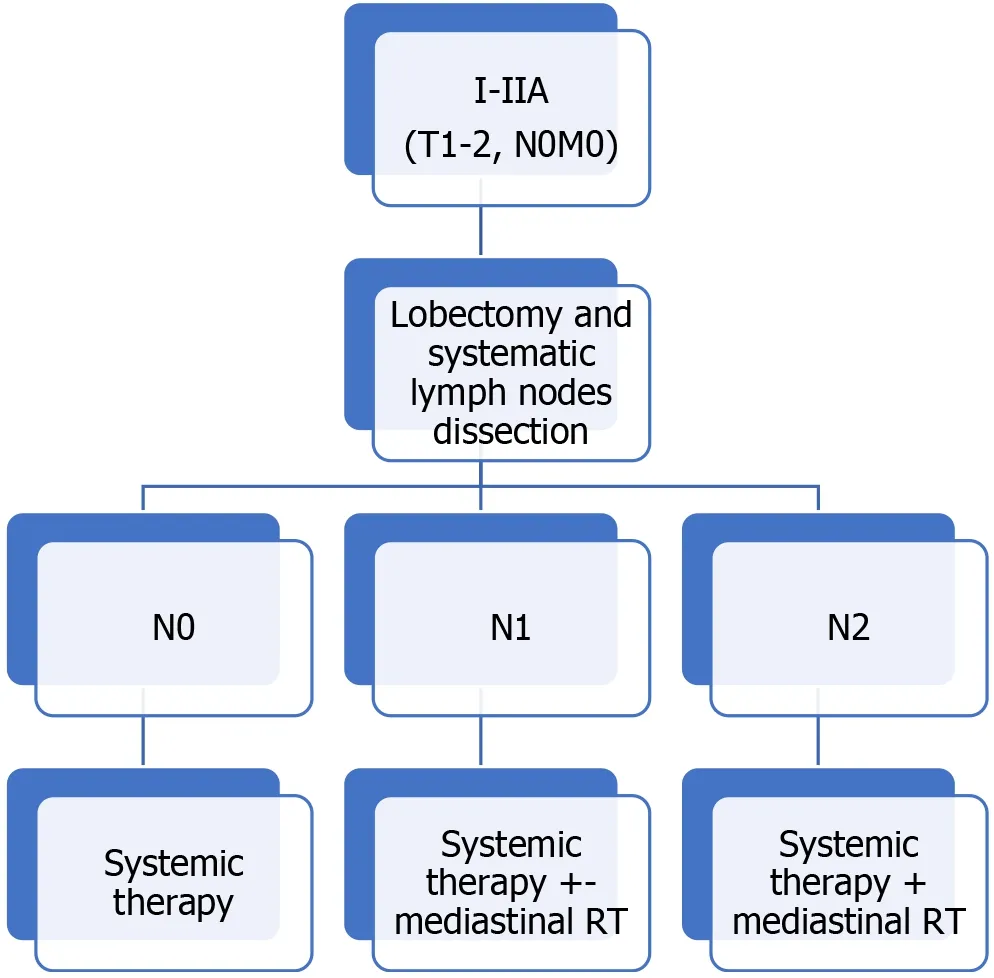
Figure 1 Therapeutic algorithm. Indications for adjuvant treatment. TNM: Tumor-node-metastasis; RT: Radiotherapy.
Extensive disease
Consolidative TRT:Prior to the introduction of immunotherapy, the main treatment for ES-SCLC was platinum-based PCh, preferably with carboplatin rather than cisplatin due to the more tolerable toxicity profile. The median OS ranges from 8-13 mo[78].
Although platinum-based PCh is the mainstay of treatment for ES-SCLC, 80% of patients present residual intrathoracic disease or progression. For this reason,consolidative TRT has been evaluated in these patients. This approach should be considered in patients with ES-SCLC who present residual thoracic disease and low volume extrathoracic metastatic disease with a complete or good response to PCh(level of evidence I, grade of recommendation A). One meta-analysis found that consolidative TRT prolongs both OS and PFS, with only a small increase in the risk of esophagitis[79].
ES-SCLC with new-onset brain metastasis (BM):BM are treated with whole-brain RT(WBRT). In asymptomatic patients, the recommended approach is PCh followed by WBRT. For symptomatic patients, the recommendation is WBRT +/- concomitant PCh.
In selected cases with only a few BM, stereotactic radiosurgery (SRS) followed by active surveillance with MRI can be considered in order to avoid PCI/WBRT; SRS can also be performed to treat new BM[80]. The FIRE SCLC study[81]was a multicenter cohort study comparing SRS to WBRT. In that study, although WBRT prolonged the interval to brain progression, it did not improve OS.
ENCEPHALON (NCT03297788) is an ongoing phase II RCT designed to compare WBRT to SRS in patients with 1-10 BM. Another trial (NCT03391362) is currently underway to compare SRS in patients diagnosed with ES-SCLC and 1-6 BM.
Oligometastatic disease:The number of metastases is a prognostic factor[82], but the differences in OS between oligometastatic and polymetastatic SCLC remain unclear. In one trial, patients with stage IV SCLC and ≤ 4 extracranial metastases after response to PCh were randomised to PCI with or without consolidative TRT and irradiation of extracranial metastases. TRT reduced the risk of the first thoracic recurrence (62.5%vs25.8%) and the risk of recurrence in previous metastases (78.1% to 41.9%).
Elderly patients
The incidence of SCLC increases as a function of age, with patients ≥ age 70 accounting for approximately one-third of cases. Data on the treatment of these patients are limited because the elderly are underrepresented in clinical trials[83]. The use of CRT in elderly patients has been limited because these patients are less likely to be able to tolerate the potential toxicity of treatment due to lower functional reserve levels.However, elderly patients with LS-SCLC who are in good general condition can be treated with CRT and modern RT techniques such as 3-dimensional (3D)-RT or intensity modulated RT (IMRT). A RCT found that disease-free survival and response rates among elderly patients were similar to those observed in patients younger than age 70[84]. Even though 5-year OS was higher in younger patients, 16% of patients ≥ age 70 remained alive at 5 years. In the CONVERT trial, elderly and younger patients presented comparable OS outcomes (29vs30 mo;P= 0.38)[85]. Although many elderly patients with LS-SCLC have comorbidities, they are generally able to tolerate CRT as well as younger patients, though they present a higher risk of asthenia and myelosuppression[86]. Patients with ES-SCLC with good PS and adequate supportive treatment can tolerate standard PCh.
DEFINITION OF VOLUMES AND ORGANS AT RISK
The ESTRO/ACROP guidelines for the target volume delineation for non-SCLC(NSCLC) categorised the recommendations as follows: Mandatory (M), recommended(R); optional (O); and discouraged (D)[87]. Some of these indications have been adopted for the treatment of SCLC[52].
Treatment planning should be based on computed tomography with the patient placed in the treatment position (M) with IVC (R) and a slice thickness ≤ 3 mm (M).Preferably, 4D-CT (R) should be used to evaluate the gross tumour volume (GTV)displacement in the respiratory cycle, or with breathing coordination methods (ABC)or gating techniques (R).
The primary GTV (GTVp) is the macroscopic tumour visible on CT or 18F-FDG PET/CT. The LN GTV (GTVn) corresponds to the LNs involved prior to PCh (M) that are visible on CT or 18F-FDG PET/CT (short axis > 10 mm on CT or with significant uptake), and/or have been histologically confirmed. Grouped nodes with a short axis< 10 mm adjacent to the tumour are considered to be involved (R).
The GTVp and GTVn must be contoured separately (O). The recommended lung window parameters (R) are: W = 1600 and L= -600 for lung parenchymal tumour. W =400 andl= 20 for involved LNs/tumours invading the mediastinum.
At present, there are no validated data to support the fusion of diagnostic 18F-FDG PET/CT with treatment planning CT[88]. There may be discrepancies between these images, for the following reasons: (1) The GTV on 18F-FDG PET/CT frequently corresponds to areas of normal tissue on the CT. The 18F-FDG PET/CT images are acquired over several minutes, which allows us to define the motion of the primary tumour. When these images are associated with those obtained in a 3D planning CT,some areas of the GTV on 18F-FDG PET/CT may correspond to “air” areas on the CT due to lesion movement; (2) When the tumour is contiguous to areas of similar density, it must be defined by 18F-FDG PET/CT; in cases in which FDG uptake is not due to the tumour (e.g., heart, active infection), this should be contoured on CT; and (3)LNs that are enlarged on CT but negative on 18F-FDG PET/CT imaging should not be considered part of the GTV unless pathological data is available. Small nodes without uptake adjacent to the primary tumour or those located between pathological nodes,or with evident growth on consecutive CT scans, should be considered part of the GTV[89,90].
Disease confined to the chest
Postoperative RT:To date, postoperative RT (PORT) after surgery and sequential PCh of early -stage SCLC (N0) has not been shown to improve OS[91-93].
PORT can improve 5-year OS in pN2 disease[94-96], but does not significantly improve OS in stage pN1[75]. A MTA[97]concluded that PORT improves 1-3 year LC rates in pN1 and pN2 and 1-5 year OS in pN2 disease. However, PORT provides no benefit in pN0[98,99]. Clinical guidelines recommend PORT for the treatment of microscopic or macroscopic residual disease (R1 or R2) or pN2+ disease (level of evidence V, grade of recommendation C)[100,101].
The recommended treatment volumes are as follows: Bronchial stump, ipsilateral hilum, preoperatively involved regions, and pathologically-positive areas[102-104]. The treatment fields proposed in the randomised Lung ART trial for PORT in patients with pN2+ NSCLC[105]could be applied to patients with SCLC. However, PORT should be used cautiously in the treatment of SCLC given the negative results reported in a recent RCT in NSCLC[106]. Consequently, the treatment approach should be individualised and determined by consensus decision at the thoracic tumour board at each hospital.
The margin added to the clinical target volume (CTV) to define the planning target volume (PTV) will depend on the immobilization system used and other centrespecific factors. A 5 mm margin added to the involved LNs may be sufficient[107]. A 3 mm margin may be recommended for nodes < 2 cm and 12 mm for nodes > 2 cm[108].
Extracranial stereotactic radiation therapy
Respiratory motion should be compensated for by defining the internal target volume(ITV) based on reconstruction of the phases of the respiratory cycle obtained through 4D-CT imaging[109]. Published series for SBRT do not differentiate between the GTV and CTV. The CTV margin for the PTV is 5 mm (range, 3-7 mm).
Radical RT
In the 1980s, studies were performed to determine whether delimitation of the GTV should be based on the pre- or post-induction PCh tumour volume[110], with the available evidence suggesting that there were no apparent differences in OS or relapse patterns. One study[111]evaluated 59 patients treated with neoadjuvant CT followed by CRT; 31 patients received TRT to the pre-PCh volume and 28 to the post-PCh volume.The use of the post-PCh volume did not increase out-of-field margin failures or thoracic recurrences. These data suggest that reduced fields are an acceptable strategy.
In a recent RCT[112]patients with stage I-III SCLC (92.9% stage III) underwent neoadjuvant PCh and were then randomised to TRT to the primary pre-PCh GTV(control arm) or the post-PCh GTV (experimental arm). Initially involved nodal regions were included in both treatments. There were no differences in locoregional PFS or OS at 3 and 5 years, although significant grade 3 esophagitis was observed in the pre-PCh arm. In the CONVERT and CALBG30610/RTOG 0538 trials, the treatment volume was the residual post-PCh GTV.
Another controversy surrounds the use of elective irradiation of mediastinal and supraclavicular LNs[27,28]. The study conducted by Baaset al[113]was the first to omit elective mediastinal irradiation. The volume irradiated was the involved primary tumour and LNs with a diameter ≥ 1 cm. In that study, 16% of patients developed local recurrence within the RT field. Updated data from that trial[114]reported out-of-field nodal recurrences in two patients and in-field recurrences in 9 (sole site of recurrence in 4). From the out-of-field relapses, one occurred in the ipsilateral supraclavicular fossae. The incidence of recurrences in the elective mediastinum is low (range, 2%-11%) and in more than 50% of cases these occur in the ipsilateral supraclavicular fossae, which underscores the crucial importance of accurate staging (with 18F-FDG PET/CT and/or supraclavicular ultrasound)[115]. In the CONVERT trial, elective mediastinal irradiation was not permitted. In the CALBG 30610/RTOG 0538(NCT00632853) trial, the CTV included the ipsilateral hilum. Staging with 18F-FDG PET/CT was not mandatory in either trial.
The post-PCh primary GTV and pre-PCh nodal areas should be included in the treatment volume. For the primary GTV, if the tumour invades the mediastinum, the pre-PCh volume should be contoured at the tumour-parenchyma interface; only the residual post-PCh GTV should be considered. If CR in the primary tumour has been achieved, the primary CTV (CTVp) should be contoured on the pre-PCh image.Elective irradiation of areas located between involved mediastinal stations is optional,as is irradiation of the ipsilateral supraclavicular fossae in cases with extensive mediastinal involvement (level of evidence II, grade of recommendation B).
To account for microscopic disease after neoadjuvant CT, an adequate GTVp margin would be 1.4 mm. To reduce the irradiation volume, the CTV can be omitted with no significant differences in the recurrence rate. Micrometastases in the CTV may be small enough to be sterilized by PCh. In most studies, a 5-8 mm margin is added to the GTVp or ITV to establish the CTVp. When contouring the CTVn, the entire area encompassing the pathological nodes should be included with a 5-8 mm margin. The PTV margin is generally obtained by adding 5-10 mm to the CTV.
Metastatic disease
The optimal treatment volume for consolidation TRT in ES-SCLC has not been established[116-121]. Table 5 shows the volumes used in recent studies. According to ESTRO/ACROP recommendations, the following should be included: Post-CT GTVp,the hilar region, and the mediastinum (not only the involved area), in all cases accounting for the decreased nodal volume after Ch (R).
PCI
The indication for PCI in ES-SCLC is controversial[122]. Standard WBRT is recommended. To protect the hippocampus, this structure should be contoured according to the RTOG atlas[123]on a T1-weighted 3D contrast-enhanced MRI. For hippocampal protection, the planning CT should have a slice thickness ≤ 1.25-1.3 mm to ensure correct fusion with the MRI. In this case, the CTV should be defined as the whole brain minus the bilateral hippocampus + 5 mm[124,125]. To define the PTV, a 3-5 mm margin is added to the CTV to protect the outer table of the skull to avoid alopecia.
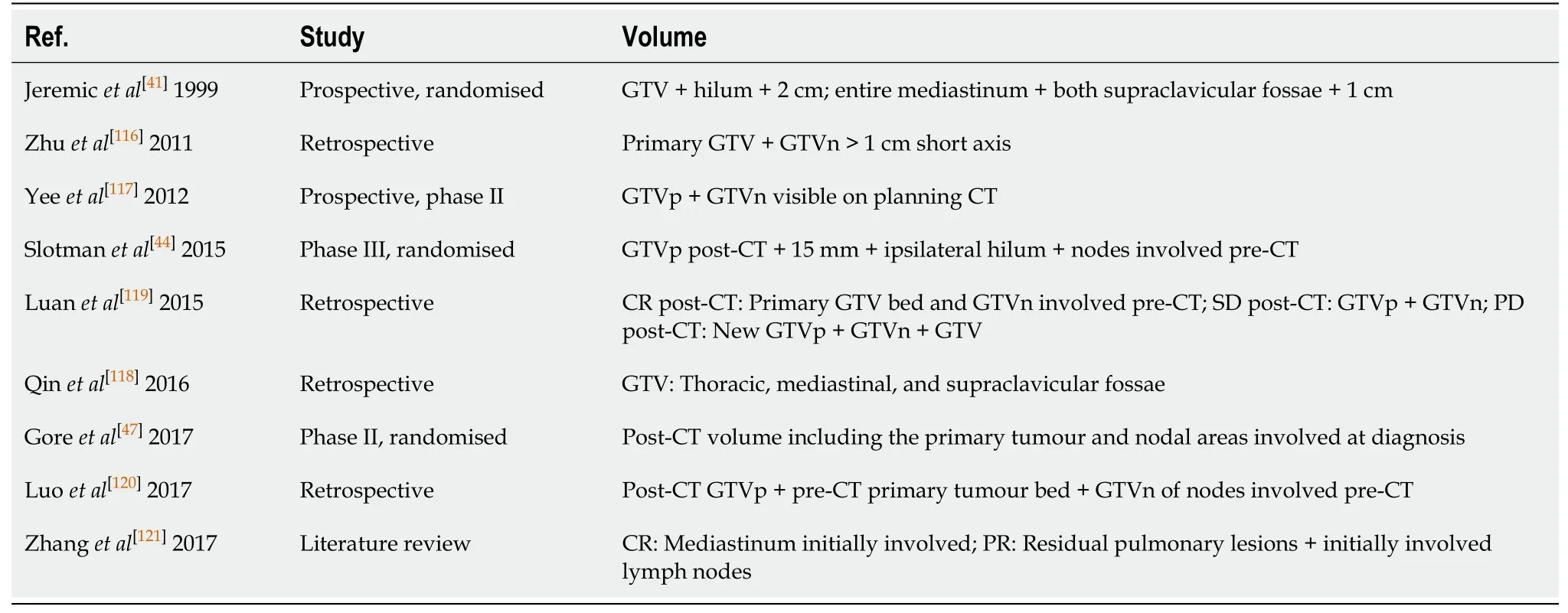
Table 5 Planning volumes in the principal studies of concurrent chemoradiotherapy in small cell lung cancer
Organs at risk
A fundamental aspect of RT planning in SCLC is to delineate the organs-at-risk (OAR)with adequate dose limitations.
Lung:The dose limits on the dose/volume histogram (DVH) for the lung are based on both lungs minus the target volume (GTV)[126,127].
DVH dosimetric parameters in TRT [V5, V13, V30, V20 and mean lung dose (MLD)]may be associated with the risk of pneumonitis, mainly grade ≥ 3. The most commonly used parameters are the V20 and MLD. When V20 is limited to ≤ 30%-35% and MLD ≤20-23 Gy, the reported rate of radiation pneumonitis is ≤ 20%[128]. The incidence of grade 5 pneumonitis is extremely low and associated with dose fractions totalling > 2 Gy, elevated V20 values, and lower lobe tumours.
Based on RTOG 0813 and 0915 recommendations, the 2016 SABR guidelines 2016,and data from TG 101[126], the recommended dose limits for SBRT per fraction are as follows: One fraction: 7 Gy/1500 cc, 7.4 Gy/1000 cc; three fractions: 11.6 Gy/1500 cc,12.4 Gy/1000 cc, V20 < 10%, V12.5 < 15%; four fractions: 11.6 Gy/1500 cc, 12.4 Gy/1000 cc, V20 ≤ 12%, MLD < 6 Gy; five fractions: 12.5 Gy/1500 cc, 13.5 Gy/1000 cc,V12.5 < 15%, V20 < 10%; and eight fractions: V12.5 < 15%, V20 < 10%.
Trachea-proximal bronchial tree:The trachea should be contoured in the mediastinal window, including all layers. The upper limit is the cricoid cartilage and the lower limit is the upper border of the proximal bronchial tree (2 cm above the carina). The bronchial tree includes 2 cm distal from the trachea, the carina, the right and left main bronchi to the lower-lobe bronchi. Contouring ends at the segmental bifurcation.
The RTOG recommends the following dose/volume parameters for the trachea and ipsilateral bronchus: 20.2 Gy (Dmax)/10.5 Gy (< 4 cm3) in one-fraction; 30 Gy(Dmax)/15 Gy (< 4 cm3) in three fractions; 34.8 Gy (Dmax)/15.6 Gy (< 4cm3) in four fractions; 105% of the prescribed dose to the PTV/18 Gy (< 4 cm3) in five fractions; and 44 Gy (Dmax) in eight fractions[129].
Esophagus:All layers of the esophagus should be contoured from the cricoid to the gastroesophageal junction. Several factors-concomitant boost; AHF-RT; concurrent CRT-may increase the risk of severe acute esophagitis (grade ≥ 3), which can occur in up to 15%-25% of cases[130]. The V60 is the most accurate predictor of grade 3 esophagitis[131]: V60 < 0.07% = 4%, V60 0.07%-17% = 10%, and V60 > 17% = 22%.Dmean < 34 Gy may be associated with ≥ grade 3 acute esophagitis in 5%-20% cases;V35 < 50%, V50 < 40%, and V70 < 20% may limit ≥ grade 2 esophagitis to less than 30%[132]. The maximum dose is a better predictor of severe late toxicity than Dmean. In SBRT treatments, the incidence of ≥ G3 esophagitis is low; studies of single-fraction RT suggest that D2.5 cm3to the esophagus < 14 Gy will maintain a rate less than 5% and a Dmax < 22 Gy[133]. Other studies with one or more fractions report dose/volume limits as follow: D5cc <14.5 Gy; D2cc 15-20 Gy; and Dmax < 19 Gy[134].
Spinal cord:The spinal cord is contoured in the mediastinal window from the cricoid to below L2. The structure should be contoured following the bony limits of the spinal canal. Another possibility is to delineate the visible spinal cord on the CT and apply a 2 mm margin for the DVH analysis to estimate all uncertainties. In tumours located close to this organ, or for SBRT treatments, fusion with MRI is useful[126,127]The use of a planning OAR volume margin around a critical organ such as the spinal cord is sufficient to avoid overdose (level of evidence IV, grade of recommendation C).
In normofractionated treatments (1.8-2 Gy/day) that include the cord circumference, myelopathy rates of 0.2%, 6%, and 50% have been described after 50 Gy, 60 Gy, and 69 Gy, respectively. The NCCN recommends Dmax ≤ 50 Gy. In SBRT for tumours in which only part of the spinal cord is irradiated, the risk of myelopathy is < 1% with Dmax 13 Gy/1 fraction or 20 Gy/3 fractions. RTOG 0915 establishes limits of 14 Gy in one fraction and 30 Gy in 5 fractions; a strict limit of 22 Gy in five fractions should also be considered[135]. Although the main variable associated with myelopathy is the maximum dose, the volume irradiated is also important. When the D0.1cc parameter is used, lower dose levels are established in each fraction.
Brachial plexus:The brachial plexus is divided into five nerve roots, including C5 at the exit through the neural hole, and C4-C5 to T1 as they exit through the T1-T2 foramen. To delineate the brachial plexus in the mediastinal window setting, it is preferable to use IV contrast to distinguish the nerves from the adjacent vessels. If no contrast is used, the subclavian and axillary arteries and veins should be used for reference. In the treatment of apical tumours with SBRT, CT-MRI fusion is recommended[126,127]. The upper limit is the exit point of the C5 root through the C4-C5 neural foramen, while the lower limit is the subclavian and axillary neurovascular bundle not including the vessels to identify the lateral border, and the second rib as the medial limit; external limit: Space between the anterior and middle scalene muscles from the vertebral body of C5 to its insertion at the first rib; internal limit: At the space at the conjunction of the lateral border of the spinal canal and the space or soft tissue between the scalene muscles at the vertebral body. To reduce the risk of radiationinduced plexopathy[136], the Dmedian should be ≤ 69 Gy with a Dmax at 2 cm3< 75 Gy.The risk of plexopathy is greater for brachial plexus doses > 26 Gy in 3-4 fractions, and for maximum doses > 35 Gy and V30 > 0.2 cm3.
Heart:The entire pericardial sac should be contoured, including the atria and ventricles. The upper limit is below the aortic arch (aorto-pulmonary window) and the lower limit is the cardiac apex, excluding the pulmonary artery trunk, aorta, and superior vena cava[126]. The trade-off between protecting the heart and the likelihood of controlling the lung cancer must always be considered.
In normofractionated RT, the risk of toxicity for Dmean values < 10 Gy, 10-20 Gy,and ≥ 20 Gy is 4%, 7%, and 21%, respectively. V50 values ≤ 25% and Dmean ≤ 20 Gy are recommended and the risk of pericarditis increases with pericardial Dmean > 26 Gy and V30 > 46% according to QUANTEC. In SBRT, dose/volume limits[135]are as follows: Four fraction treatments: Dmax < 45 Gy, V40 ≤ 1 cm3, and V20 ≤ 5 cm3. RTOG 0618, 0813, and 0915 used Dmax values of 22 Gy/16 Gy < 15 cm for single fractions,Dmax 30 Gy/24 Gy < 15 cm3in 3 fractions, Dmax 34 Gy/< 15 cm3in 4 fractions, and 105% of the dose prescribed at PTV/32 Gy < 15 cm3in 5-fraction regimens[129].
Great vessels:The great vessels are contoured in SBRT, including the aorta and vena cava, from 10 cm above the PTV to 10 cm below it. Dose limits are Dmax 37 Gy/31 Gy< 10 cm3in single fraction treatments, Dmax 45 Gy/39 Gy < 10 cm3in three fractions,Dmax 49 Gy/43 Gy < 10 cm3in four fractions and 105% of the dose prescribed at PTV/47 Gy < 10 cm3in five fractions.
Chest wall:In peripheral tumours treated with SBRT, the chest well is considered an OAR. Each rib should be contoured at 5 cm from the PTV, excluding the intercostal space. Descriptive parameters are: Dmax 30 Gy/22 Gy < 1 cm3in a single fraction,Dmax 36.9 Gy/28.8 Gy/< 1 cm3in three fractions, and Dmax 40 Gy/32 Gy < 1 cm3in four fraction schemes.
PCI IN LIMITED AND ES DISEASE
The capacity of standard PCh agents (cisplatin-etoposide) to penetrate the blood-brain barrier is limited and 60% of patients will develop BM within two years of diagnosis,which explains the importance of PCI. The value of PCI is supported by the highest quality scientific evidence. Aupérinet al[33]performed a MTA of seven clinical trials(mostly LS-SCLC), finding that PCI resulted in an absolute decrease of 25.3% in BM and a 5.4% improvement in 3-year OS.
A RCT conducted in Japan compared PCI to MRI-based observation in patients with ES-SCLC[122], finding that PCI did not provide any survival benefit, thus calling into question the role of PCI in these patients. Nevertheless, while PCI is considered standard in LS-SCLC, some patients-such as those older than age 75 or with high risk factors for neurotoxicity due to pre-existing disorders-may opt for close follow-up with MRI.
Prior to initiating PCI after completion of CRT, a brain MRI should be performed for re-evaluation. In patients with chest CR, MRI performed after CRT shows an incidence of BM ranging from 20%-32%[137,138].
Another MRI-based study found that the cumulative incidence (prior to PCI) of BM was 6.67% 4 mo after the initial treatment, rising to 12.2% at month 5 (1 mo later) until gradually reaching 21.82% at month 9[139]. These data indicate that PCI should be administered after completion of CRT. BM were detected in 10% of SCLC patients in the computed tomography eravs24% in the MRI era. The emergence of MRI reduced the use of PCI from 42% of patients to only 13%[140].
Recent data from non-randomised studies suggest that PCI significantly benefits patients with LS disease, with better OS in patients re-evaluated with MRI[141].
Data from retrospective studies involving patients with LS-SCLC who achieve CR after CRT suggest that PCI does not improve OS, provided that MRI and SRS are available; however, those same data show that PCI reduces the incidence of BM[142].SRS is an effective treatment for selected cases of SCLC with BM[143].
A MTA of seven randomised trials involving > 2000 patients found that PCI improved OS and significantly decreased the incidence of BM compared to observation[144,145]. However, it is important to note that those trials were highly heterogeneous in terms of the OS analyses due to the wide range of imaging tests utilized.
PCI is the standard treatment for most patients with LS-SLC who respond to radical CRT (level of evidence I, grade of recommendation A). Although the MTA by Aupérinet al[33]included only patients who achieved CR after CRT, the role of PCI can be extrapolated to patients with partial response based on current radiological tests. The NVALT-11/DLCRG-02 Study and SWOG S1827 phase III (MAVERICK) noninferiority trial (NCT04155034) are currently being performed to determine whether active surveillance with MRI yields similar survival outcomes to MRI plus PCI[146].
The use of PCI in patients with ES-SCLC is controversial. The EORTC study[38]showed that PCI reduced the incidence of BM from 40% to 15%, prolonged median survival (from 5.4 mo to 6.7 mo) and improved OS rates (27% to 13%). However, prior to initiation of PCI, no imaging test was required in that study. In the study conducted by Takahashiet al[122]all patients underwent MRI prior to PCI. The cumulative incidence of BM in the two arms (PCIvsobservation) was 48% and 69%, respectively.Despite the lower rate of BM with PCI, this did not improve OS (Table 6).
The PCI dose in ES-SCLC is 2500 cGy in 10 fractions (level of evidence I, grade of recommendation A) and 2000 cGy in 5 fractions[38]; however, the neurocognitive effects of the latter scheme have not been fully investigated.
Although two MTA and one pooled analysis have shown that PCI improves OS in patients with ES-SCLC[157-149], the result of another MTA called into question the value of PCI[150]. Consequently, the decision to use PCI or not should be discussed with the radiation oncologist. Patients with ES-SCLC who progress after PCh should not receive PCI.
PCI is associated with several acute toxicities, including alopecia, headache, fatigue,and, in rare instances, nausea and vomiting. Long-term sequelae such as severe memory loss, intellectual impairment, or even dementia and ataxia (both rare) have been reported. Chronic neurotoxicity (CNT)[151]may be associated with age, high total doses, dose fractions > 3 Gy, and concomitant PCh. In the prospective RTOG 0212 trial[152], age was the most significant factor for the development of CNT, with 83% of patients over age 60 developing CNT at 12 mo compared to only 56% of younger patients.
A recent systematic review assessed the risk factors for CNT after PCI[153],concluding that there is a lack of sufficient quality data to define these factors.However, disease-related factors (paraneoplastic syndromes, undiagnosed cerebral micrometastases, steroid use,etc.) are known to influence the development of CNT.Numerous factors have been shown to exacerbate the toxicity of cancer treatment,including treatment-related factors (CT or CT-induced anemia); smoking; excessive alcohol intake; comorbidities (e.g., depression and anxiety); hypertension;hyperlipidemia; and diabetes caused by vascular damage and cerebral hypoperfusion.
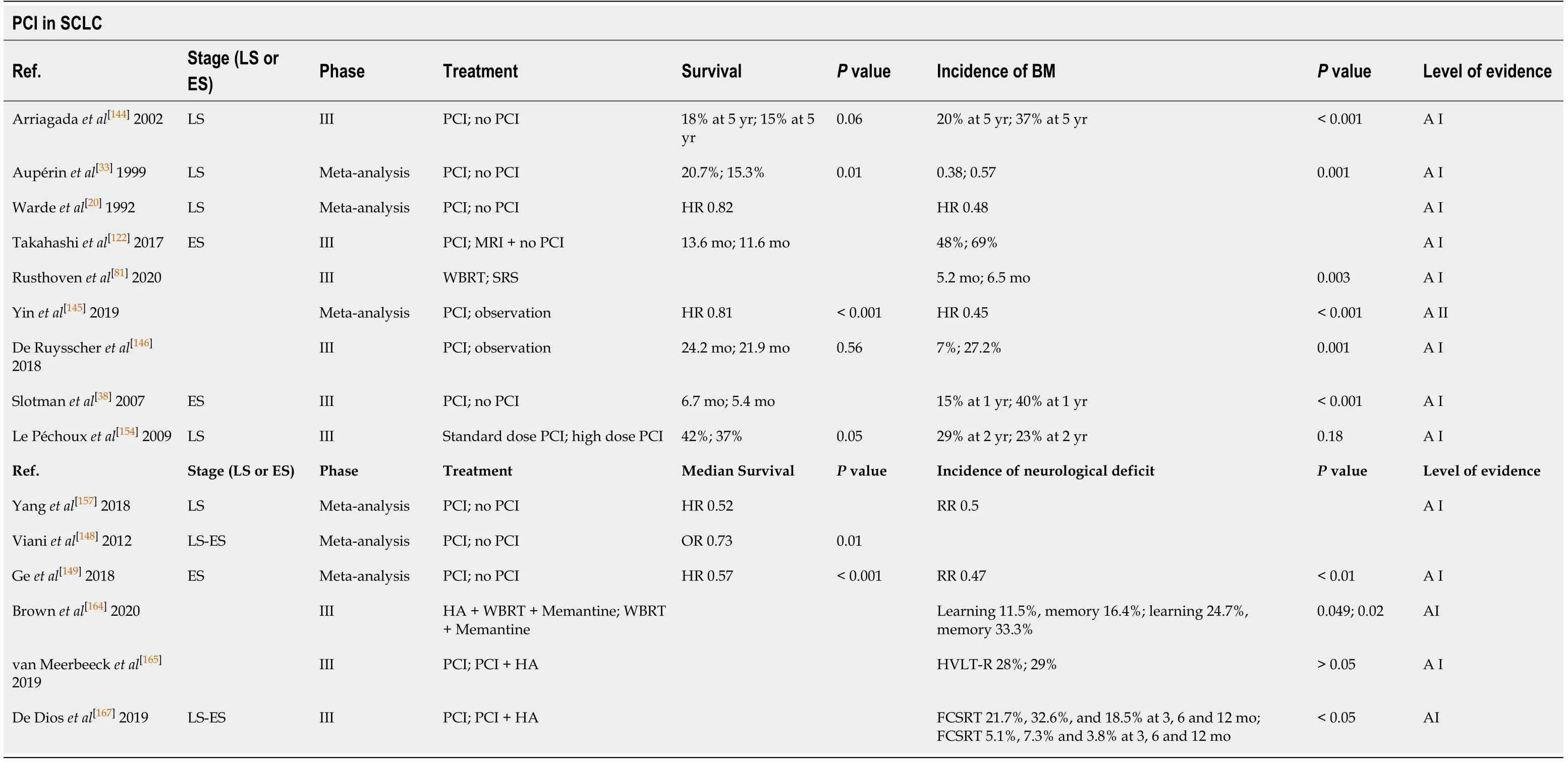
Table 6 Summary of the main studies of prophylactic cranial irradiation in small cell lung cancer
A dose escalation study[154]found no significant reduction in the 2-year incidence of BM at doses > 25 Gy, but did find an increase in mortality. A neurocognitive analysis of data from RTOG 0212 (2500 vs 3600 cGy) showed a significant increase in CNT with higher doses[152].
In patients with stage I SCLC, the risk of BM is minimal and there does not appear to be any survival benefit associated with PCI in surgically-treated stage pT1-2N0M0 disease[155-157]. Consequently, follow-up in this patient population should be limited to brain MRI.
Few studies have evaluated the impact of PCI on survival outcomes in elderly patients. Eatonet al[158]evaluated PCI in 1926 patients, finding that this treatment improved OS in patients ≥ 75 years of age but not in those ≥ age 80. Other series suggest that patients older than 65-70 years of age-especially those with large tumours and/or women-are unlikely to achieve an OS benefit even after a good response to CRT[159,160]. One study suggested that patients with LS-SCLC and incomplete response to CRT may not benefit from PCI[161]. Active surveillance with brain MRI is preferable in elderly patients and in those with limited functional status,pre-existing neurocognitive disorders, or significant comorbidities.
Neurocognitive deterioration associated with PCI[162]is caused in part by irradiation of the hippocampus. For this reason, hippocampal sparing techniques have been evaluated, with promising results in metastatic patients treated with WBRT and hippocampal avoidance[163,164]. As a result, several multicenter RCTs are currently underway. NCT01780675 is a trial[165]performed to assess the safety of PCI and its impact on memory with or without hippocampal avoidance in patients with SCLC.That trial included 168 patients, finding that the rate of cognitive decline [Hopkins Revised Verbal Learning Test (HVLT-R)] at 4 and 8 mo did not significantly differ for PCI alonevsPCI with hippocampal avoidance (28%vs29%). Moreover, the incidence of metastases did not significantly differ between the groups, and none of the patients developed BM in the hippocampus[166].
The Spanish PREMER trial (NCT02397733) reported contrary results. Among the 118 eligible patients, a significant decrease in memory was observed in the PCI groupvsthe hippocampal avoidance group, as follows: At 3 mo: 21.7%vs5.1%; at 6 mo: 32.6vs7.3%; and 12 mo: 18.5vs3.8%). That trial used the Free and Cued Selective Reminding Test (FCSRT) to test neurocognitive function, but this test (FCSRT) was not used in the other randomised trial[167].
The study endpoints of the NRG-CC003 trial (NCT02635009) were 12-mo intracranial relapse and 6-mo impairment of delayed recall on the HVLT-R[168].
The latest NCCN guidelines recommend hippocampal avoidance and the use of memantine during and after PCI because this delayed the time to cognitive deterioration in patients who received WBRT for BM (RTOG 0614)[169].
For the radiation oncologist, the decision to recommend PCI or not is challenging, as it is not always clear which patients are likely to benefit and which can be offered MRI surveillance. In some cases, surveillance with MRI may be preferred, especially for patients who show a CR to the initial treatment[80]. Risk assessment should be individualized and decision-making should be shared with patients[100,101].
RT TECHNIQUES
Image-guided radiation therapy (level of evidence IV, grade of recommendation C)
Published evidence on the role of image-guided radiation therapy (IGRT) in SCLC is limited. One study involving a series of 132 patients with SCLC[170]found no significant differences in OS between patients treated with or without IGRT. No toxicity information was reported.
IMRT (level of evidence IV, grade of recommendation C)
There are no randomised studies comparing IMRT to 3D-RT in LS-SCLC. Data from retrospective studies[171]suggest that OS is comparable, although IMRT is associated with a significantly lower rate of percutaneous feeding tube placement due to esophagitis (5%vs17%).
Volumetric modulated arc therapy (level of evidence IV, grade of recommendation C)
Comparative dosimetric studies between IMRT and volumetric modulated arc therapy(VMAT), including patients with SCLC[172], show that each technique has specific advantages depending on the tumour location. In peripheral tumours, the lung V5 (%)is lower with VMAT than IMRT while the lung V30 (%) is lower with IMRT. In central locations with mediastinal involvement, VMAT has a lower V20 (%) than IMRT.
Proton therapy (level of evidence III, grade of recommendation D)
The first prospective study of proton therapy with concomitant CT in LS-SCLC was published in 2017[173]. The median dose was 63.9 cobalt gray equivalents (range, 45-66.6) in 33-37 fractions administered once [n= 18 (60.0%)] or twice daily [n= 12(40.0%)]. Compared to IMRT, proton RT achieved a statistically significant reduction in mean doses to the spinal cord, heart, and lung, but not in mean esophageal dose or V20. At a median follow-up of 14 mo, LC and OS survival rates were 85% and 72% at one year and 63% and 58% at two years, respectively. There was only one case each(3.3%) of grade ≥ 3 esophagitis and pneumonitis. Grade 2 pneumonitis and esophagitis occurred in 10% and 43% of patients, respectively.
SABR
SABR treatment o ptions include 60 Gy delivered in 3 fractions, 48 Gy in 4 fractions,and 50 Gy in 5 fractions for peripheral lesions. Doses of 40-45 Gy in 15 daily fractions or 50-55 Gy in 20-25 daily fractions are potential alternative treatment schemes during periods such as the recent COVID-19 pandemic[101,174](level of evidence III, grade of recommendation D).
Compensation for treatment interruption
At least five studies have been published on compensation for treatment interruptions in SCLC. Of these, four have shown that delays in completing RT can have deleterious effects on the following: (1) Significant loss of locoregional control and worse OS/PFS outcomes, especially in patients who do not receive PCI after TRT and in patients with a SER (start and end of RT) interval ≥ 30-31 d[175-177]; (2) Significant decrease in 5-year OS, especially in men, (> 6 times higher)[178]; (3) Delays > 7 d for hypofractionated (40 Gy in 15 fractions) and normofractionated (50 Gy in 25 fractions) schemes, as well as delays > 29-30 d in patients treated with accelerated regimens (Turrisi scheme) appear to be deleterious[175-177]; (4) The only study that does not show a deleterious effect(subanalysis of CALGB-9235, F-III) involved normofractionated CRT (50 Gy), with RT initiated in the 4thcycle of CT (possible effect due to accelerated repopulation at the start of “late” RT after CT[179]; and (5) No values have been reported for Tk (time from the start of RT at which accelerated repopulation begins) or K (estimated loss of biological efficacy in Gy per day of delay that would need to be added to compensate).Similarly, compensation techniques for the Turrisi scheme have not been described.
The potential loss of radiobiological effectiveness due to treatment interruption should be considered as a risk exposure to potentially avoidable threats. Thus, this would not be subject to the rules of evidence-based medicine, which refers to the evaluation of the benefits of therapeutic/diagnostic techniques[180]. However, the following recommendations can be made: (1) Avoid and/or compensate if the interruption is > 7 d in hypofractionated (40 Gy, 15 fractions) or normofractionated (50 Gy, 25 fractions) schemes. By extension, this rule should apply to normofractionated schemes > 60 Gy (perhaps even when the interruption is > 5-7 d); (2) Avoid/compensate if total treatment time > 29 days in patients treated with accelerated schemes (Turrisi); (3) Avoid/compensate for interruptions, especially in men; and (4)compensation methods: In the absence of published studies, the following recommendations should be considered as general guidelines. For normofractionated schemes, any compensation method would be, in principle, appropriate. However,each case should be evaluated individually; the absence ofTkandKvalues makes it difficult to perform radiobiological calculations in cases in which it is necessary. For this reason, it would be preferable to use double treatment sessions and/or to perform RT on holidays/Saturdays whenever possible. For hyperfractionated schemes,compensation is more challenging for the same reasons, especially given the nature of such schemes. In addition to considering treatment on holidays/Saturdays, a reasonable alternative would be to increase the number of sessions (add 1-2 more days of treatment, which would mean 32 to 34 fractions rather than 30), or to increase the dose per fraction for the remaining sessions (increased the dose from 1.5 to 1.6-1.8 Gy/fraction), or to combine both approaches. In all cases, it is essential to assess OAR tolerances. If it is not feasible to continue with AHF-RT, another alternative would be to switch to a hypofractionated scheme (≥ 2.67 Gy/d).
REIRRADIATION IN SCLC
Very few studies have been published on reirradiation (reRT) in lung cancer. As a result, most of the available data come from retrospective studies, which are highly heterogeneous in terms of histology (both SCLC and NSCLC), disease stage,treatments received (time interval between RT treatments, doses, fractionations,techniques, use of PCh or not,etc.) and in the parameters used to assess treatment response[181].
Approximately 30% of patients with SCLC are diagnosed with LS disease; of these 30%, will progress locally[32]. LC is an important factor that may influence OS. Thoracic recurrence can produce symptoms that affect the patient's quality of life and may even require emergency treatment. The review that included the largest number of lung cancer patients (any histological type) to date was published in 2017[182]. That study reviewed data from 13 clinical trials involving 435 patients treated with 3D-RT or IMRT and another 10 trials (253 patients) of SBRT.
Below we review and discuss the published reports on reRT that provide specific data for SCLC. One of the limited recommendations from these studies is the need to assess the indication for reRT in SCLC independently from other histologies due to the unique behavior of SCLC[183]. Reirradiation is especially indicated to treat hemoptysis and vena cava syndrome, but it is less effective for dyspnea[184]. Hypofractionated schemes are recommended, with doses > 40 Gy (EQD2)[185]. If the patient is in poor general condition (PS > 3) or presents extrathoracic metastases, reRT should only be performed in selected symptomatic patients. In these cases, reRTvssupportive treatment should be assessed.
In terms of the recommended doses and fractionations for reRT, reported treatment schemes range from 25-37.5 Gy in 9-15 fractions for symptomatic patients or those with metastatic disease. In general, the reported benefits of reRT in terms of symptom palliation lasts only for approximately 1 mo, probably due, in part, to the short OS in these patients [media survival: 1.7 mo (1.0-4.0)]. The risk of chronic toxicity is greater if there are overlapping fields in central tumours. Cumulative doses of 90-150 Gy in central structures should be avoided. If less than 6 mo have elapsed between one RT treatment and reRT, the spinal cord dose should be < 50 Gy (EQD2)[184]. However, if more than 6 mo have passed, doses of 40-45 Gy have been used in 20-25 fractions, with a safe cumulative mean dose of 87.4 Gy.
The following factors are associated with better OS: KPS > 70%; asymptomatic patient without metastatic disease; reRT dose > 40 Gy (EQD2); cumulative dose > 90 Gy (EQD2)[185]. Predictors of good response are PS, small recurrence, and a long interval between RT.
Based on the available data, we can conclude that reRT for SCLC at palliative doses(< 40 Gy) is useful to treat symptoms such as hemoptysis, vena cava syndrome, and rib pain; however, this technique should only be used in selected patients. In asymptomatic patients without distant disease and good PS, higher doses may improve both quality of life and OS. Thus, we recommend treating selected asymptomatic patients without metastatic disease with radical intent RT; in other cases, we recommend considering hypofractionated reRTvssupportive treatment to prevent toxicity.
APPENDIX: LEVELS OF EVIDENCE
The present review was carried out by Spanish radiation oncologists from the GOECP/SEOR to review RT and combined treatment of SCLC. This review is based on a quality analysis of the published literature according to internationally-accepted classification of the levels of recommendation and the grades or quality of scientific evidence[188].
杂志排行
World Journal of Clinical Oncology的其它文章
- Thromboembolic events in metastatic testicular cancer treated with cisplatin-based chemotherapy
- Overview of recent advances in metastatic triple negative breast cancer
- Systemic adverse effects and toxicities associated with immunotherapy: A review
- Cardiovascular risk management in cancer survivors: Are we doing it right?
