Bidirectional link between diabetes mellitus and coronavirus disease 2019 leading to cardiovascular disease:A narrative review
2021-04-02
Vijay Viswanathan,M Viswanathan Hospital for Diabetes,M Viswanathan Diabetes Research Centre,Chennai 600013,India
Anudeep Puvvula,Annu’s Hospitals for Skin and Diabetes,Nellore 524101,Andhra Pradesh,India
Ankush D Jamthikar,Department of Electronics and Communications,Visvesvaraya National Institute of Technology,Nagpur 440010,Maharashtra,India
Luca Saba,Department of Radiology,University of Cagliari,Monserrato 09045,Cagliari,Italy
Amer M Johri,Department of Medicine,Division of Cardiology,Queen’s University,Kingston,ON K7L 3N6,Canada
Vasilios Kotsis,3rd Department of Internal Medicine,Hypertension Center,Papageorgiou Hospital,Aristotle University of Thessaloniki,Thessaloniki 541-24,Greece
Narendra N Khanna,Department of Cardiology,Indraprastha APOLLO Hospitals,New Delhi 110020,India
Surinder K Dhanjil,Stroke Diagnosis and Monitoring Division,AtheroPoint™ LLC,CA 95661,United States
Misha Majhail,Jasjit S Suri,Stroke Diagnosis and Monitoring Division,AtheroPoint™,Roseville,CA 95661,United States
Durga Prasanna Misra,Department of Clinical Immunology and Rheumatology,Sanjay Gandhi Postgraduate Institute of Medical Sciences,Lucknow 226014,Uttar Pradesh,India
Vikas Agarwal,Departments of Medicine,Sanjay Gandhi Postgraduate Institute of Medical Sciences,Lucknow 226014,Uttar Pradesh,India
George D Kitas,Academic Affairs,Dudley Group NHS Foundation Trust,Dudley DY1 2HQ,United Kingdom
George D Kitas,Arthritis Research UK Epidemiology Unit,Manchester University,Manchester M13 9PL,United Kingdom
Aditya M Sharma,Division of Cardiovascular Medicine,University of Virginia,Charlottesville,VA 22908,United States
Raghu Kolluri, OhioHealth Heart and Vascular,Ohio,OH 43082,United States
Subbaram Naidu, Electrical Engineering Department,University of Minnesota,Duluth,MN 55812,United States
Abstract Coronavirus disease 2019 (COVID-19) is a global pandemic where several comorbidities have been shown to have a significant effect on mortality.Patients with diabetes mellitus (DM) have a higher mortality rate than non-DM patients if they get COVID-19.Recent studies have indicated that patients with a history of diabetes can increase the risk of severe acute respiratory syndrome coronavirus 2 infection.Additionally,patients without any history of diabetes can acquire newonset DM when infected with COVID-19.Thus,there is a need to explore the bidirectional link between these two conditions,confirming the vicious loop between “DM/COVID-19”.This narrative review presents (1) the bidirectional association between the DM and COVID-19,(2) the manifestations of the DM/COVID-19 loop leading to cardiovascular disease,(3) an understanding of primary and secondary factors that influence mortality due to the DM/COVID-19 loop,(4) the role of vitamin-D in DM patients during COVID-19,and finally,(5)the monitoring tools for tracking atherosclerosis burden in DM patients during COVID-19 and “COVID-triggered DM” patients.We conclude that the bidirectional nature of DM/COVID-19 causes acceleration towards cardiovascular events.Due to this alarming condition,early monitoring of atherosclerotic burden is required in “Diabetes patients during COVID-19” or “new-onset Diabetes triggered by COVID-19 in Non-Diabetes patients”.
Key Words:COVID-19; Diabetes mellitus; Bidirectional association; Cardiovascular disease; Atherosclerotic burden; Imaging tools
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a global pandemic and an ongoing international public health emergency caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1].Our understanding of the COVID-19 epidemic is limited.However,the information gleaned from previous viral outbreaks may shed light on new approaches to prevent and cure this pandemic.As of November 5,2020,there are over 48.5 million laboratory-confirmed cases of COVID-19 in around 200 countries,with nearly 1.2 million deaths,mostly in comorbid and high-risk groups[1].
Diabetes mellitus (DM) is a highly prevalent metabolic disorder,affecting more than 400 million people globally[2-4].It is now also considered an independent risk factor of COVID-19[5-9].Long-standing DM leads to macrovascular and microvascular complications that ultimately affect patients’ quality of life[10].DM has a long history of being associated with several other infections[11],such as the 2008 epidemic SARS-CoV-1[12],the 2009 pandemic influenza A (H1N1)[13],and the 2010 epidemic Middle East respiratory syndrome-related coronavirus (MERS-CoV)[14].Similarly,it has been shown that DM is associated with the current COVID-19 pandemic[5,15-18].A study of 20982 COVID-19 patients by the Chinese Centre for Disease Control and Prevention showed a 5% prevalence of DM.Further,in an Italian study,Onderet al[6]reported that out of 355 COVID-19 patients,36% had DM.Similarly,another study in the United States by Bhatrajuet al[8]reported that in 24 patients,58% were diabetic.There is,therefore,an extensive range (5.3%-58%) of reported prevalence of DM in COVID-19 patients[5],which could be attributed to the fact that the studies were conducted in different countries (or geographical locations),assessed significantly different sample sizes,and had different objectives.
The majority of people suffering from COVID-19 escape major complications,but a significant minority develops severe illness leading to death.Several factors have been implicated in the development of severe illness,including (1) older age,(2)comorbidities,(3) professional risk of exposure to SARS-CoV-2 at work,and (4)socioeconomic and household conditions.People of any age can get the COVID-19 infection,including children,but typically,COVID-19 related serious complications are present in those over 60 years[19,20].A supporting study from the Centers for Disease Control and Prevention,United States[21],consisting of 4226 people,reported that 80%of deaths were in people aged ≥ 60 years that required intensive care unit admission and long-term care.People of any age with a history of serious past chronic health problems are more vulnerable to COVID-19,possibly because of a weak immune system.Such comorbidities include long-standing heart and brain problems[22,23],kidney impairment[24],cancer[19],immunological disorders[20],obesity[25,26],and diabetes[27].
Obesity is a major risk factor for many diseases,and an increasing number of reports show obesity as a risk factor for COVID-19[25,26],similar to what had been seen with previous coronavirus infections such as SARS-CoV-1 and MERS[28].Our observations point to the reason for comorbidity (that includes diabetes) as one of the highly probable causes of COVID-19 mortality.The top seven countries in diabetes prevalence (India,United States,Pakistan,Bangladesh,Indonesia,Mexico,and Brazil)are listed in the top 8% of countries contributing COVID-19 deaths globally.This suggests that DM and its comorbid conditions may be a major contributor to COVID-19-related mortality[1].Front-line workers,including drivers,sanitation handlers,police,security guards,doctors,and paramedics,come into contact with the public more frequently and may have a higher chance of COVID infection[29,30].In addition,poor living conditions,discrimination,lifestyle,and low socioeconomic status are associated with a higher risk of severe COVID-19 related infection,complications,and death[31,32].
Recent studies have also indicated a plausible reverse association between DM and COVID-19,which means that COVID-19 patients without a history of DM could experience a new onset of diabetes[33-35].To investigate this association,an international group of diabetes researchers from the CoviDIAB Project (covidiab.e-dendrite.com)has set up a global registry of patients with COVID-19-associated diabetes.Although there is a high prevalence of DM in COVID-19,a possible bidirectional association and the link between these two conditions are still unclear.Therefore,to prevent long term cardiovascular events,it is essential to collect more evidence from different peerreviewed studies and validate this bidirectional mechanism between DM and COVID-19.
We analyzed this possible bidirectional hypothesis by splitting it into two unidirectional flows.We hope that this will lead to a better understanding of these two global health emergencies.In section 2,we address the question “How does diabetes increase the viral entry of SARS-CoV-2?”,while in section 3,we address the question“How can COVID-19 infections lead to new-onset diabetes or the worsening of preexisting diabetes?” Table 1 provides an evidence-based summary of studies supporting sections 2 and 3.Section 4 addresses the question “How can the interplay between DM and COVID-19 increase the risk of cardiovascular disease (CVD)?”.Further,it also discusses the role of vitamin D (Vit D) during the COVID-19 pandemic.Finally,section 5 presents the role of atherosclerosis imaging for diabetes patients during the COVID-19 pandemic and long term follow-up of survivors[36,37].
HOW DOES DM INCREASE THE VIRAL ENTRY OF SARS-COV-2?
Three possible pathways that might increase COVID-19 susceptibility in patients with DM are depicted in Figure 1.Increased susceptibility to COVID-19 infection may occur through (1) medication-induced angiotensin-converting enzyme 2 (ACE2) expression(pathway-I),(2) impaired immunity (pathway-II),and (3) increased furin levels(pathway-III).In the first pathway,patients with DM exhibit a high prevalence of increased blood pressure and CVD[38].Hence,along with hypoglycemic agents,these patients are mostly treated with antihypertensive medications like angiotensinconverting enzyme inhibitors (ACE-I),angiotensin-II type I receptor blockers (ARB)[39],and lipid-lowering drugs such as statins[40].Hypoglycemic agents,such as (1)glucagon-like peptide-1 agonists (e.g.,liraglutide)[41]and (2) thiazolidinediones (e.g.,pioglitazone)[42,43],facilitate viral entry following overexpression of ACE2.A new hypoglycemic agent,sodium-glucose cotransporter 2 inhibitors (SGLT2i),has been used for treating type 2 diabetes[44].SGLT2i may also promote cellular viral entry by increasing ACE2 levels indirectly,mainly when used alongside ACE-I.Moreover,using SGLT2i in patients with COVID-19 may cause serious complications such as dehydration and could increase the risk of diabetic ketoacidosis (DKA)[45-47].Antihypertensive medications such as ACE-I and ARBs are also associated with an increase in ACE2 expression[48-51].Increased expression of ACE2 receptors by the epithelial cells of the lung [alveolar type 2 (AT2) cells][52,53],intestine (enterocytes)[54],kidneys (proximal tubule cells)[52],and heart (myocardial cells)[52]facilitates the human cell entry of SARS-CoV-2[41-43,45,48-51].Additionally,Hodgsonet al[55]showed that patients with DM and hypertension treated with ACE-I and ARB are more susceptible to SARS-CoV-2 infection due to higher expression of ACE receptors.Furthermore,experimental animal studies demonstrated increased expression of ACE2 receptors by using statins[56,57].In an experimental rabbit model,atorvastatin use resulted in overexpression of ACE2 receptors in both the heart and the kidney[56].A similar study in diabetic rats treated with fluvastatin resulted in overexpression of ACE2 receptors in the heart and blood vessels[57].Recently Motaet al[57]reported in their study that frequently used drugs in patients with DM,like glucagon-like peptide-1 receptor agonist,thiazolidinediones,anti-hypertensives such as ACE-I,and lipid-lowering drugs such as statins,hike ACE2 expression,increasing the risk of COVID-19.On the other hand,it was shown that people who were on insulin had less ACE2 expression(Table 1)[58].Furthermore,Abbaset al[59]and Muniangi-Muhituet al[60]also validated that “Drug-induced” causes an increase in ACE2 expression in DM,which further increases the possibility of SARS-CoV-2 viral entry.These studies suggest that a medication-induced ACE2 overexpression may play a role in the pathophysiology of COVID-19,as shown in pathway-I (Table 1).

Table 1 Summary of studies describing potential linkages between diabetes mellitus and increasing propensity for coronavirus disease 2019 infection or diabetes mellitus triggered in response to viral infection,supporting sections 2 and 3
The second pathway (marked as II in Figure 1) is related to the impaired immune response in DM patients.Patients with DM have an increased susceptibility to infections[61].The presence of hyperglycemia and oxidative stress in DM inhibits (1)neutrophil chemotaxis,(2) phagocytosis,and (3) macrophage activity[55,58,62-66].Furthermore,in DM,impairment of natural killer cells and interferons (IFN-γ) has been observed.Additionally,the SARS-CoV-2 virus primarily infects monocytes and dendritic cells that results in a weakened immune system (Table 1)[60,67].All these play a vital role in increasing susceptibility to viral proliferation in COVID-19 patients,especially those with poor blood glucose control[55,61-63].Hence,with the above explanation,we can conclude that there is a possible association between impaired immune response and an increased risk of COVID-19 infection in diabetes patients.Recently,Kalraet al[68]also supported the same pathway II in their newly published article.
In the third pathway (marked as III in Figure 1),DM increases the presence of furin,which is a type-1 membrane-bound protease belonging to the “proprotein convertase subtilisin/Kexin” receptor family[55].Interestingly,recent studies showed an increased furin level in DM patients facilitating the cellular entry of SARS-CoV-2 (Table 1)[58,69,70].Furthermore,furin is associated with the cleavage and priming of the spike protein of SARS-CoV-2 (S1 and S2 proteins),thereby mediating viral entry in the host cell[71,72].The presence of furin is likely associated with the replication of SARS-CoV-2 in patients with DM[16,69].Although pathway-III is well seen and better understood compared with pathway-I and pathway-II,the cumulative effect of the three pathways validates the possible link that indicates the increased cellular entry of SARS-CoV-2 in patients with pre-existing DM.
HOW CAN COVID-19 LEAD TO NEW-ONSET OR WORSENING OF PREEXISTING DM?
This section illustrates the reasons causing “new-onset DM” or “worsening of preexisting DM” in post-COVID-19 cases.It has been observed that patients that were not having a prior history of diabetes,but when infected by COVID-19,lead to severe complications such as DKA[33-35].It has been shown that DKA occurs mainly due to total or subtotal insulinogenic (reduced insulin levels) and the overproduction of counter regulators,which favors the production of ketones[34,73].Further,DKA is most commonly observed in patients with type 1 DM but may also occur in type 2 diabetes[74,75].In an observational study,Liet al[33]reported that COVID-19 infection also induces DKA in patients with diabetes.Henceforth,we hypothesized the three plausible series of pathways of new-onset DM or worsening of pre-existing diabetes after COVID-19 infection,which is depicted in Figure 1 (pathways-IV,V,and VI).A zoomed version of Figure 1 indicating the bidirectional association between DM and COVID-19 is provided in Supplementary Figure 1.
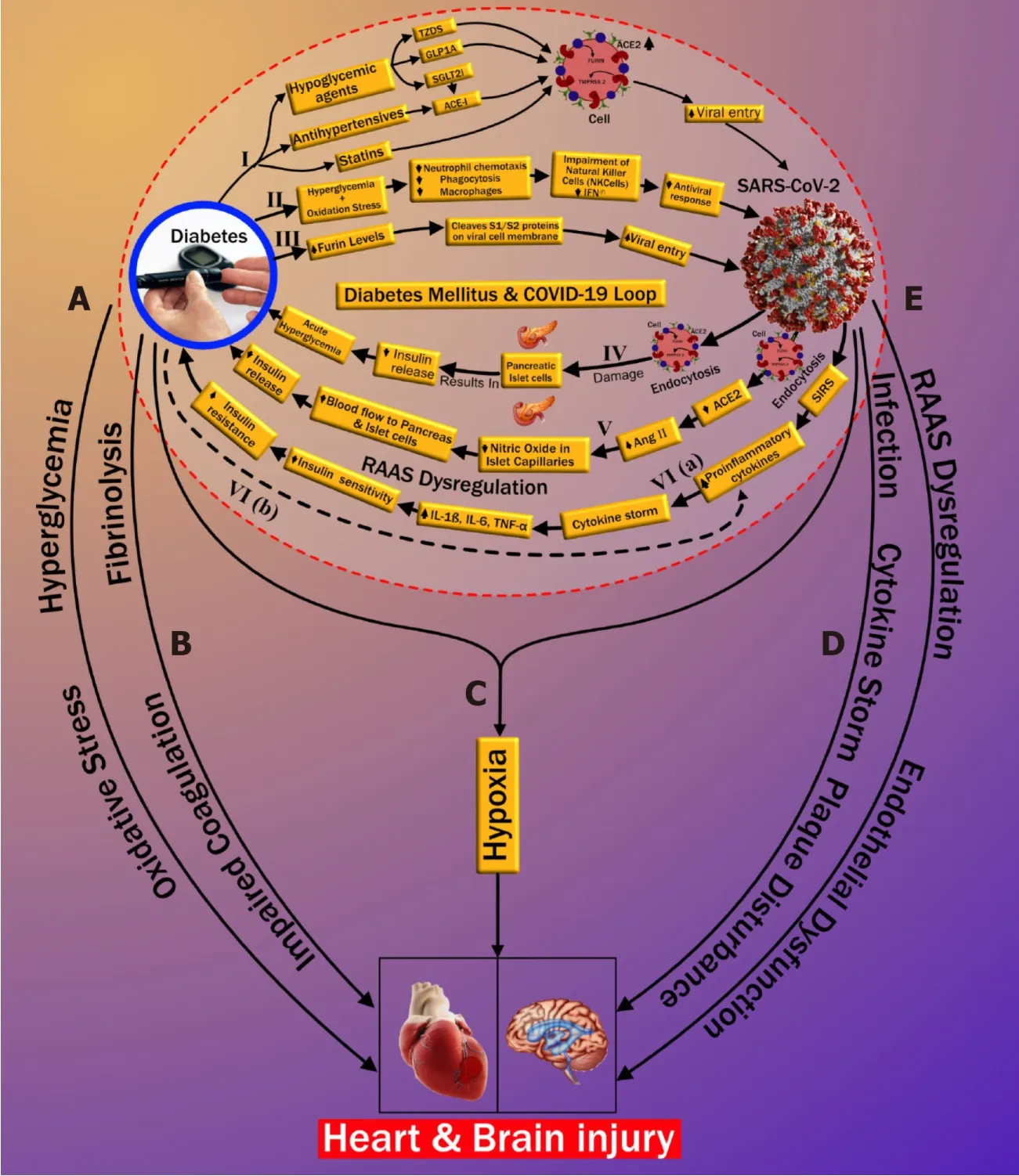
Figure 1 The plausible bidirectional link between diabetes mellitus and coronavirus disease 2019.
Pathway IV explains the effect of COVID-19 causing insulin-dependent DM.It is well known that viral infections are associated with the development of pancreatic autoantibodies leading to insulin-dependent DM or type 1 DM.These respiratory viruses were identified as one of the potential causative pathogens in “the environmental determinants of diabetes in the young” (TEDDY) study[76,77].SARS-CoV-2 uses the ACE2 receptor as an entry gate[78]into the pancreas.An interesting study by Thaweeratet al[79]showed that the ACE2 receptors are more densely populated in the endocrine area when compared with the exocrine area of the pancreas through ACE2 immunostaining of pancreatic tissue[79].The main function of the exocrine area of the pancreas is to facilitate blood glucose regulation[80].SARS-CoV-2 enters into the pancreas thereby triggering autoimmunity and resulting in pancreatic cell destruction;this is particularly prevalent in severe COVID-19 cases[81-84].This pathway,activated due to viral infection,may lead to the production of cross-reactive antibodies against pancreatic cells (molecular mimicry hypothesis)[85,86].Thus,the hypothesis of the bidirectional involvement of DM-COVID-19 holds,which states that the SARS-CoV-2 infection results in direct damage to pancreatic islet cells,leading to the impairment of insulin levels[79,87]and potentially triggering DM (shown in pathway-IV in Figure 1).Recently Abbaset al[59]also validated the existence of this pathway (Table 1).Additionally,Baracchiniet al[66]and Motaet al[58]also mentioned this pathway in their recent work on COVID-19 (Table 1).Balasubramanyamet al[88]and Rubinoet al[35]further asserted their views on this pathway,establishing its validation.
Pathway V suggests that endocytosis of SARS-CoV-2 decreases ACE2 levels that causes the increase of angiotensin II (AngII) levels,which is a potent vasoconstrictor.Constriction of vessel lumen may be due to inhibition of nitric oxide in the endothelium of islet capillaries[89].This results in a decrease in blood supply to the pancreas.Islet cells receive 15% of the total blood supply to the pancreas,even though they constitute only 1%-2% of pancreatic volume[90].Hence,a decrease in the blood flow to the pancreatic islets due to vasoconstriction may impair insulin secretion in the pancreas[91](see pathway V of Figure 1).This further confirms the bidirectional association between COVID-19 and DM,also listed in Table 1.
In pathway VI,increased proinflammatory cytokines due to COVID-19 are higher in patients with DM in comparison with patients without DM.This likely contributes to a poorer prognosis when both diseases coexist[92].The severe illness that accompanies COVID-19 causes a systemic inflammatory response.This can be seen even with mild COVID-19 infection,resulting in an increase of proinflammatory cytokines such as interleukin (IL)-6,IL-1β,and tumor necrosis factor alpha (TNF-α)[93].Increased proinflammatory cytokines result in decreased insulin sensitivity,which then leads to hyperglycemia.Further obesity,a significant coexisting condition associated with type 2 diabetes,is linked to the development of insulin resistance (IR).Obesity and type 2 diabetes further aggravate the proinflammatory cytokine response,which worsens the IR[93](see pathway VI in Figure 1).Wanget al[70]reported that SARS-CoV-2 infection in patients with diabetes results in increased levels of stress hormones such as glucocorticoids that can lead to a hyperglycemic state.An acute rise in glycemic levels may result in life-threatening complications like ketoacidosis.Finally,the systemic inflammatory state associated with COVID-19 may plausibly worsen the pre-existing IR state in such individuals,manifesting as overt DM[94].The long-term sequelae of this process are currently unknown,and clinical studies are needed to validate the hypothesis further.However,our hypothesis is supported by some recent studies that also indicate a similar thought that “the association between COVID-19 and hyperglycemia is because of metabolic inflammation and exaggerated cytokine release” (Table 1)[60,95].This thought emerged because of the potential role of SARSCOV-2 in the impairment of insulin secretion,leading to hyperglycemia.Reddyet al[96]presented a case study of two patients positive with COVID-19 and no personal history of diabetes.The authors indicated precipitation of DKA,which can occur in newly diagnosed diabetes patients.Palet al[97]also provided an overview of this bidirectional interaction between DM and COVID-19,where COVID-19 may lead to diabetes and,in turn,further increases the severity of COVID-19.These studies validate our thought process and indicate a need for a global study to investigate this bidirectional hypothesis.The recent announcement of the CoviDIAB project will shed light on this possible hypothesis of the bidirectional association between both of these global healthcare emergencies[35,98].
HOW CAN THE INTERPLAY BETWEEN DM AND COVID-19 INCREASE THE RISK OF CVD?
Throughout the world,DM is one of the leading causes of mortality and morbidity due to its association with several microvascular and macrovascular complications,which include CVD.Since there is a positive correlation between DM and COVID-19,it is imperative to understand its implication on CVD risk.Several studies have found that patients with COVID-19 and DM are at increased risk of vascular complications[99,100].Poor glycemic control with the presence of IR plays a vital role in the worsening of CVD risk in DM patients[101].As supporting evidence,the study by Madjidet al[102]highlighted the identification of heart damage through high levels of troponin in the blood due to COVID-19 leading to mortality of the patient.Further,it was shown that there is a role for inflammation of the heart due to COVID-19,e.g.,myocarditis,vascular inflammation,and cardiac arrhythmias.Other supporting evidence by Javanmardiet al[103]is a meta-analysis showing the prevalence of preexisting diseases in COVID-19 patients.The data in this study were pooled from 10 articles having 76993 patients and showed a prevalence of 7.87% [95% confidence interval (CI) 6.57-9.28] diabetes,16.37% (95%CI:10.15-23.65) hypertension,12.11%(95%CI 4.40-22.75) CVD,and 7.63% (95%CI 3.83-12.43) smoking history,respectively,in patients infected with SARS-CoV-2.Further,Azaret al[104]have shown that the presence of pre-existing diseases such as DM,hypertension,and CVD are more likely to be associated with an increased risk of mortality in COVID-19 patients.Azaret al[104]focused on the cytokine storm concept that showed the connection between DM and COVID-19.Further,they showed that the higher basal levels of proinflammatory cytokines were seen in diabetic patients,which resulted in a cytokine storm with an increase in viral infection.They demonstrated the link between high levels of IL-6 and the AMP-activated protein kinase (AMPK)/mechanistic target of rapamycin signaling pathway and their role in exacerbating diabetes-related complications and IR.Both statements in the article support pathway VI of our article,which shows the possibility of aggravating preexisting diabetes or new-onset diabetes in COVID-19 due to cytokine storm.Additionally,they highlighted the role of the ACE2 receptor during viral binding to the host cell,thereby causing an increased risk of viral uptake in diabetes patients.
The work of Azaret al[104]is one of the bases of our hypothesis,where we discuss the two-way relationship between DM and COVID-19,i.e.triggering of COVID-19 on the new onset of DM and worsening glycemic levels of DM.Further,our study demonstrated the importance of early imaging to prevent CVD among all patients with COVID-19.On the contrary,the studies by Madjidet al[102]and Javanmardiet al[103]did not directly support the concept of bidirectional relationship.Additionally,Sattaret al[105]showed that there was worsening of cardiac events in COVID-19 patients with preexisting cardiac conditions,such as coronary artery disease,hypertension,and DM[105].Furthermore,six different studies across various hospitals in China reported the prevalence of comorbid conditions in COVID-19 patients.Out of 1527 COVID-19 admissions,9.7% had diabetes,leading to increased CVD prevalence by 16.4%[7,106-110].
In the previous two sections,we have explained the possible bidirectional link between DM and COVID-19.Current data from many countries such as China,Italy,and the United States have shown that COVID-19 can lead to mild symptoms in most individuals.However,a minority of individuals suffer from severe complications due to underlying chronic complications (explained in detail in section I,page 3)[111].Possible reasons for increased CVD risk in known DM patients infected with COVID-19 are explained in five subsections (labeled as pathways A to E) of Figure 1.The first two subsections discuss the possible connection between DM and CVD,which includes (1) oxidative stress due to chronic hyperglycemia (subsection A) and (2)increased coagulation activity (subsection B).Subsection C shows how COVID-19 and DM jointly affect CVD due to hypoxia.In the last two subsections (D and E),we show the possible pathways between COVID-19 and CVD.This includes the role of (1)cytokine storm (subsection D) and (2) renin-angiotensin-aldosterone system (RAAS)dysregulation along with endothelial dysfunction (subsection E).
Oxidative stress
Oxidative stress is defined as the pathology of hyper-production of “reactive oxygen species” (ROS) and the counterbalancing part of the endogenous antioxidant defensive system[106].Chronic hyperglycemia and IR in DM result in the production of proinflammatory cytokines and an increase of “advanced glycation end products”(AGEs)[61,107].High levels of AGEs increase CVD risk two-fold when compared with low AGE levels[112].A further increase in AGE levels results in ROS production,which accelerates AGE production,producing a cyclic effect[113].Increased ROS results in oxidative stress as a systemic manifestation that plays a vital role in DM[108].These further result in endothelial dysfunction due to (1) nitric oxide inhibition in the endothelial cells of the blood vessels and (2) increased inflammation and fibrosis[109],eventually leading to increased risk of atherosclerotic CVD (marked as subsection A in Figure 1).Interestingly,many studies found that AMPK has a protective role in cardiac injury by acting against oxidative stress and turns into a potential therapeutic target in patients with diabetes and COVID-19[110,114].Besides this link between oxidative stress,DM,and CVD,some recent studies have also pointed out a possible role of oxidative stress in the pathogenesis of COVID-19 related infections[112,113,115,116]; however,this is beyond the scope of this review.
Increased coagulation activity
Increase in coagulation activity occurs due to the loss of fibrinolytic activity associated with DM.In general,the fibrinolytic process helps to degrade clots and remove them from blood vessels.It counters clot formation and risk occlusion in blood vessels by eliminating fibrin from the vasculature.In patients with DM,there is an increase in clotting factors and a relative reduction of the fibrinolytic system[117].The impaired coagulation in DM is associated with alterations of the fibrin network and increased antifibrinolytic proteins[118].Hence the reduced coagulation activity in patients with DM causes endothelial dysfunction by triggering platelet activation and aggregation,which further favors atherosclerotic plaque formation[119],increasing cardiovascular risk (marked as subsection B in Figure 1).
Hypoxia
There is clear evidence that SARS-CoV-2 causes pulmonary as well as extrapulmonary complications like CVD[120].Primary SARS-CoV-2 enters through the respiratory route and anchors to AT2 cells in the alveolar pulmonary epithelium[121].This fusion is occurring due to the presence of ACE2 receptors on the surface of AT2 cells and the resulting development of respiratory symptoms as the most common clinical presentation of COVID-19 patients[122].Infected AT2 cells further initiate the immune response by producing inflammatory mediators shown with SARS-CoV-1 and stimulate the production of proinflammatory cytokines and chemokines[123].Hyperproduction of chemokines and cytokines results in endothelial dysfunction,causing vasodilation and an increase in sub-endothelial space's vascular permeability.This further leads to diffused alveolar interstitial exudate[124]and causes pulmonary edema resulting in an alveolar gas exchange disorder known as “acute respiratory distress syndrome” (ARDS)[125].Additionally,DM patients can have reduced lung function indicated by decreased levels of “forced vital capacity” (FVC) and “forced expiratory volume in one second” (FEV1).Generally,FVC and FEV1 are vital parameters for accessing lung function[126-128].Impending lung function is associated with chronic hyperglycemia resulting in an increased risk of ARDS[126-128].Decreased lung function in DM can increase the risk of ARDS if infected with COVID-19.This was further supported by Huanget al[129],where the authors showed that roughly 30%of diabetes patients with COVID-19 developed impairment in lung function,shown as a decline in FEV1/FVC ratio.Hence,ARDS risk is increased with the coexistence of DM and COVID-19 and can further lead to depletion in the oxygen levels in the blood[130-132].
Ongoing hypoxia in myocardial cells results in myocardial ischemia and heart injury[132],and ongoing hypoxia in brain cells results in brain injury[133].Nanet al[134]showed that COVID-19 patients with comorbidity had an acute cardiac injury and needed invasive mechanical ventilation,while Kwenandaret al[135]showed that cardiovascular manifestations in COVID-19 patients like myocardial injury,arrhythmias,sudden cardiac arrest,heart failure,and coagulation abnormality occur in up to 33% of patients[135].Zunyou,Wuet al[136],and Clerkinet al[137]submitted a summary of the report to the Chinese center for disease control and prevention indicating 1023 deaths in 44672 confirmed cases with COVID-19,i.e.a case-fatality rate(CFR) of 2.3,and stating that patients with underlying CVD or hypertension had a higher CFR compared with people without comorbidities[136,137].
Cytokine storm
Guoet al[92]reported a higher risk of pneumonia in COVID-19 patients with DM when compared with patients without a history of DM.Patients with DM experience an advanced stage of illness that causes multiple organ dysfunction,triggering an exaggerated inflammatory response compared with non DM.This results in the production of proinflammatory cytokines that include IL-6,IL-7,IL-12,IL-15,IL-22,Creactive protein,and TNF-α,leading to cytokine storm[138-141].Another study by Guoet al[142]showed that cardiac injury in patients with COVID-19 had elevated troponin and C-reactive protein,suggestive of increased morbidity and mortality[122,142].Zhenget al[122]and Wuet al[143]exemplified that cardiac injury may happen due to cytokine storms caused by the inflammatory response of T helper cells.Huanget al[129]also supported that imbalance in T helper cells results in triggering cytokine storm leading to destabilization of carotid plaque and micro thrombosis.Supporting evidence by Kanget al[144]showed that significant risk of cardiac complications,such as arrhythmia,heart failure,and myocardial infarction,in COVID-19 was due to a combination of (1)hyper inflammation with cytokine release,(2) plaque instability,(3) myocardial inflammation,(4) hypercoagulable state,and (5) direct myocardial injury.Vinayagamet al[145]concluded that chronic inflammation through cytokines and chemokines promotes hypercoagulability,causing multiorgan dysfunction leading to heart and brain injury.
RAAS dysregulation
RAAS plays an important role in maintaining cardiovascular health and electrolyte balance[146]and has been well-described before.In COVID-19 patients,the SARS-CoV-2 gains entry into the cells by attaching to the ACE2 receptor of the cell.The anchoring ability of the virus is due to its spike protein,which is present on its surface[71,147,148].The dysregulation of RAAS occurs due to the loss of a counter-balance between Ang II levels and ACE2 levels after SARS-CoV-2 infection[149].ACE2 levels degrade Ang II and produce Ang (1-7),which opposes the negative impact of Ang II[132,150].ACE2 and Ang(1-7) are recognized as a cardio-cerebral protective factor[151].The reduced levels of ACE2 receptors and increased levels of Ang II after a SARS-CoV-2 infection can lead to atherosclerotic CVD in two possible ways.First,an increased Ang II level causes stimulation of the adrenal gland,triggering the production of the mineralocorticoid hormone aldosterone[146],which causes Na (sodium) and water retention in the collecting duct of the kidney[152].This increases blood volume and blood pressure[153],causing endothelial dysfunction that progresses to atherosclerotic CVD.Second,excessive Ang II levels result in vasoconstriction,proinflammation,prothrombotic,and proliferative effects[132,150].This has a detrimental effect on the blood vessels,thereby leading to endothelial cell damage and subsequent atherosclerotic cardiovascular events.Additionally,DM patients using ACE inhibitors and ARBs have increased ACE2 expression,which is beneficial to vascular health by reducing profibrotic and proinflammatory function.But,increased ACE2 levels promote the entry of SARS-CoV-2 infection that potentially results in a loss of ACE2 in blood vessels in diabetes patients causing vascular complications like CVD[154].Recently,Suriet al[23]showed that COVID-19 is an independent risk factor for developing CVD due to hypoxia,cytokine storm,and RAAS dysregulation.
Role of CVD risk factor in COVID-19 patients with/without DM
In Table 2,we briefly illustrated the difference in COVID-19 severity between patients with DM and non-DM.We have shown that DM has an added risk in patients with COVID-19.There are many reasons to explain why COVID carries a worse prognosis in DM patients.They include age,culture,comorbidities like hypertension and preexisting CVD,higher body mass index,and proinflammatory and pro-coagulable state,all of which may contribute to the risk of worse outcomes[155].
A meta-analysis by Santosoet al[156]showed a total of 2389 patients taken from 13 studies that had a cardiac injury and were associated with a higher risk of mortality when affected by COVID-19.Further,the authors stated that these patients required intensive care unit admission during the COVID-19 period[156].In another metaanalysis by the same group (see Huanget al[157]),which included 6452 patients with DM from 30 studies,DM associated with the worst outcome and mortality when affected by COVID-19.Additionally,Pranataet al[158]showed in a total of 4448 patients from 16 studies that 77% of people had poor health outcomes due to cerebrovascular disease,and 60% of people had poor health outcomes due to CVD.
Role of Vit D during COVID-19 pandemic
Vit D has many beneficial roles in the maintenance of musculoskeletal health,and its deficiency causes calcium malabsorption resulting in fractures[159].This can be prevented by a daily intake requirement of 800-2000 IU of Vit D,co-administrated with calcium,thereby reducing the risk of fracture by 15%-30%.This range of doses is recommended by major organizations during pre-COVID times[160].Interestingly in recent publications on COVID-19 by Ilieet al[161]and Rhodeset al[162],the authors showed that low Vit D levels are associated with higher mortality rates in SARS-CoV-2 infections.Further ecological studies have shown that major risk factors of low Vit D levels are older age,higher latitudes,winter season,less sunlight exposure,and dietary habits.Vit D is responsible for the modulation of innate and adaptive immunityviaVit D receptor (VDR) and CYP27B1 (enzyme converting it to active metabolite calcitriol),and both are expressed in immune cells[163,164].
Many studies showed that the major role of Vit D in COVID-19 is that it lessens the cytokine production after SARS-CoV-2 infection including IL6,TNF-α,and IFN-β[165].Other anti-viral properties include modulation of macrophage chemotactic protein1,IL 8,type 1 IFN,TNF-α,and lowering of ROS[166].Ongoing clinical trials on pharmaceutical interventions of 2019 Novel Coronavirus Research Compendium[167]andprimary registry trials of World Health Organization[168]include trials of Vit D supplementation in COVID-19 infection.

Table 2 Cardiovascular severity in diabetes and non-diabetes coronavirus disease 2019 patients
ROLE OF ATHEROSCLEROSIS IMAGING IN DIABETES PATIENTS DURING COVID-19
Figure 2 shows the cytokine storm leading to atherosclerosis and pathway “E” leading to endothelial dysfunction and atherosclerosis formation.Both DM and COVID-19 are associated with vascular wall damage[169](such as plaque erosion or atherosclerotic plaque vulnerability),and this elevates the risk of atherosclerotic CVD events[170-172].Vinciguerraet al[173]reported that atherosclerosis may be an ideal pathogenetic substrate for high viral replication ability,leading to adverse cardiovascular outcomes.Atherosclerosis is generally initiated by damage to the endothelial cells[174].Recent histological findings suggest the involvement of COVID-19 viral elements in endothelial cell damage and the accumulation of inflammatory cells leading to endothelial cell death[175].The damage to endothelial cells and the hyperdynamic circulation due to COVID-19 may lead to atherosclerotic plaque instability and rupture[176].
Similarly,the formation of atherosclerotic thrombus in the arteries has been reported in recent studies[177].Lapergueet al[177]reported COVID-19 patients that showed the development of large thrombus in the cervical carotid artery with underlying mild non-stenosing atheroma.Indeset al[178]also reported an elevation of risk for acute arterial thromboembolic complications in patients with COVID-19 infection.Esenwaet al[179]presented a radiology-pathology case series of three COVID-19 patients and reported that a disproportionately high intra-luminal thrombus in the carotid artery showing mild-to-moderate atherosclerotic disease with intimal thickening and plaque calcification.Mohamudet al[180]presented a case study of six COVID-19 patients that had atherosclerotic plaque vulnerability and the development of thrombotic events.This was the result of the inflammatory response and cytokine storm,which has also been considered as an important phenomenon for cardiac events in our review (Figure 2).Similarly,Alkhaibaryet al[181]reported complete occlusion of the left common carotid artery and left middle cerebral artery of the COVID-19 patients.

Figure 2 Pathways linking coronavirus disease 2019 to heart/brain injury.
These studies have also indicated the presence of vascular risk factors such as hypertension and DM that exaggerate the risk of cardiovascular and stroke events in COVID-19 patients.Since the COVID-19 driven cytokine storm is associated with atherosclerotic plaque instability,it is essential to screen such patients for the presence of arterial plaque burden.Imaging techniques have shown promising results in screening,diagnosis,and patient management during this pandemic.COVID-19 symptoms of the patients and seriousness have helped to decide which imaging technique is most appropriate:Portable and non-portable[23].In COVID-19 patients,non-invasive carotid ultrasound may be adopted for low-risk patients to investigate the presence of carotid atherosclerotic plaque[182,183],which is also considered as a surrogate marker CVD[184-186]and also used for CVD risk assessment in diabetic patients[187-197].Similarly,magnetic resonance imaging and X-rays can be useful for screening of medium risk patients[198-200].Most of the studies prefer the use of these non-portable imaging techniques.For critical cases,intravascular ultrasound imaging,computed tomography angiography,and ventriculography are generally followed for arterial imaging[201-203].Among all these techniques,ultrasound is a portable,less expensive,and radiation-free imaging technique and,therefore,can be adopted for the screening of atherosclerotic plaque in patients with COVID-19[204].Several studies used ultrasound to detect the rupture-prone atherosclerotic plaque and the tissue characterization of such plaque for the prevention of future cardiac events[205-207].Furthermore,studies have used ultrasound-based imaging techniques for CVD risk assessment in patients with diabetes[187-189,191,193,195,196,208-212]and,thus,could also be adopted for diabetic patients with COVID-19.Several other imaging-based studies have shown the interaction of plaque measurements in diabetes patients[196,211,212].In the imaging category,plaque area was recently measured and correlated with hemoglobin A1c in diabetes patients[213].These further assert the role of atherosclerotic imaging and phenotype measurements in post-COVID-19 patients.Image-based risk calculators can be adapted for 10-year risk assessment on diabetes patients,which can be adapted for post-COVID-19 follow-up[211,214].
In the current pandemic,it is also equally important for radiologists and medical practitioners to follow the guidelines while conducting an imaging-based screening of COVID patients.These include isolating the imaging equipment,taking the images through the isolation room glasses,and using disposable sterile protection to imaging probes[23].
CONCLUSION
We showed clearly the six pathways between DM and COVID-19,establishing the DM-COVID-19 loop.Three pathways were unidirectional from DM to COVID-19 and vice-versa establishing the bidirectional flow.Further,we demonstrated the effect of this DM-COVID-19 loop on CVDs,causing the acceleration towards cardiovascular and cerebrovascular events.The mini-review also shows why only a minority group of people develop severe complications,unlike the majority group of people who escape.The review also sheds light on the role of Vit D during the COVID-19 pandemic.Finally,the review presents the role of vascular imaging for tracking atherosclerotic burden during COVID-19 and on long term follow-up patients.We conclude that (a)DM/COVID-19 loop is detrimental to the patient’s heart and brain and (b) early monitoring of atherosclerotic burden is required in “Diabetes patients during COVID-19” or “new-onset Diabetes triggered by COVID-19 in Non-Diabetes patients”.
杂志排行
World Journal of Diabetes的其它文章
- Diabetes and COVID-19:Diseases of racial,social and glucose intolerance
- Progress on haptoglobin and metabolic diseases
- Anti- and non-tumor necrosis factor-α-targeted therapies effects on insulin resistance in rheumatoid arthritis,psoriatic arthritis and ankylosing spondylitis
- Causal effect of education on type 2 diabetes:A network Mendelian randomization study
- Altered spontaneous brain activity in patients with diabetic optic neuropathy:A resting-state functional magnetic resonance imaging study using regional homogeneity
- Effectiveness of cognitive behavior therapy for sleep disturbance and glycemic control in persons with type 2 diabetes mellitus:A community-based randomized controlled trial in China
