噻吩和吡啶取代三芳基三唑的两个铜配合物的合成与晶体结构
2021-03-12聂荣荣朱敦如
李 刚 冯 哲 聂荣荣 朱敦如*,,2
(1南京工业大学化工学院,材料化学工程国家重点实验室,南京 211816)
(2南京大学配位化学国家重点实验室,南京 210023)
0 Introduction
Over the past few decades,3,4,5-triarylsubstituted 1,2,4-triazoles have attracted considerable attention in coordination chemistry[1]because these triazole derivatives can be used as flexible bridging ligands and linkers between transition metal ions[2-5].In particular,some Fe(Ⅱ)complexes with substituted triazoles can show fascinating spin crossover properties and may find potentialapplicationsin molecularswitches,display devices,sensors and data storages[6-9].In our previous work,a series of 3,4,5-triarylsubstituted 1,2,4-triazoles with the pyridyl,phenyl,pyrrolyl,imidazolyl,benzimidazolyl and quinolyl groups and their metal complexes have been reported[10-24].However,only a Co(Ⅱ)complex with thienyl substituted triaryltriazoles has been reported until now[25-26].As a continuation of our exploration of asymmetrically substituted 1,2,4-triazoles,we prepared two new triaryltriazoles with a thienyl group:3-(2-pyridyl)-4-phenyl-5-(2-thienyl)-1,2,4-triazole(L1)and 3-(2-pyridyl)-4-(p-chlorophenyl)-5-(2-thienyl)-1,2,4-triazole(L2)(Scheme 1).Herein we report the syntheses,crystal structures and spectral characterization of two mononuclear copper(Ⅱ)complexesbasedonthedesignedligands:trans-[Cu(L1)2(MeOH)2](ClO4)2(1)andtrans-[Cu(L2)2(ClO4)2]·2MeCN(2).
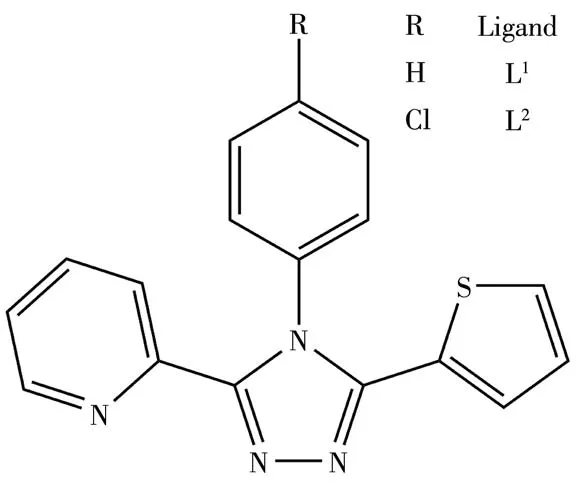
Scheme 1 Structures of ligands L1 and L2
1 Experimental
1.1 Materials and measurements
All chemicals and solvents purchased were of analytical grade.Solvents were purified by conventional methods.Melting points were determined with an X-4 digital microscope melting-point apparatus(Beijing)and are uncorrected.Elemental analyses(C,H,N,S)were carried out with a Thermo Finnigan Flash 1112A elemental analyzer.FT-IR spectra were recorded on a Nicolet Avatar 380 FT-IR instrument using KBr disks from 4 000 to 400 cm-1.1H NMR spectra were measured on a Bruker AM 400 MHz spectrometer in DMSO-d6solution.Electrospray ionization mass spectra(ESIMS)were recorded with a LCQ ADVANTAGE MAX mass spectrometer.Powder X-ray diffraction(PXRD)data were collected on a Bruker D8 ADVANCE diffractometer using CuKαradiation(λ=0.154 06 nm)at 40 kV and 40 mA in a range of 5°~50°.
1.2 Characterization of ligands L1 and L2
Ligands L1and L2were synthesized based on our reported method[21].
3-(2-pyridyl)-4-phenyl-5-(2-thienyl)-1,2,4-triazole(L1):m.p.201~203℃.Anal.Calcd.for C17H12N4S(%):C,67.08;H,3.97;N,18.41;S,10.53.Found(%):C,67.34;H,4.24;N,18.65;S,10.81.IR(KBr,cm-1):3 072(w),1 585(m),1 489(s),1 438(m),1 002(m),798(m),722(m).1H NMR(400 MHz):δ8.31(d,1H,Py-H),8.03(d,1H,Py-H),7.91(t,1H,Py-H),7.66(d,1H,thienyl-H),7.54(t,1H,Ph-H),7.51(d,2H,Ph-H),7.47(d,2H,Ph-H),7.35(t,1H,Py-H),7.01(d,1H,thienyl-H),6.71(d,1H,thienyl-H).ESI-MS:m/z=305.08,[L1+H]+.
3-(2-pyridyl)-4-(p-chlorophenyl)-5-(2-thienyl)-1,2,4-triazole(L2):m.p.192~194 ℃ .Anal.Calcd.for C17H11N4SCl(%):C,60.26;H,3.27;N,16.54;S,9.46.Found(%):C,60.42;H,3.42;N,16.39;S,9.72.IR(KBr,cm-1):3 054(w),1 590(m),1 498(s),1 443(s),1 089(m),997(m),841(m),721(m).1H NMR(400 MHz):δ8.33(d,1H,Py-H),8.08(d,1H,Py-H),7.93(t,1H,Py-H),7.69(d,1H,thienyl-H),7.59(d,2H,Ph-H),7.57(d,2H,Ph-H),7.36(t,1H,Py-H),7.06(t,1H,thienyl-H),6.83(d,1H,thienyl-H).ESI-MS:m/z=339.05,[L2+H]+.
1.3 Syntheses of complexes 1 and 2
trans-[Cu(L1)2(MeOH)2](ClO4)2(1):A solution of Cu(NO3)2·3H2O(0.05 mmol)and NaClO4(0.1 mmol)in anhydrous MeOH(4 mL)was added to a solution of L1(0.1 mmol)in MeOH(4 mL).The mixture was stirred for 4 h at room temperature.The green precipitate was separated by filtration,and washed with water,then dried under vacuum to obtain complex 1 in a yield of 82%.The green block-shaped single crystals of 1 suitable for X-ray diffraction were obtained by slow evaporation from MeOH solution.Anal.Calcd.for C36H32Cl2CuN8O10S2(%):C,46.23;H,3.45;N,11.98;S,6.86.Found(%):C,46.52;H,3.51;N,11.77;S,6.54.IR(KBr,cm-1):3 449(m,b),3 082(w),2 917(w),1 617(w),1 562(m),1 493(s),1 388(m),1 112(vs),929(w),859(w),731(m),626(s).
trans-[Cu(L2)2(ClO4)2]·2MeCN(2):The prepared procedure for 2 was the same as that for 1 except using L2(0.1 mmol)to replace L1.The well-shaped single crystalsof2 suitable forX-ray diffraction were obtained by slow evaporation from MeCN solution.Yield:87%.Anal.Calcd.for C38H28Cl4CuN10O8S2(%):C,44.65;H,2.76;N,13.70;S,6.27.Found(%):C,44.27;H,2.93;N,13.82;S,6.41.IR(KBr,cm-1):3 077(w),2 921(w),2 347(w),1 613(m),1 566(m),1 493(s),1 114(vs),1 098(s),930(w),859(m),726(m),621(s).
1.4 Crystal structure determination
Suitable single crystals of 1 and 2 were selected for lattice parameter determination and collection of intensity data on a Bruker SMART APEXⅡCCD diffractometer with a graphite-monochromated MoKα(λ=0.071 073 nm)radiation using aφ-ωscan mode at 296(2)K.Multi-scan absorption correctionswere applied to all intensity data using SADABS.The structures were solved by direct methods and refined onF2by full-matrix least squares procedures using SHELXTL software[27].All non-hydrogen atoms were anisotropically refined.All hydrogen atoms were fixed in calculated positions and refined isotropically.The ClO4-ions in 1 were disordered over two positions with an occupancy of 0.677(14)for O2,O3,O4 and O5 and 0.323(14)for O2A,O3A,O4A and O5A.The two O atoms of coordinated ClO4-in 2 were also disordered over two positions with an occupancy of 0.548(7)for O2 and O3 and 0.452(7)for O2A and O3A.The crystallographic data of 1 and 2 are summarized in Table 1 and the selected bond lengths and angles are given in Table 2.

Table 1 Crystal data and structure refinements for 1 and 2
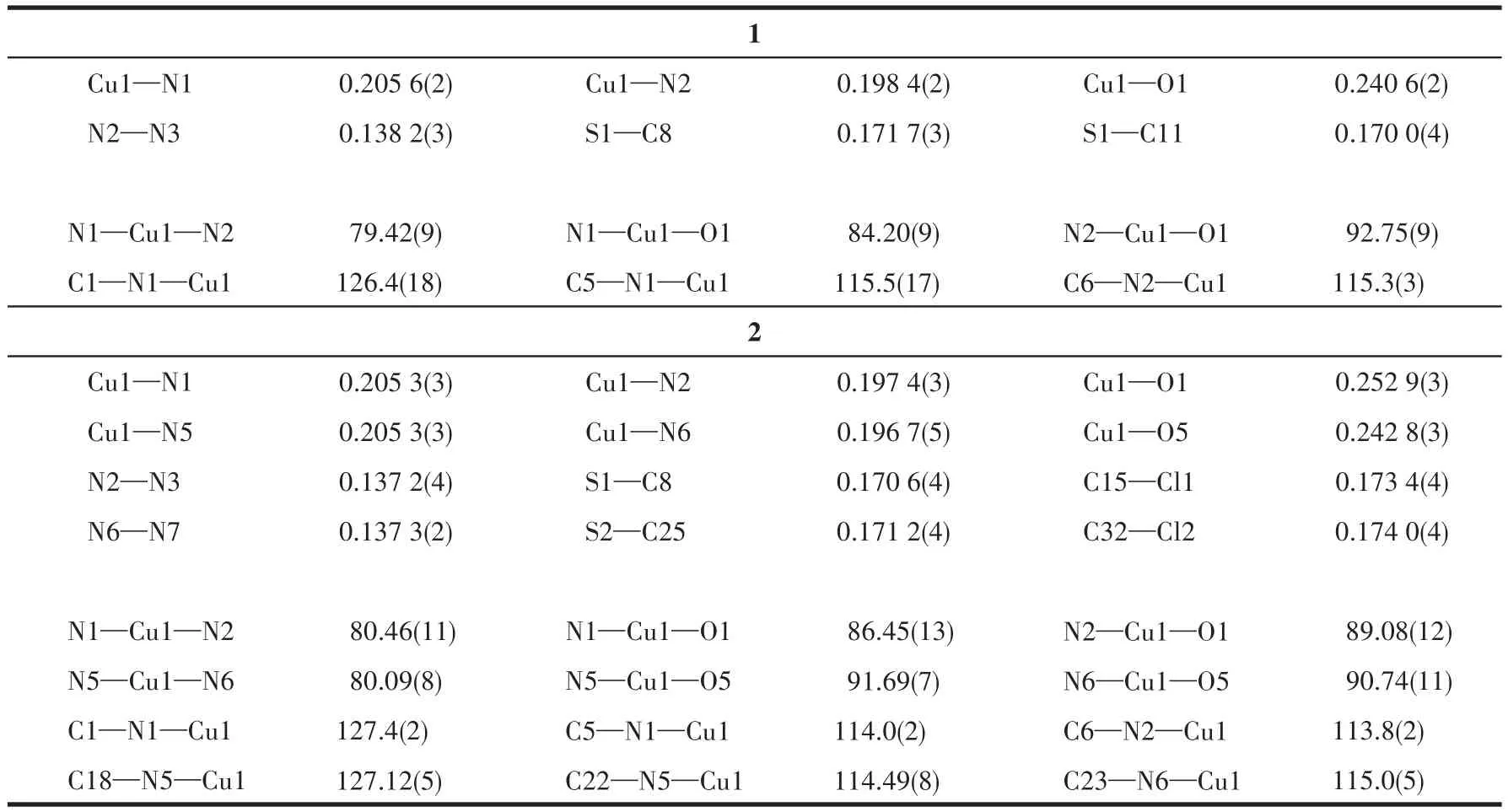
Table 2 Selected bond distances(nm)and bond angles(°)for 1 and 2
CCDC:2423470,1;2423471,2.
2 Results and discussion
2.1 Synthesis
Asymmetrically thienyl substituted 3,4,5-triaryl-1,2,4-triazoles(L1and L2)reacted with Cu(NO3)2·3H2O and NaClO4in molar ratio of 2∶1∶2 to lead the formation of two mononuclear complexes,trans-[Cu(L1)2(MeOH)2](ClO4)2(1)andtrans-[Cu(L2)2(ClO4)2]·2MeCN(2),which are stable in air.The yields for 1 and 2 were 82% and 87%,respectively.The elemental analyses were satisfactory and indicate that 1 contains one Cu(Ⅱ),two L1ligands,two MeOH molecules and two ClO4-ions,and 2 has one Cu(Ⅱ),two L2ligands,two MeCN molecules and two ClO4-anions.
2.2 Crystal structures of 1 and 2
Although both 1 and 2 crystallize in the monoclinic space groupP21/c(Fig.1),Z=2 andc=1.566 7(4)nm in 1 whileZ=4 andc=3.404 6(5)nm in 2.Thus,there is an inversion center at the Cu(Ⅱ)ion in 1 but none occurs in 2.The asymmetric unit of 1 is composed of half Cu(Ⅱ) cation,one L1ligand,one coordinated MeOH molecule and one ClO4-anion,which is consistent with the elemental analysis result.The Cu1 ion of 1 is coordinated by four nitrogen atoms from two L1ligands in the equatorial plane and two oxygen atoms from two MeOH molecules in the axial positions to form a distorted octahedral[CuN4O2]geometry.Each L1ligand coordinates to Cu1 ion via N1 atom of the pyridyl and N2 atom of the triazole,similar to the coordinated modes in some related Cu(Ⅱ)complexes[14,16,24].The thienyl group of L1ligand does not take part in coordination,which is similar to the Co(Ⅱ)complex with a thienyl substituted triaryltriazole:trans-[CoL2(NCS)2](L=3-(2-pyridyl)-4-(p-methoxyphenyl)-5-(2-thienyl)-1,2,4-triazole)[25].However,the S1 atom of thienyl group in 1 does not orient to the phenyl ring,which is quite distinct from that observed in thetrans-[CoL2(NCS)2]complex[25].The distance of Cu1—O1 is 0.240 6(4)nm.The Cu—N bond lengths are within the normal ranges found for the octahedral Cu(Ⅱ)complexes[28-31].Nevertheless,the Cu—N bond to the triazole nitrogen is 0.007 3 nm shorter than that to the pyridyl one.The analogous feature has been observed in the similar Cu(Ⅱ)complexes[24].The ligand L1in 1 is nonplanar.The triazole makes dihedral angles of 6.2(4)°,29.7(6)°and 64.6(3)°with the pyridyl ring,the thienyl ring and phenyl ring,respectively.

Fig.1 Projection of structures of 1 and 2 with 30% thermal ellipsoids probability
Different from 1,the asymmetric unit of 2 consists of a Cu(Ⅱ)cation,two L2ligands,two ClO4-ions and two MeCN molecules.The Cu(Ⅱ)ion of 2 is coordinated by four nitrogen atoms from two L2ligands in the equatorial plane and two oxygen atoms from two ClO4-in the axial positions to build a distorted octahedral[CuN4O2]geometry.Each L2ligand coordinates to Cu1 atom via N1(or N5)atom of the pyridyl and N2(or N6)atom of the triazole whereas the thienyl group of L2ligand is also uncoordinated,which is very similar to the coordination mode of L1in 1.The distance of Cu1—O1(0.253 nm)is longer than that of the Cu1—O5(0.242 8 nm).The Cu—N bond lengths are within the normal ranges observed for some octahedral Cu(Ⅱ)complexes,whereas Cu—N bond to the triazole nitrogen is 0.008 6 nm shorter than that to the pyridyl one.The triazole ring containing N2 atom forms dihedral angles of 2.4(2)°,6.9(9)°and 89.2(8)°with the pyridyl ring with N1 atom,the thienyl ring with S1 atom and phenyl ring with Cl1 atom,respectively.And the triazole ring having N6 atom makes dihedral angles of 4.1(7)°,9.6(4)°and 87.6(3)°with the pyridyl ring with N5 atom,the thienyl ring with S2 atom and phenyl ring with Cl2 atom,respectively.Therefore,the pyridyl,triazole and thienyl ring of L2in 2 is almost coplanar.Notably,the S atoms(S1 and S2)of thienyl groups in 2 orient to thepchlorophenyl ring,which is similar to that found in thetrans-[CoL2(NCS)2]complex[25].
There are abundantintermolecularhydrogen bonds interactions in 1:(1)between MeOH and ClO-4anion (O1—H1A…O4);(2)between pyridyland triazole ring(C1—H1…N3i);(3)between phenyl and ClO4-anion(C13—H13…O2iiiand C17—H17…O2i);(4)between pyridyl and ClO4-anion(C3—H3…O5ii).In addition,there are an intramolecular edge-to-face C—H…πinteraction involving C4—H4 and phenyl ring(H4…π0.308 nm and ∠C4—H4…π=148°)and an intermolecular edge-to-face C—H…πinteraction involving MeOH and pyridyl ring(H18C…π0.326 nm and ∠C18—H18C…π=139°)(Fig.2,Table 3).These interactions further connect the mononuclear 1 to form a 3D framework(Fig.3).
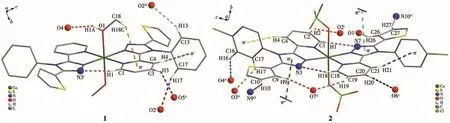
Fig.2 Hydrogen-bonding,C—H…π and π…π interactions in 1 and 2
Complex 2 has richer C—H…O interactions including:(1)between pyridyl and ClO4-(C2—H2…O2i,C19—H19…O7iiand C20—H20…O6v);(2)between thienyl and ClO4-(C9—H9…O7iiand C26—H26…O1i);(3)between phenyl and ClO4-(C16—H16…O4ivand C17—H17…O3iv).There are two kinds of C—H…N interactions in 2 including:(1)between pyridyl and triazole(C1—H1…N7 and C18—H18…N3);(2)between thienyl and MeCN(C10—H10…N9iiiand C27—H27…N10vi).In addition,there are two kinds ofπ-πstacking interactions between triazole rings and the MeCN molecules(π…π(C≡N9)v0.331 6 nm,π…π(C≡N10)v0.330 4 nm)(Table 3,Fig.2).All the interactions assemble the mononuclear 2 into a 3D network(Fig.3).

Table 3 Hydrogen-bond geometry and C—H…πinteractions in 1 and 2

Fig.3 Three dimensional frameworks of 1 and 2
2.3 IR spectra
In the IR spectra of 1 and 2,a broad band at 3 447 cm-1in 1 is due to the O—H stretching vibrations of MeOH,while a weak band at 2 347 cm-1in 2 is assigned to characteristic C≡N stretching vibrations of MeCN[32].A medium band at 1 562 cm-1in 1 or 1 566 cm-1in 2 can be assigned to the coordinated pyridyl ring vibrations.In 1(or 2),the bands around 1 112(or 1 114),929(or 930),626(or 621)cm-1can be assigned to the IR-allowed ν mode,IR-forbiddenνmode and the non-degenerate ClO3symmetrical bending frequency of the ClO4-anions,respectively[33].Additionally,the stretching vibration of Cl-C(Ph)in 2 is found at 1 098 cm-1[34].These features are in consistent with the X-ray analysis results.
2.4 PXRD
The PXRD patterns and simulated ones of 1 and 2 are shown in Fig.4.The experimental patterns are in well agreement with the simulated ones from the single X-ray crystal data,revealing that the high phase purity of the bulk products of 1 and 2.

Fig.4 PXRD patterns of 1 and 2
3 Conclusions
Two new Cu(Ⅱ)complexes based on the thienyl and pyridyl substituted triarytriazole,trans-[Cu(L1)2(MeOH)2](ClO4)2(1)andtrans-[Cu(L2)2(ClO4)2]·2MeCN(2),have been synthesized and characterized by IR,PXRD and X-ray crystallography.The structural analyses reveal that two complexes have a similar pseudooctahedral[CuN4O2]core with two MeOH molecules in thetrans-positions in 1 but two ClO4-ions in thetranspositions in 2.
