3D printed tacrolimus suppositories for the treatment of ulcerative colitis
2021-03-22FrnisoOteroEspinrAlvroGoynes
,Frniso J.Otero-Espinr,Alvro Goynes
aDepartment of Pharmacology,Pharmacy and Pharmaceutical Technology,Faculty of Pharmacy,University of Santiago de Compostela,Santiago de Compostela 15782,Spain
b Department of Pharmaceutics,UCL School of Pharmacy,University College London,London WC1N 1AX,UK
c Pharmacy Department,University Clinical Hospital Santiago de Compostela(SERGAS)(CHUS),Santiago de Compostela 15706,Spain
dFabRx Ltd.,Ashford,Kent TN24 0RW,UK
eDepartamento de Farmacología,Farmacia y Tecnología Farmacéutica,I+D Farma Group(GI-1645),Universidade de Santiago de Compostela,Santiago de Compostela 15782,Spain
ABSTRACT Ulcerative colitis is a global health problem,affecting millions of individuals worldwide.As an inflammatory condition localised in the large intestine,rectal delivery of immunosuppressive therapies such as tacrolimus is a promising strategy to maximise drug concentration at the site of action whilst minimising systemic side effects.Here,for the first time,self-supporting 3D-printed tacrolimus suppositories were prepared without the aid of moulds using a pharmaceutical semi-solid extrusion (SSE) 3D printer.The suppositories were printed vertically in three different sizes using combinations of two lipid pharmaceutical excipients(Gelucire 44/14 or Gelucire 48/16)and coconut oil.Although both suppository formulations had the appropriate viscosity characteristics for printing,the Gel 44 formulation required less energy and force for extrusion compared to the Gel 48 system.The Gel 44 disintegrated more rapidly but released tacrolimus more slowly than the Gel 48 suppositories.Although the tacrolimus release profiles were significantly different,both suppository systems released more than 80%drug within 120 min.DSC and XRD analysis was inconclusive in determining the solid-state properties of the drug in the suppositories.In summary,this article reports on the fabrication of 3D printed selfsupporting suppositories to deliver personalised doses of a narrow therapeutic index drug,with potential benefits for patients with ulcerative colitis.
Keywords:3D printed drug products Semi-solid extrusion 3D printing Inflammatory bowel disease Suppository drug delivery Pressure assisted syringe M3dimaker
1.Introduction
Inflammatory bowel disease is a group of incurable chronic inflammatory disorders of the gastrointestinal tract,of which Crohn’s disease and ulcerative colitis are the most common[1,2].Specifically,ulcerative colitis is characterised by inflammation of the colon and the rectum.Despite being an inflammatory condition of the distal gastrointestinal tract,the majority of efforts for new treatments have revolved around the development of oral therapies[3-5].However,oral delivery in ulcerative colitis is challenging as potentially only a fraction of the administered drug might reach the intended site of action.In contrast,rectal delivery can enhance therapeutic efficacy and safety by maximising drug concentrations at the disease site,reduce systemic side effects,and increase the rate of response[6,7].
Studies have previously reported the use of tacrolimus suppositories as a treatment in therapy-resistant ulcerative proctitis (ulcerative colitis limited to the rectum) [8-10].Tacrolimus is a calcineurim inhibitor widely used as an immunosuppressive agent [11].Treatment with tacrolimus induces a rapid clinical response and mucosal healing in hospitalised patients with steroid-refractory ulcerative colitis,and its use is also supported in patients with Crohn’s disease refractory to conventional therapies [12-14].The oral administration of tacrolimus is related with long-term toxicity (especially hypertension and renal dysfunction),and the higher the serum trough levels,the more likely it is that a patient will suffer an adverse effect (therapeutic range 5-20 μg/l)[15].This fact,together with the low oral bioavailability of tacrolimus,makes it a suitable candidate for inclusion in a rectal dosage form.However,there are currently no commercially available tacrolimus suppositories,warranting their extemporaneous compounding in pharmaceutical services.
In current practice,suppositories are prepared by a moulding technique,which requires several steps and a relatively long time for hardening.Additionally,it is common for hospital pharmacists to compound suppository formulations from tablets designed for oral administration when a suppository formulation is not available.This leads to a number of risks,such as compounding errors and inaccurate dosing [16].In light of these issues,threedimensional (3D) printing has been explored as a novel manufacturing technology for the manufacture of bespoke formulations including suppositories.3D printing is an additive manufacturing technology that enables the production of individualised objects in a layer-by-layer manner.In the pharmaceutical field,3D printing offers the opportunity to make a significant technological contribution in the design and manufacture of medicines [17].When compared to conventional manufacturing processes,this technology offers unique benefits for the manufacture of solid drug products,such as patient-tailored medicines[18],retentive devices [19,20],and customised drug release formulations [21,22].Within the pharmaceutical field five main 3DP technologies are currently used;powder bed ink jet printing [23],fused deposition modelling (FDM) [24],selective laser sintering (SLS) [25],stereolithography (SLA) [26],and semi-solid extrusion(SSE)[27,28].
SSE 3D printing is based on the deposition of semisolids (gel or paste) in sequential layers through a syringebased tool-head nozzle to create the 3D object,and it is highly relevant to print objects using soft materials [29,30].Compared to other 3D printing techniques,SSE is suitable for thermolabile compounds since the printing process does not require high temperatures [31].Moreover,excipients and drugs can be added directly to the gel base without the need for intermediate steps,such as the preparation of drug-loaded filaments in FDM [32].This technique has been used to manufacture polypills with well-defined and separate controlled release profiles for the different drugs incorporated into the multi-active tablet [33],as well as immediate release tablets with high drug loading[34].Moreover,SSE was the first 3D printing technology used to prepare personalised printlets(3D printed tablets) in a hospital setting.The dosage forms were chewable printlets used for the treatment of a rare metabolic disease,and they were well accepted by paediatric patients,demonstrating the feasibility of this approach to prepare oral tailored-dose therapies[35].The ability of SSE to print soft materials could be exploited to prepare lipid based formulations [29].The use of self-emulsifying drug delivery systems (SEDDS) and self-microemulsifying drug delivery systems(SMEDDS)is one of the most popular approaches for enhancing the solubility of poorly water-soluble drugs [36].SEDDS and SMEDDS are isotropic mixtures of oil,surfactant,cosurfactant and drug which form kinetically stable oil-inwater(O/W)emulsions.The lipophilic drugs are solubilised in the small lipid droplets,which have a large interfacial surface area for drug absorption[37].In this regard,drug-loaded solid SMEDDS (S-SMEDDS) intended for oral administration were successfully prepared using SSE 3DP[38].This approach could allow for the preparation of novel lipid-based formulations with a defined dose,shape,size and drug release profile tailored to the needs of each patient.
As such,by implementing SSE 3D printers in hospital settings,different suppository sizes may be printed to suit the patient’s comfort.Given that discomfort was the main reason for non-adherence to suppositories in ulcerative colitis treatment,the ability to tailor suppositories according to the patient’s comfort can enhance adherence and consequently treatment outcomes [39].Additionally,pharmacists could design a personalised suppository containing a precise dose of drug for each patient.This is especially useful in the case of narrow therapeutic index drugs,such as tacrolimus.Previous work has described the fabrication of suppository moulds and shells with different shapes and geometries [40-42],but to date no study has directly printed the suppositories without the need for moulds or covers.Enabling the direct preparation of self-supporting suppositories would obviate the need for moulds,reducing both time and material costs required to prepare the suppositories.Additionally,since a reason for the poor and variable oral bioavailability of tacrolimus is its low water solubility (4-12 μg/ml in water) [43],the preparation of tacrolimus suppositories via SSE 3D printing with the drug solubilised within SEDDS,would increase its water solubility and consequently its effectiveness.
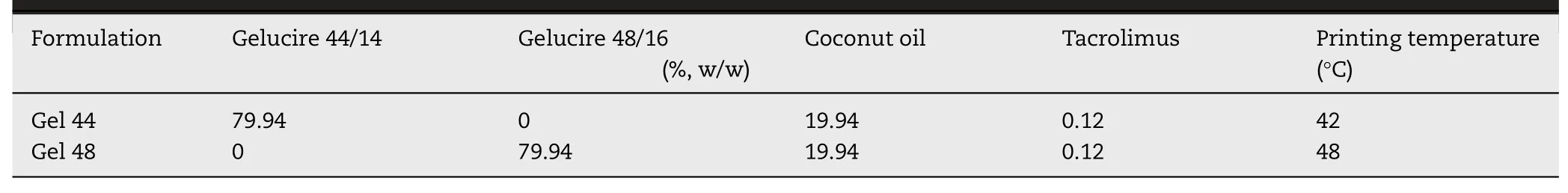
Table 1-Formulation composition and printing temperature.
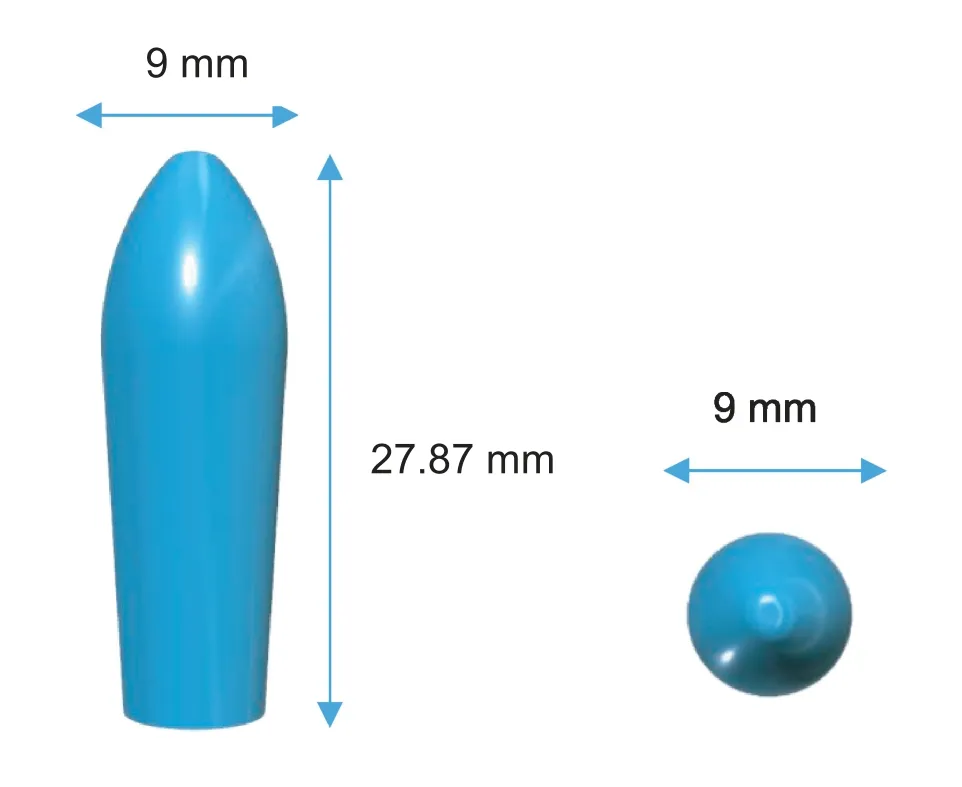
Fig.1-3D model of a suppository from two different views:a side view(left)and a top view(right).
Therefore,this study reports,for the first time,the use of a SSE 3D printer to fabricate self-supporting and self-emulsifying suppositories loaded with the immunosuppressant drug tacrolimus for the treatment of ulcerative colitis.Moreover,the versatility of lipid excipients was exploited to modulate the melting range of the suppositories,as well as their disintegration times and drug release kinetics.
2.Materials and methods
2.1.Materials
Tacrolimus was purchased from Guinama S.L.U.(Spain).Coconut oil was obtained from Acofarma (Spain).Gelucire 44/14 and Gelucire 44/16 were kindly donated by Gattefosse España SA(Spain).
2.2.Design of the 3D models
The software 123D Design (Autodesk Inc.,USA) was used to design the templates of the suppositories with 3 different sizes (diameter×height):the smallest size was 8 mm×24.77 mm,the medium size was 9 mm×27.87 mm(Fig.1)and the largest size was 12 mm×36 mm.
2.3.Semi-solid extrusion 3D printing
Pre-selected ratios of lipid excipients and drug were mixed in a glass beaker and placed on a heating plate (Table 1).The tacrolimus dose was selected according to published literature [8].The mixtures were heated until the mixtures were melted and remained under magnetic stirring until complete solubilisation of the drug in the lipid excipients occurred.The masses were immediately transferred to a 20 ml extrusion syringe with a tapered extrusion tip(0.58 mm orifice)and allowed to solidify at room temperature.Then,the syringe was placed into the pharmaceutical 3D printer with the semi-solid extrusion tool (M3DIMAKER,FabRx Ltd,UK).The previously prepared 3D models of the suppositories were transformed into .gcode files using Cura software (v 15.04.6,Ultimaker Utrecht,Netherlands) using the following printing parameters:0.5 mm layer height;2.4 mm shell thickness;25 mm/s flow speed;1.2 mm nozzle size;room temperature of build plate and chamber;printing temperatures as showed in Table 1.Suppositories were printed in two different positions,vertical and horizontal.Finally,3D printed suppositories were allowed to solidify at room temperature and subsequently stored in the fridge at 4°C.
2.4.Characterisation of the 3D printed suppositories
2.4.1.Scanning electron microscopy(SEM)
SEM images of the suppositories surface were taken in the horizontal position with ZEISS EVO LS15 at 20Kv using a BSD detector (backscattered electron detector)/SE (secondary electron detector),under conditions of variable pressure.
2.4.2.Syringe extrusion force studies
The syringe extrusion force studies were performed in sextuplicate by using Shimazdu Autograph AGS-X universal tester with a load cell (SSM-DAM-1000N) with a maximum capacity of 1 kN.Each extrusion syringe loaded with the mixture of drug and lipid excipients was heated up at the printing temperature (42°C for Gel 44 and 48°C for Gel 48),placed in a physical support and immobilised.A ram (15 mm diameter)was moved to apply pressure at a displacement rate of 10 mm/min on the plunger of the syringe and the load cell was utilised to measure extrusion force during each extrusion run.TRAPEZIUM X Materials Testing Software in compression mode was used for collecting extrusion force data.
2.4.3.Drug loading
Approximately 1.7 g of the drug-loaded suppositories was dissolved in 50 ml of ethanol absolute at 37°C under magnetic stirring until a clear transparent solution was obtained.The resulting solution was centrifuged for 30 min at 12 500 rpm and the supernatant was collected and analysed by HPLC.The amount of drug in solution was determined using a Hewlett Packard 1050 Series HPLC system (Agilent Technologies,UK).The validated assay entailed injecting 20 μl samples for analysis using a mobile phase,consisting of water-acetonitrile(35:65,v/v)through a Poroshell 120,EC-C(4.6 mm×100 mm,4 μm) and at 60 °C.The mobile phase was pumped at a flow rate of 1 ml/min.A wavelength of 210 nm was employed for the quantification of tacrolimus.All measurements were made in triplicate.
2.4.4.Fourier transform-infrared spectroscopy(FTIR)
FTIR spectroscopy was performed using FTIR model Varian 670-IR attached to an attenuated total reflectance (ATR)accessory(GladiATR,PIKE Technologies).ATR was fitted with a single monolithic diamond at 45°internally reflected incident light providing a sampling area of 1.5 mm in diameter with a sampling depth of several microns.Drug,lipid excipients and suppository formulations were analysed.A small amount of the sample was directly placed on the diamond disk.Sample was scanned for absorbence over the wavenumber range of 4000 to 400 cmat a resolution of 4 cm.
2.4.5.Differential scanning calorimetry(DSC)
The thermal properties of raw materials (drug and lipid excipients)and suppository formulations were determined by DSC Q100 (TA Instruments,New Castle,DE,USA).Samples were heated from room temperature to 300°C at 10°C/min under nitrogen flow (50 ml/min).The calibration for cell constant and enthalpy was done with indium (T=156.6 °C,Δ
H=28.71 J/g) according to the manufacturer’s instructions.Aluminium (TA) pans and lids (Tzero) were used with an average sample mass of 7-9 mg.TA Advantage software(version 2.8.394) and TA Instruments Universal Analysis 2000 were used to collect and analyse the data.2.4.6.X-ray diffraction analysis(XRD)
XRD measurements of the powdered samples were performed using a Philips diffractometer (Almelo,The Netherlands)fitted with a Philips PW1710 control unit,a Vertical Philips PW1820/00 goniometer and an Enraf Nonius FR590 generator operating at 40 kV and 30 mA.The X-ray were obtained from a Cu sealed tube and the radiation was monochromated with a monochromator of graphite (λ
(K)=1.5406 ˚A).The diffractograms were obtained in the 2θ
angle range 2°-50°with a step of 0.04°and a counting time of 6 sec per step.The samples were mounted into a sample holder substrate(silicon single crystal)to skip the dispersion that could be produced by a glass substrate.2.4.7.Determination of disintegration time
The test was performed in distilled water at 37 °C using the USP disintegration apparatus slightly modified to meet the requirements of the method described in the European Pharmacopeia[44].Each suppository was placed between the two perforated plates of the basket,which was inserted into a transparent plastic sleeve.The suppository disintegration rig was then placed in the glass beaker containing 1 l of distilled water at 37 °C.The mean values were calculated from three parallel measurements.
2.4.8.Determination of self-emulsification time
The emulsification time of SEDDS suppositories was determined according to USP XXIII,dissolution apparatus type II.Each formulation was melted and then added dropwise to 500 ml of purified water at 37°C.Gentle agitation was provided by a standard stainless-steel dissolution paddle at 50 rpm.Self-emulsification time was recorded as the time taken by the formulation to form a clear solution in water.The mean values were calculated from three parallel measurements.
2.4.9.Determination of droplet size and ζ potential
Solid formulations (50 mg) were added to 100 ml of ultrapure water under constant stirring until the emulsion was formed.Droplet size distribution (mean diameter of lipid droplets and polydispersity index) andζ
potential (the charge of droplets) were determined using a Zetasizer Nano (Malvern Instrument Limited,Worcestershire,UK).All measurements were performed in triplicate.2.4.10.Transmission electron microscopy(TEM)
TEM images of microemulsion droplets were taken using a transmission electron microscope JEOL JEM-1011.First,50 mg of SEDDS suppositories were placed in 100 ml of distilled water under magnetic stirring until the emulsion was formed.Then,a drop of diluted SEDDS was then deposited on the holey film grid,stained by 1%aqueous solution of phosphotungstic acid and observed after drying.
2.4.11.In vitro drug release
In vitro
drug release profiles of the 3D printed suppositories were obtained using a USP-II mini paddle apparatus (Model PTWS,Pharmatest,Hainburg,Germany).The studies were conducted in 100 ml pH 8 phosphate buffer (0.1 M) and the paddle speed was set at 100 rpm with a temperature of 37±0.5°C (n
=3).At pre-determined time points (20,40,60,80,100,120 min),1 ml aliquots were withdrawn and 0.5 ml of ethanol absolute were added to the samples to solubilise the tacrolimus that could be trapped inside the lipid droplets.Then,the samples were centrifuged for 30 min at 12 500 rpm and the supernatant was collected and analysed by HPLC as described in Section 2.4.3.Tacrolimus release profiles from the suppositories were fitted to the Gompertz growth model(Eq.1):
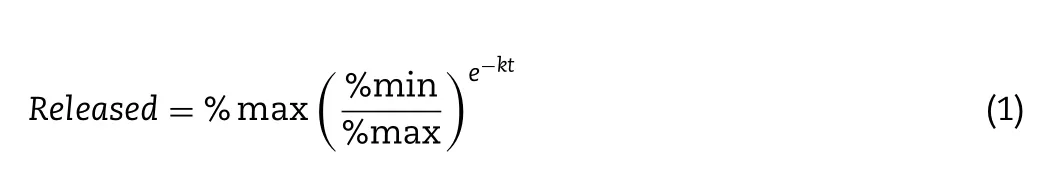
%
max is the maximum percentage of drug released,%min is the minimum percentage of drug released andk
is a constant.Gompertz growth model was fitted to drug release profile by non-linear regression analysis using the program GraphPad Prism(version 7.0,CA,USA).Furthermore,the dissolution profiles of Gel 44 and Gel 48 formulations were compared using an ƒsimilarity test,which is calculated using Eq.2 [45].The similarity factor ƒis a logarithmic transformation of the sum-squared error of differences betweenT
andR
over all the considered time points: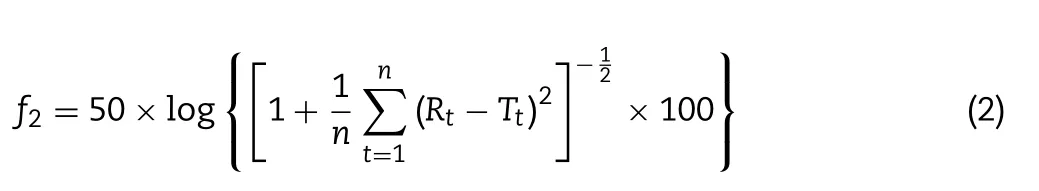
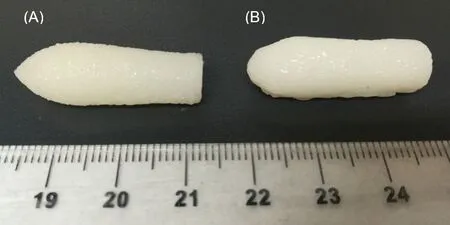
Fig.2-Gel 44 suppositories printed in(A)vertical position and(B)horizontal position(scale is in cm).
whereR
andT
are the release profiles of the reference and test formulations at time pointt
respectively,andn
the number of dissolution time points considered.The ƒvalue ranges from 0 to 100 and,in general,the values higher than 50 indicate similarity of the dissolution profiles [46].Moreover,as the ƒvalue decreases below 50,the variation between the dissolution profiles increases,indicating that the formulations have different release profiles[47].2.4.12.Statistical analysis
The Mann-Whitney non-parametric test was used to evaluate the statistical significance of the difference between the obtained results from the two formulations (energy and extrusion force required to extrude each formulation,disintegration time,self-emulsifying time tests,drug loading,droplet size andζ
potential).All analysis were executed using the program GraphPad Prism (version 7.0,CA,USA).P <
0.05 was considered statistically significant.3.Results and discussion
This study proved that it was feasible to prepare drug-loaded suppositories without a mould or other physical support using 3DP technology.The printed suppositories were well-defined and with acceptable consistency for normal handling.No material slumping was observed during the printing process,and it was not necessary to use a refrigerated build plate to facilitate the solidification of the printed layers.During the printing process,the suppositories quickly solidified in less than 1 min without the need for additional cooling or drying steps.Two different compositions of printing material were tested(Table 1).
The suppositories were printed in two positions,vertically(Fig.2A) and horizontally (Fig.2B).The printing time for the suppositories printed vertically was around 4 min 30 s and 2 min 16 s for those printed horizontally.Although printing the suppositories in the horizontal plane was faster due to the lower number of layers,the shape and resolution of the suppositories was better when they were printed vertically(Fig.2A).
SEM images indicate that the deposition of the individual layers are connected to each other forming a solid low porosity object (Fig.3).However,SEM images also showed that the deposition of layers when printing the suppository in vertical position (Fig.3A) was better than when printing horizontally(Fig.3B).This is because when printing horizontally,some layers of material have no support underneath due to the convex shape of the suppository and are partially deposited in the air.Thus,some layers have irregularities that affect the final shape of the suppository.Therefore,for the rest of the studies,all the suppositories were printed vertically.Suppositories with two different compositions were printed in three different sizes to demonstrate that it is possible to produce personalised suppositories containing different doses(Fig.4).
Preliminary studies were performed using only Gelucire 44/14 or Gelucire 48/16,but these excipients alone did not have adequate properties,such as the appropriate viscosity for printing.To improve the printability properties of both Gelucire,coconut oil was employed as plasticizer.Both Gelucire 44/14 and Gelucire 48/16 were completely miscible with coconut oil when melted.
The printing temperature was set at 42°C for Gel 44 formulation and to 48°C for Gel 48,being adequately controlled by the 3D printer avoiding solidification of the material in the nozzle tip.The mixtures from the syringes were heated until they reached a viscosity low enough to extrude through the tip of the nozzle.If the material was too extensively melted,the 3D printed suppository structure would not be able to hold its shape.But if it was too viscous,the nozzle would clog and the material would not flow.Extrusion force studies,interpreted as the force required to make the material pass through the extrusion tip at the printing temperature,were performed to obtain the energy and maximum extrusion force in order to evaluate the ease of extrusion of the mixtures at printing temperatures.Statistical analysis showed that the extrusion force and energy required to extrude Gel 48 was significantly higher (P <
0.05) than that required to extrude Gel 44 even though the printing temperature was higher for Gel 48(Table 2).The mean weight of suppositories was between 1.7 and 1.81 g (Table 2).The drug loading values of the suppositories represented 98.03% of the theoretical drug loading value for Gel 44 and 108.53% for Gel 48 (Table 2).The values suggest that tacrolimus did not undergo any degradation either during the mixture preparation or the printing process.The difference could be attributable to inadequate mixing of drug and excipients;however,the values differ less than 10%from theoretical values.Moreover,no statistically significant differences were found between both formulations.
FTIR spectroscopy was performed to investigate possible interactions between the drug and the selected lipid excipients in their formulations (Fig.5).The IR patterns of Gel 44 and Gel 48 are practically the same as Gelucire 44/14 and Gelucire 48/16.The proportion of Gelucire in both formulations is around 80% (w/w) and tacrolimus is only 0.12% (w/w),so the IR peaks of the drug practically disappear and cannot be detected in the final Gel 44 and Gel 48 formulations.
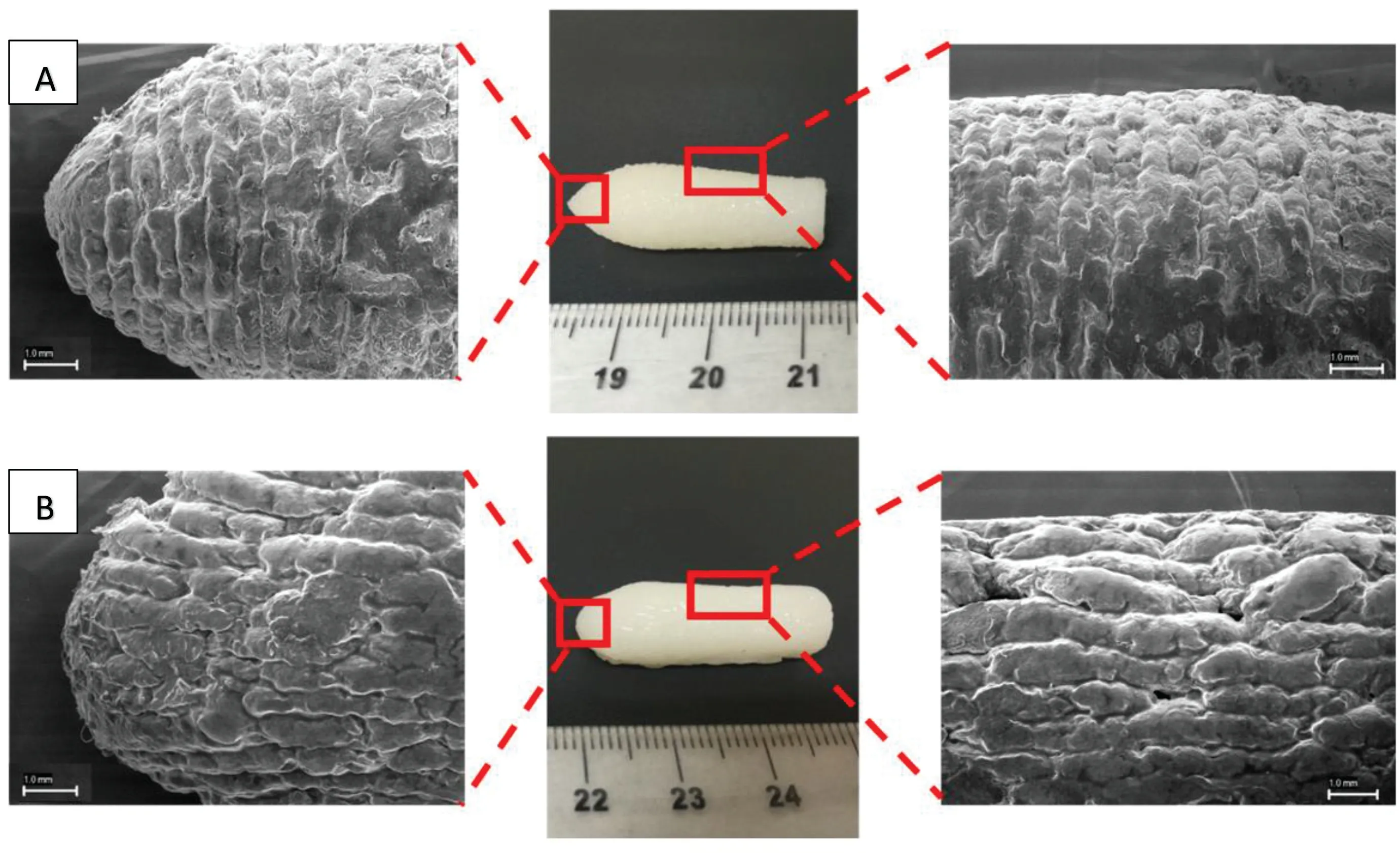
Fig.3-SEM images of different sections of Gel 44 self-emulsifying suppositories printed in(A)vertical position and(B)horizontal position.
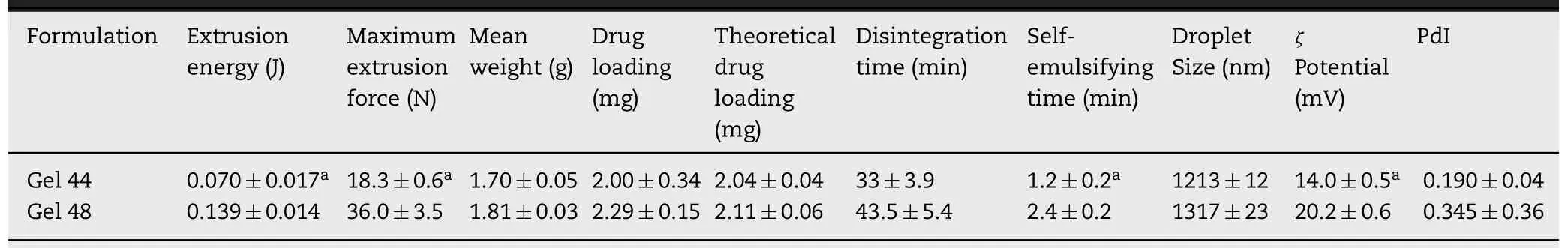
Table 2-Characterisation of the 3D printed suppositories(mean±SD).
DSC and X-ray analysis were employed to investigate the physical state of the drug in the final formulations.The DSC thermographs show that tacrolimus raw material melts around 130 °C while the melting point of both Gelucire 44/14 and 48/16 is between 40 and 50 °C (Fig.6).The melting peaks for Gelucire 44/14 and Gelucire 48/16 are almost the same as their respective Gel 44 and Gel 48 formulations.It was not possible to determine the melting point of tacrolimus in the formulations which indicates that the drug may be forming a solid solution with the lipid excipients.Other explanations are that during the DSC study while the temperature increases the drug is completely dissolved in the melted polymer or that the drug content is below the detection limit of the DSC equipment.The X-ray diffractograms of Gelucire 44/14,Gelucire 48/16,Gel 44 and Gel 48 showed very similar structural behaviours.In particular,the peaks of Gelucire 48/16 and Gel 48 are sharper than those of Gelucire 44/14 and Gel 44,which may be due to the higher degree of crystallinity of Gelucire 48/16 and Gel 48.Tacrolimus showed the characteristic peaks of crystalline structures.However,it is not possible to elucidate the physical state of the drug in the formulations;the absence of sharp peaks in the diffractograms of the tacrolimus formulations suggests that either the drug is in its amorphous state or it is below the limit of detection of the XRD instrument(Fig.7).
The disintegration time was determined following the method described in the European Pharmacopeia [44]to evaluate the time required to disintegrate the suppository formulations.Gel 44 suppositories disintegrate faster compared to Gel 48 suppositories (Table 1),however,no statistically significant differences were found between both formulations.On the other hand,when the self-emulsification time was evaluated,Gel 44 showed a statistically significant(P<
0.05)faster emulsification time compared to Gel 48(Table 2).To characterise the formed system,the globule size of both self-emulsifying suppository formulations and theζ
potential of the emulsion droplets were measured (Table 2).The mean size of lipid droplets of both formulations was between 1 and 1.5 μm,and the stability of the system was given byζ
potential of lipid droplets,this being −14 mV for Gel 44 and−20 mV for Gel 48,which means that the system formed by Gel 48 was more stable due to its higher charge.No statistically significant differences were found between the droplet size of both emulsions.Conversely,the differences between theζ
potential of lipid droplets of both emulsions were statistically significant(P <
0.05).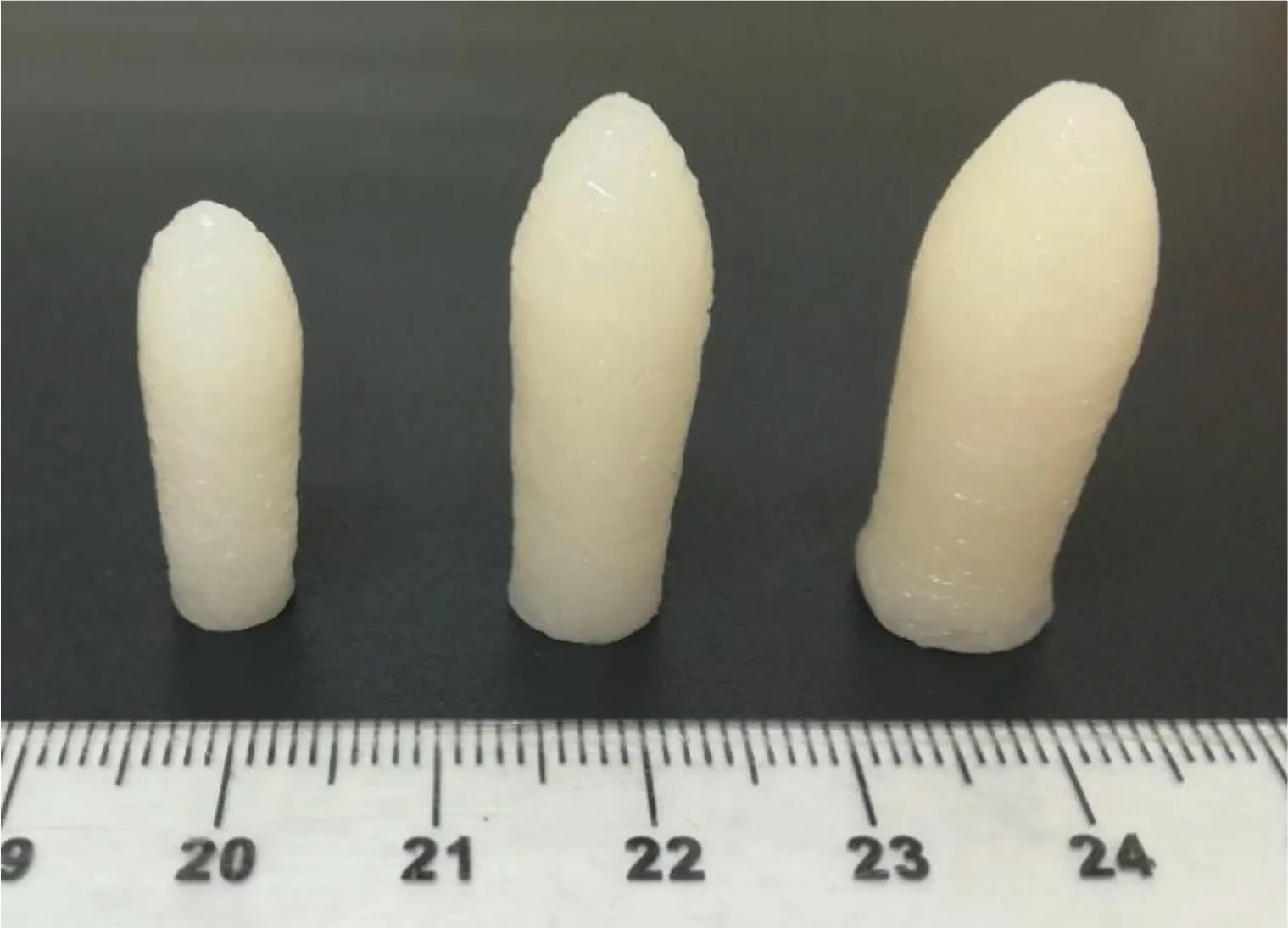
Fig.4-Picture of Gel 44 suppositories printed in three different sizes(from left to right:small,medium and large)as an example of personalisation(scale is in cm).
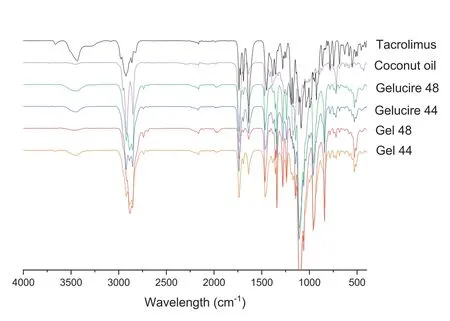
Fig.5-FTIR spectra of the drug(tacrolimus),the lipid excipients(coconut oil,Gelucire 44 and Gelucire 48)and the formulations(Gel 44 and Gel 48).
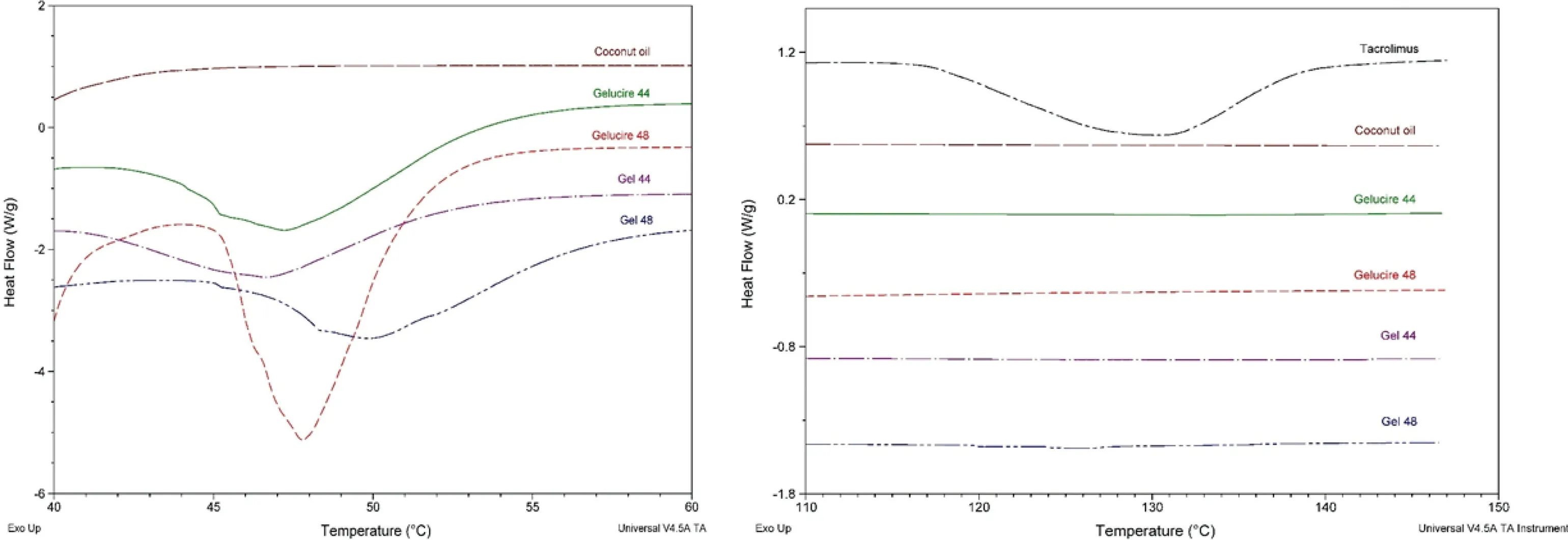
Fig.6-DSC curves for the drug(tacrolimus),the lipid excipients(coconut oil,Gelucire 44 and Gelucire 48)and the formulations(Gel 44 and Gel 48).
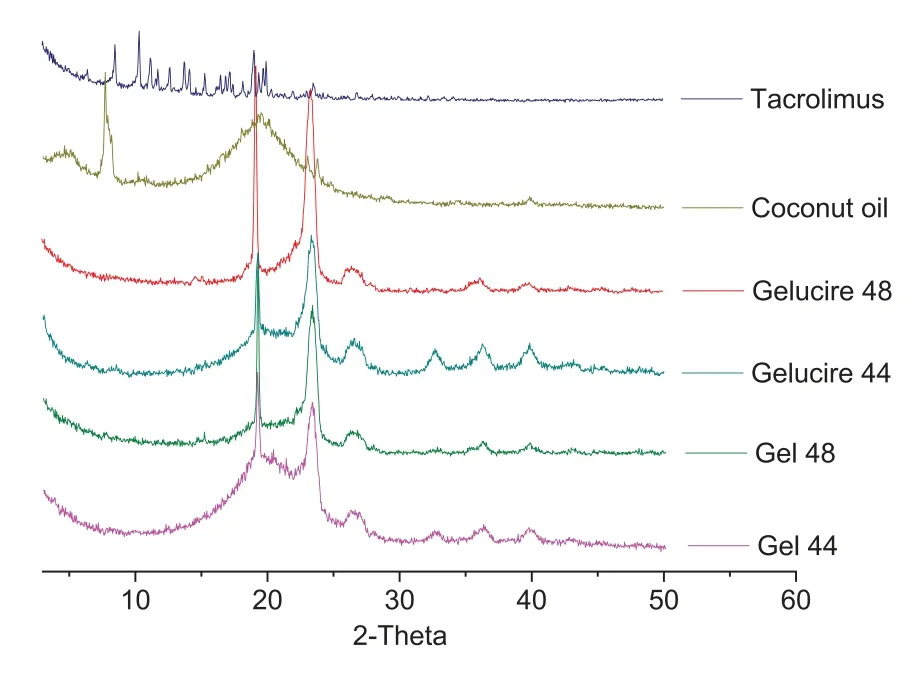
Fig.7-X-ray spectra of the drug,the excipients(coconut oil,Gelucire 44 and Gelucire 48)and the formulations(Gel 44 and Gel 48).
Although it is reported in the literature that Gelucire 44/14 forms microemulsions and Gelucire 48/16 forms micellar systems in aqueous media [48,49],the larger particle sizes obtained in this study may be due to the inclusion of coconut oil as a plasticiser to facilitate the printing process.Moreover,the low polydispersity index of Gel 44(0.190±0.04)is indicative of a homogeneous monodisperse population,whereas the polydispersity index value for Gel 48(0.345±0.36)is representative of a more heterogeneous population.Regarding the globule morphology and structure,TEM images were obtained to directly visualise the lipid droplets (Fig.8).The images show globules with spherical shapes and sizes consistent with the dynamic light scattering measurements.
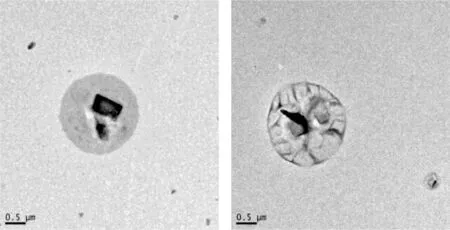
Fig.8-Representative TEM microphotographs of lipid droplets formed after emulsification of Gel 48(right)and Gel 44(left)fixed with phosphotungstic acid.The black spots are traces of staining with phosphotungstic.
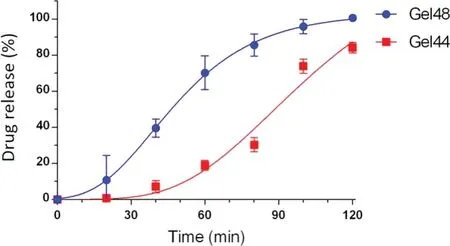
Fig.9-Drug release profile of Gel 44 and Gel 48 suppositories in phosphate buffer pH 8.
The drug dissolution profiles of both suppositories are shown in Fig.9.Gel 48 suppositories released more than 50% of tacrolimus within the first 60 min compared to Gel 44 suppositories,which required 90 min to reach the same percentage of drug released.A ƒsimilarity value of 21 was obtained,which indicates that Gel 44 and Gel 48 suppositories have different drug release profiles (ƒvalues between 50 and 100 indicate parity).Although Gel 44 showed a faster disintegration and emulsification,a delayed release was observed in comparison to Gel 48.It is hypothesised that the lower hydrophilic-lipophilic balance of Gel 44 (HLB=10.4)compared to Gel 48 (HLB=11.2) indicates a slightly higher lipophilicity of Gel 44,which would increase the retention of tacrolimus (lipophilic drug) and explain the longer duration of drug release [50].The experimental data was fitted to the Gompertz growth model (Eq.1),because it is a sigmoid function which could describe the lag time and the slow growth at the beginning of a time period.The high correlation coefficients obtained with this fitting support the choice of this model,R
=0.9996 for Gel 48 andR
=0.9747 for Gel 44.One important aspect of suppository formulations is that the suppository base materials should melt at body temperature to release drugs to the site of application[51,52].Conventional rectal suppositories are formulated using polyethylene glycol(PEG),which possess various issues including a relatively high melting point,inability for loaded drugs to absorb rapidly into mucous membranes and potential rectal irritation [53].SMEDDS could be used to overcome these problems.A study that compared SMEDDS suppositories with commonly used PEG suppositories found that SMEDDS suppositories showed a narrower melting point,which is important for maintaining the suppository shape during storage and insertion into the rectum.Moreover,SMEDDS suppositories released a lower amount of drug in the initial phase than PEG suppositories,displaying a more prolonged release of the drug in the rectum [54].The mixture of lipid excipients that have been selected in this study to prepare the suppositories results in a self-emulsifying system in which the tacrolimus is solubilised in the small droplets of oil [55].The materials displayed adequate viscosity for printing at low temperatures,which can make this formulation adequate for thermolabile compounds.Moreover,the rapid solidification of the suppositories precludes the need for post manufacturing time to allow the formulations to harden.
Previous studies have demonstrated the potential of 3D printing to tailor oral medicines to individual patients [56-59],and the feasibility of this technology to prepare tailored medicines in a hospital setting [35].In the present study,we have investigated the potential of SSE 3D printing for the development of customised lipid-based suppositories with different sizes and containing a specific dose of drug.Although Gel 48 suppositories released drug at a faster rate,better printing properties and better miscibility of Gelucire 44 provided by its lower melting point makes Gel 44 a more suitable formulation.Overall,we have demonstrated that with SSE 3D printing it is possible to fabricate suppositories with the selected drug dose in a single step process,without the need for moulds.This approach represents a more rapid and cost-efficient approach to prepare drug loaded suppositories tailored to each patient’s needs.This is of special interest for drugs with a narrow therapeutic index[60],such as tacrolimus [11],since dose requirements can be markedly different amongst patients of different age groups,for example,children and the elderly[61],which increases the risk of adverse effects[62].
4.Conclusions
This work demonstrates,for the first time,the use of a SSE 3D printer to fabricate self-supported lipid-based suppositories loaded with tacrolimus intended for the treatment of inflammatory bowel disease patients.Different sizes of 3D printed tacrolimus suppositories were produced with precise doses,giving rise to the possibility of manufacturing suppositories tailored to each patient’s needs without the need for moulds.The suppositories printed using combinations of two lipid excipients (Gelucire 44/14 or Gelucire 48/16) and coconut oil exhibited adequate viscosity for printing.The Gel 44 suppositories had a faster disintegration time but slower drug release rate than the Gel 48 suppositories.Although the drug release profiles were significantly different,both suppository types released more than 80% of tacrolimus within 120 min.The local delivery of precise doses of tacrolimus may reduce the risk of adverse effects without losing the immunosuppressive effect.The unprecedented flexibility of 3D printing technologies could provide new manufacturing opportunities to produce personalised dosage forms on-demand.
Conflicts of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors would like to thank Prof.Carmen Álvarez-Lorenzo for her support in the DSC analysis.Authors also thank Gattefosse España S.A.(Madrid,Spain) for the generous gift of samples of Gelucire 44/14 and Gelucire 48/16.This research was funded by Xunta de Galicia grant number GRC2013/015 and GPC2017/015.Authors would like to thank the use of RIAIDT-USC analytical facilities.
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Black phosphorus-based nano-drug delivery systems for cancer treatment:Opportunities and challenges
- Unraveling GLUT-mediated transcytosis pathway of glycosylated nanodisks
- A biomimetic antitumor nanovaccine based on biocompatible calcium pyrophosphate and tumor cell membrane antigens
- 3D printing-based drug-loaded implanted prosthesis to prevent breast cancer recurrence post-conserving surgery
- Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs
- Trends of nanotechnology in type 2 diabetes mellitus treatment
