Insight into Medicinal Chemistry Behind Traditional Chinese Medicines: p-Hydroxybenzyl Alcohol-Derived Dimers and Trimers from Gastrodia elata
2021-03-12YananWangMinZhangXueZhouChengboXuChenggenZhuYuheYuanNaihongChenYongchunYangQinglanGuoJiangongShi
Yanan Wang ·Min Zhang ·Xue Zhou ·Chengbo Xu ·Chenggen Zhu ·Yuhe Yuan ·Naihong Chen ·Yongchun Yang ·Qinglan Guo ·Jiangong Shi
Abstract From an aqueous extract of “tian ma” (the steamed and dried rhizomes of Gastrodia elata), ten new compounds gastrodibenzins A-D (1- 4) and gastrotribenzins A-F (5- 10), along with known analogues (11- 20), having structure features coupling between two and three p-hydroxybenzyl-derived units via carbon- and/or ether-bonds, were isolated and characterized by spectroscopic data analysis.Meanwhile, the new compounds 5a,6a,8a,22, and 23, as well as the known derivatives 13a,14a,15,17- 21,24,25, and p-hydroxybenzyl aldehyde were isolated and identifi ed from a refluxed aqueous solution of p-hydroxybenzyl alcohol.Methylation of 5a and 6a in methanol and ethylation of 6a,8a,13a, and 14a in ethanol produced 5 and 6 and 7,8,13, and 14, respectively.using ultra-performance liquid chromatography high-resolution electrospray ionization mass spectrometry (UPLC-HRESIMS) analysis of the refluxed solutions of p-hydroxybenzyl alcohol and the refluxed extracts of the fresh G.elata rhizome and “tian ma” extracts indicated consistent production and variation of the dimeric and trimeric derivatives of p-hydroxybenzyl alcohol upon extracting solvents and refluxing time.In various assays, the dimeric and trimeric derivatives showed more potent activities than p-hydroxybenzyl alcohol itself and gastrodin, which are the main known active constituents of “tian ma”.These results revealed for the first time that the more eff ective dimers and trimers can be produced through condensation of the co-occurring p-hydroxybenzyl alcohol during processing and decocting of the G.elata rhizomes, demonstrating insights into medicinal chemistry behind application protocols of traditional Chinese medicines.
Keywords Orchidaceae·Gastrodia elata·p-Hydroxybenzyl alcohol dimer·p-Hydroxybenzyl alcohol trimer·Gastrodibenzins·Gastrotribenzins·Medicinal chemistry behind Chinese medicines
1 Introduction
The steamed and dried rhizomes ofGastrodia elataBlume (Orchidaceae) is a precious and important tonic herbal medicine, named “tian ma” in Chinese, having health benefi ts enhancing strength and virility as well as improving memory and blood circulation [1].Since antiquity this traditional medicine is mainly used for the treatment of various neuralgic and nervous disorders in China [2].The plantG.elatais an endangered holomycotrophic species living on several symbiotic mycorrhizal fungi at specifi c stages of its life cycle [3].To satisfy medicinal utilization and to protect the wildly endangered species and ecological environment, starting in the late 1950′s, tremendous eff orts were made and succeeded in agricultural cultivation of this plant by Chinese scientists [3,4].Meanwhile, considerable chemical and pharmacological studies of the raw material and the processed “tian ma” led to isolation and identifi cation of around 100 constituents with diverse chemical structures and biological activities from extracts of this herbal medicine [2,5— 8].Most the constituents feature characteristic structures deriving from or modifi ed byp-hydroxybenzyl alcohol [2,5— 8].Notably, vanillyl alcohol and vanillin exhibited antiepileptic and anticonvulsant activities similar to that of the water extract ofG.elatarhizomes, which promoted the clinic application of vanillin as an antiepileptic drug in China [9— 13] 3 .The major component of “tian ma”, gastrodin and its synthetic acetate were developed for the treatment of neurasthenia, neurasthenic syndrome, angioneurotic headache, and insomnia [14— 20].Recent pharmacological studies showed that 4-hydroxybenzyl analogues [21— 36] and gastrodin [37— 42] possessed various in vivo and in vitro neurological activities.Gastrodin was found also to have eff ects on cardiac hypertrophy and fibrosis [43] and vasodilation [44] as well as on anti-cancer immune response [45].However, some studies showed that after removing of gastrodin the aqueous extract of “tian ma” retained the anti-hypoxia, sedative, hypnotic and anti-inflammatory eff ects and that at high dosages gastrodin did not exhibit the eff ects [46,47].The occurrence of antiepileptic vanillin in “tian ma” was questioned until 2006 [48,49].Meanwhile, new constituents from “tian ma” and their bioactivities were frequently discovered [2,5— 8].
According to the theory of traditional Chinese medicines (TCM), the drug materials are commonly processed and/or decocted to detoxify and/or to enhance effects of the herbal medicines.Chemical reaction must take place during processing and/or decocting to alter the chemical compositions of final decoctions used for the treatment of patients.This suggests that the aqueous decoction of the processed drug material might contain more benefit components for patients.Thus, there are important secrets of medicinal chemistry hiding behind processing and/or decocting protocols in TCM though these are yet to be confirmed in many cases including “tian ma” [50— 58].Because the previous phytochemical studies of “tian ma” were performed mostly by extracting the drug material with ethanol or methanol [2,5— 8], the extracting protocol completely differed from that of conventional application by decocting with water.Therefore, an aqueous extract of “tian ma” (the steamed and driedG.elatarhizomes [50— 58]) was investigated as part of our project to investigate chemical diversity and biological activities of several commonly used TCM [59— 70].Previously we reported 27 new and 40 known chemical constituents of the aqueous extract, along with their bioassays and pharmacological activities [71— 79].Especially we found that severalp-hydroxybenzyl-modified gastrodins from the extract could be produced from a coupling reaction of the co-occurringp-hydroxybenzyl alcohol and gastrodin in H2O under refluxing [80].This unraveled production of the new components during processing and decocting of “tian ma” in the classical application protocol.A further investigation resulted in characterization of ten new compounds gastrodibenzins A-D (1- 4) and gastrotribenzins A-F (5- 10) as well as ten known derivatives (11- 20) (Fig.1) from the remaining subfractions of the extract.Viewing the structures of 4- 20, these compounds may be derived from condensations of two or threep-hydroxybenzyl alcohol units at different positions via carbon- and/or ether-bonds, followed by etherification with the solvents MeOH or EtOH (4- 14).With the speculation, a refluxed H2O solution ofp-hydroxybenzyl alcohol was isolated to yield 5a,6a,8a,13a,14a,15,17- 19,21- 25 (Fig.1), andp-hydroxybenzaldehyde.UPLC-HRESIMS analysis of the refluxed methanol solutions of 5a and 6a and ethanol solutions of 6a,8a,13a, and 14a confirmed production of 5 and 6 and 7,8,13, and 14, respectively.Subsequent UPLC-HRESIMS analysis of the refluxed H2O, MeOH, and EtOH solutions ofp-hydroxybenzyl alcohol and the extracts of the freshG.elatarhizomes and “tian ma” provides insights into medicinal chemistry behind the processing and decocting protocols of TCM.Herein described are details.
2 Results and Discussion
2.1 Isolation and Structure Elucidation of 1- 20
The pulverized “tian ma” (the steamed and air-driedG.elatarhizomes) was extracted by ultrasonicating with H2O.After concentrated, the aqueous extract was chromatographed over macroporous adsorbent resin, eluting with a gradient increasing EtOH in H2O to give fractions A-D.Fraction C was chromatographed over MCI gel, with successive elution using H2O, 30% EtOH, 50% EtOH, 95% EtOH, and Me2CO, to yield subfractions C1-C5.Further separation of the subfractions by column chromatography (CC) over Sephadex LH-20 and normal phase silica gel, middle-pressure liquid chromatography (MPLC) over reversed phase (C18) silica gel, and reversed phase highperformance liquid chromatography (RP-HPLC) aff orded compounds 1- 20 (see ‘ Experimental’ section).
Compound 1, a white amorphous powder, showed infrared (IR) absorptions for hydroxy (3392 cm -1 ) and aromatic ring (1614 and 1514 cm -1 ) functionalities.High resolution electrospray ionization mass spectrometry (HRESIMS) atm/z311.1254 [M + Na] + (calcd.for C17H20O4Na, 311.1254), together with the nuclear magnetic resonance (NMR) spectroscopic data (Table 1), determined the molecular formula of 1 as C17H20O4.The 1 H NMR spectrum of 1 showed resonances assignable to a 2-substituted 4-hydroxy-5-methoxybenzyloxy unit atδH7.46 (brs, 4-OH), 6.94 (s, H-6), 6.61 (1H, s, H-3), 3.82 (s, 5-OCH 3), and 4.38 (s,H2-7); ap-hydroxybenzyl unit atδH8.13 (brs, 4′-OH), 6.99 (d,J= 8.4 Hz, H-2′/6′), 6.74 (d,J= 8.4 Hz, H-3′/5′), and 3.84 (s,H2-7′); and an ethoxy group atδH3.47 (q,J= 7.2 Hz, OCH2CH3) and 1.15 (t,J= 7.2 Hz,OCH2CH3 ).The 13 C NMR spectrum of 1 exhibited corresponding signals to the above units (Table 1).These data indicated that 1 was a dimeric benzyl derivative containing an ethoxy group [71], which was confi rmed by 2D NMR spectroscopic data analysis of 1 (Fig.2).Especially, the heteronuclear multiple bond correlation (HMBC) spectrum of 1 showed correlations from H-3 to C-1 and C-5, from H-6 to C-2, C-4, and C-7; from H2-7 to C-2, C-6, and OCH2CH3; from 4-OHto C-3 and C-4; from 5-OCH3to C-5; and from OCH2CH3to C-7 (Fig.2).This, together with the chemical shifts of the proton and carbon resonances, revealed the presence of an ethyl 2-substituted 4-hydroxy-5-methoxybenzyl ether moiety in 1.The location of thep-hydroxybenzyl unit at C-2 in 1 was deduced from the HMBC correlations from H-3 to C-7′ and from H2-7′ to C-1, C-2′/6′, and C-3.Therefore, the structure of compound 1 was determined as ethyl 4-hydroxy-5-methoxy-2-(4′-hydroxybenzyl)benzyl ether and named gastrodibenzin A.
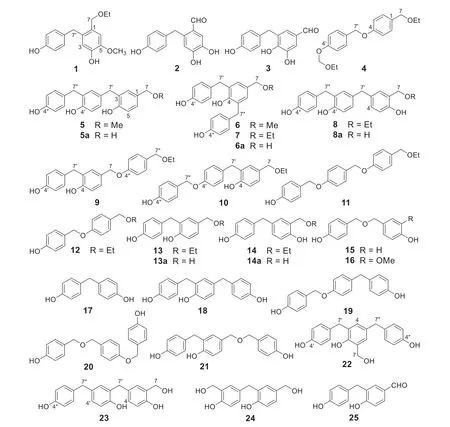
Fig.1 Structures of compounds 1- 25
Compound 2 was obtained as a yellowish amorphous powder.Its molecular formula was determined as C14H12O4by HRESIMS at m/ z 245.0807 [M + H] + (calcd.for C14H13O4, 245.0808).The NMR spectroscopic data of 2 (Table 1) indicated the presence of a 2-subustituted 4,5-dihydroxybenzaldehyde unit, in addition to the p-hydroxybenzyl identical to that in 1.This was verifi ed by the HMBC correlations from H-3 to C-1 and C-5; from H-6 to C-2, C-4, and C-7; from H-7 to C-2 and C-6; from 4-O H to C-3 and C-5; and from 5-O H to C-4 and C-6, in combination with their chemical shifts.Meanwhile, the connection between the two units was demonstrated by the HMBC correlations from H-3 to C-7′ and from H2-7′ to C-1, C-2′/C-6′, and C-3.Therefore, the structure of 2 was determined as 4,5-dihydroxy-2-(4′-hydroxybenzyl)benzaldehyde and named gastrodibenzin B.
Compound 3, a brownish amorphous powder, is an isomer of 2 as indicated by its spectroscopic data (Experimental and Table 1).Comparison of the NMR spectroscopic data between 3 and 2 suggested that the p-hydroxybenzyl was at C-3 of the 4,5-dihydroxybenzaldehyde unit in 3 instead of at C-2 in 2.The suggestion was confi rmed by 2D NMR data analysis of 3, particularly by the HMBC correlations (Fig.2) from H-2 to C-4, C-6, C-7, and C-7′; from H-6 to C-2, C-4, and C-7; from H-7 to C-2 and C-6; and from H2-7′ to C-2, C-2′/6′, and C-4, together with their chemical shifts.Therefore, the structure of compound 3was determined as 4,5-dihydroxy-3-(4′-hydroxybenzyl)benzaldehyde and named gastrodibenzin C.

Table 1 The NMR spectroscopic data of compounds 1- 4
Compound 4, a white amorphous powder, has the molecular formula of C19H24O4as determined by HRESIMS atm/z339.1572 [M + Na] + (calcd.for C19H24O4Na, 339.1567).The NMR spectroscopic data of 4 (Table 1) indicated the presence of two inequivalentp-oxybenzyloxys, two inequivalent ethoxys, and an isolated dioxymethylene.In the HMBC spectrum of 4 (Fig.2), the correlations from H 2 -7 to C-1 and C-2/C-6, from H-2/6 to C-4, and from H2-7′ to C-1′, C-2′/C-6′, and C-4, together with their chemical shifts demonstrated a head—tail connection of the twop-oxybenzyloxys via an ether bond between C-4 and C-7′.The HMBC correlations from H2-7 to the methylene carbon of one ethoxy unambiguously positioned the ethoxy at C-7.Moreover, the HMBC spectrum displayed the correlations from the dioxymethylene protons to C-4′ and the methylene carbon of the remaining ethoxy, indicating that an ethoxymethoxy unit 7 was located at C-4′ of 4.Accordingly, the structure of compound 4 was determined as ethyl 4-[4′-(ethoxymethoxy)benzyloxy]benzyl ether and named gastrodibenzin D.
Compound 5 was obtained as a white amorphous powder.Its molecular formula C22H22O 4 was determined by HRESIMS atm/z373.1397 [M + Na] + (calcd.for C22H22O4Na, 373.1410).The NMR spectra of 5 showed resonances (Table 2) attributed to ap-hydroxybenzyl, twom-substitutedp-hydroxybenzyls, and a methoxy group.This indicated that 5 was a methyl trimericp-hydroxybenzyl ether analogue.In the HMBC spectrum of 5, correlations of H-2 and H-6/C-4 and C-7; H-2′ and H-6′/C-4′ and C-7′, and H-2″ and H-6″/C-4″ and C-7″ (Fig.2), together with the splitting patterns and shifts of these proton and carbon resonances, demonstrated that the threep-hydroxybenzyls connected each other via C-3-C-7′ and C-3′-C-7″ bonds.In addition, the HMBC correlations of OCH3/C-7 and H2-7/OCH3located the methoxy group at C-7 in 5.Therefore, the structure of 5 was determined as methyl 4-hydroxy-3-[4′-hydroxy-3′-(4″-hydroxybenzyl)benzyl]benzyl ether and named gastrotribenzin A.

Fig.2 Main 1 H- 1 H COSY (thick lines) and HMBC (arrows, from 1 H to 13 C) correlations of compounds 1- 11,22, and 23
Compound 6, a white amorphous powder, is an isomer of 5 as indicated by the IR, HRESIMS (see ‘ Experimental’ section) and NMR spectroscopic data (Table 2).Comparison of the NMR spectroscopic data of the two compounds indicated the presence of two equivalentp-hydroxybenzyls, a symmetrically disubstitutedp-hydroxybenzyl, and an methoxy group in 5.This suggested that the terminal 4-hydroxybenzyl moiety at C-3′ in 5 was migrated to C-5 in 6.which was confi rmed by 2D NMR data analysis.Especially, the HMBC correlations from OH-4 and H2-7′/H2-7″ to C-4 and from OCH3and H-2/H-6 to C-7 demonstrated that the two equivalentp-hydroxybenzyls were substituted at C-3 and C-5 of the methyl 4-hydroxybenzyl ether unit to give a symmetric structure.Therefore, the structure of compound 6 was determined as methyl 4-hydroxy-3,5-di-(4′-hydroxybenzyl)benzyl ether and named gastrotribenzin B.
Compound 7 was isolated as a white amorphous powder.Comparison of the spectroscopic data between 7 and 6 revealed replacement of the methyl group in 6 by an ethyl group [δH3.41 (2H, q,J= 7.2 Hz, OCH2CH3) and 1.10 (3H, t,J= 7.2 Hz,OCH2CH 3), andδC65.6 (OCH2CH3) and 15.5 (OCH2CH3)] in 7.This was further confi rmed by the correlations of OCH2CH3/C-7 and H2-7/OCH2CH3in the HMBC spectrum of 7 (Fig.2).Thus, the structure of compound 7 was determined as ethyl 4-hydroxy-3,5-di-(4′-hydroxybenzyl)benzyl ether and named gastrotribenzin C.
Compound 8, a white amorphous powder, is an isomer of 7 having a different connection of hydroxybenzyls as indicated by the spectroscopic data (Experimental and Table 2).The HMBC spectrum of 8 showed the correlations (Fig.2) from H2-7 to C-1, C-2, and OCH2CH3; from H2-7′ to C-2′, C-4, C-6, and C-6′; and from H2-7″ to C-2′, C-2″, C-4′, and C-6″.These correlations, together with their coupling constants and chemical shifts, demonstrated C-5-C-7′ and C-3′-C-7″ linkage of the three hydroxybenzyls and location of the three hydroxy groups and the ethoxy at C-2, C-4′, and C-4″ and C-7, respectively.Hence, the structure of compound 8 was determined as ethyl 2-hydroxy-5-[4′-hydroxy-3′-(4″-hydroxybenzyl)-benzyl]benzyl ether and named gastrotribenzin D.
Compound 9 was obtained as a white amorphous powder.The spectroscopic data showed that this compound was another isomer of 7 and 8.In the HMBC spectrum of 9, the correlations (Fig.2) from H2-7 to C-2 and C-6; from H2-7′ to C-2, C-2′, C-4, and C-6′; from 4-OHto C-3, C-4, and C-5; and from 4′-OHto C-3′/5′ and C-4′, together with their chemical shifts, revealed the presence of a 4-hydroxy-3-(4′-hydroxybenzyl)benzyloxy moiety.In addition, the HMBC spectrum showed the correlations from H2-7 to C-4″, from

Table 2 The NMR spectroscopic data of compounds 5 - 11
H2-7″ to C-2″/C-6″, and OCH2CH3; and from OCH2CH3to C-7″.These correlations, in combination with their chemical shifts, unambiguously revealed the oxygen-bridged connections between C-4″ and C-7 and between C-7″ and the ethyl group.Thus, the structure of compound 9 was determined as 4-hydroxy-3-(4′-hydroxybenzyl)benzyl 4″-ethoxymethylphenyl ether and named gastrotribenzin E.
The spectroscopic data of compound 10 indicated that it was one more isomer of 7- 9.The HMBC spectrum of 10 exhibited the correlations (Fig.2) from H2-7 to C-2, C-6, and OCH2CH 3 ; from H 2 -7′ to C-2, C-2′/6′, C-3, and C-4; from H 2 -7″ to C-2″/6″ and C-4′; and from OCH 2CH 3 to C-7.These correlations, together with their chemical shifts, indicated the connection of C-3 to C-7′ as well as the ether-bond linkages of C-4′ to C-7″ and C-7 to the ethyl group in 10.Therefore, the structure of compound 10 was determined as ethyl 4-hydroxy-3-[4′-(4″-hydroxybenzyloxy)benzyl]benzyl ether and named gastrotribenzin F.
By comparison of spectroscopic data with those reported in literature, the known compounds were identified as ethyl 4-[4′-(4″-hydroxybenzyloxy)benzyloxy]benzyl ether (11) [81], ethyl 4-(4′-hydroxybenzyloxy)benzyl ether (12) [82], ethyl 4-hydroxy-3-(4′-hydroxybenzyl)benzyl ether (gastropolybenzylol C,13) [8], gastropolybenzylol B (14) [8], bis(4-hydroxybenzyl)ether (15) [82], 4-hydroxybenzyl vanillyl ether (16) [83], 4,4′-methylenediphenol (17) [82], 2,4-bis(4-hydroxybenzyl)phenol (18) [84], gastropolybenzylol A (19) [8], gastrol A (20) [85].The structure of 11 was previously determined only by UPLC/Q-TOF MS analysis [81] and identifi ed in this study by comprehensive analysis of the spectroscopic data including 2D NMR experiments (Fig.2), for which the detailed physical—chemical properties are reported (‘ Experimental’ section).
2.2 Products from a Refluxed Aqueous Solution of p-Hydroxybenzyl Alcohol and Their Etherifi cation with MeOH and EtOH
Among the 20 isolates, compounds 1- 3 and 16 containp-hydroxybenzyl and vanillyl alcohol (1 and 16) or protocatechualdehyde units (2 and 3), while the others are analogues ofp-hydroxybenzyl-derived dimers (4,12- 15 and 17) and trimers (5- 11 and 18- 20).Becausep-hydroxybenzyl alcohol, which abundantly occurs inG.elata[50— 58], is highly reactive to produce quinone methide and complex derivative via self-condensation or inter-condensation with other reactants under various conditions [86— 89], this suggests that, (a)p-hydroxybenzyl alcohol is an origin of thep-hydroxybenzyl unit in thep-hydroxybenzyl-containing chemical constituents of “tian ma”; (b) alcoholic forms of thep-hydroxybenzyl-derived dimers and trimers are generable fromp-hydroxybenzyl alcohol during processing and/or extracting of the drug material; and (c) the ethyl and methyl ethers were formed in the subsequent isolation procedure through contacting to the solvents EtOH and MeOH.To verify the suggestions, following experiments were performed: (a) an aqueous solution ofp-hydroxybenzyl alcohol was refluxed, from which the products were isolated and structurally identifi ed; (b) methylation and ethylation of thep-hydroxybenzyl alcohol-generating products were examined by UPLC-HRESIMS after refluxing of their MeOH and EtOH solutions; (c) the refluxed solutions ofp-hydroxybenzyl alcohol in H2O, MeOH, and EtOH were compared by UPLC-HRESIMS analysis using the identifi ed pure compounds as references.
As expected, all the experimental results supported the suggestions.Briefly, the dibenzyl and tribenzyl alcohols 5a,6a,8a,13a, and 14a as well as compounds 15,17- 19,21- 25, andp-hydroxybenzaldehyde were isolated from the refluxed aqueous solution ofp-hydroxybenzyl alcohol.The known compounds 13a [90],15 [82],17 [82],18 [84],19 [8],21 [90],25 [71], andp-hydroxybenzaldehyde [82] were previously reported as the “tian ma” constituents, while 14a and 24 were found as intermediates in the cure process of resol phenol—formaldehyde resins [91].The structures of the new compounds 5a,6a,8a,22, and 23 were readily determined by comparison of their spectroscopic data with those of 5- 8 (‘ Experimental’ section and Tables 2 and 3) and confi rmed by analysis of the 2D NMR spectroscopic data (Fig.2 and Supporting Information Figs.S133-S177).
UPLC-HRESIMS analysis proved that 5 and 6 were produced respectively by refluxing of 5a and 6a orp-hydroxybenzyl alcohol in methanol (Fig.3), while 7,8,13, and 14 were yielded by refluxing of 6a,8a,13a, and 14a, of which only 14 was undetectable from the refluxed EtOH solution ofp-hydroxybenzyl alcohol (Figs.4 and 5).All the isolated compounds from the refluxed H2O solution ofp-hydroxybenzyl alcohol were detectable in the refluxed MeOH and EtOH solutions (Supporting Information Figs.S178-S183).However, except for compounds 8,9, and 20, the phenolic ethers 10- 12 and 19 were undetectable in the refluxed EtOH solution ofp-hydroxybenzyl alcohol (Figs.4,5, and S178-S213), while the corresponding alcoholic forms of 9- 12 as well as compounds 19 and 20 were not obtained from the refluxed H2O solution ofp-hydroxybenzyl alcohol.This may be due to a structural instability of the phenolic ethers and/or their relative low abundance, which was preliminarily supported by interconversion of 9 and 10 in CH3CN (Fig.4).In addition, compositions and abundances of the isomeric dimers and trimers were signifi cantly varied in the sonicated and refluxed solutions (Figs.S184-S213).With increase of the refluxing time, the relative abundances of thep-hydroxybenzyl ethers 15 and 21 were signifi cantly decreased in the H2O solution, whereas the correspondingp-hydroxybenzyl-substitutedp-hydroxybenzyl alcohols 5a/6a and 13a were signifi cantly increased.Meanwhile,the relative contents of 8a,14a,22, and 23 were decreased also with increase of the refluxing time, and the contentdecreased compounds had higher relative abundances in the sonicated H2O solution without refluxing (Supporting Information Figs.S190, S191, and S193).However, relative content variations of the isomeric trimers 5a/ 6a,8a, and 21- 23 in the refluxed MeOH and EtOH solutions ofp-hydroxybenzyl alcohol (Figs.S198, S199, S205, and S206) were insignifi cant as compared with the refluxed H2O solution (Figs.S190 and S191), while the relative content variations of the isomeric dimers 13a,14a, and 15 in the refluxed MeOH solution (Fig.S201) were insignifi cant as compared with that in the H2O and EtOH solutions (Figs.S193 and S210).The aforementioned observation suggested that the preferentially formed 15 and 21 were instable in the solutions to convert into the other compounds.The suggestion was further confi rmed by sonicating and refluxing of the H2O, MeOH, and EtOH solutions of 15 and 21, respectively (Figs.S214-S232).Compounds 5 and 6 were generated in the sonicated MeOH solution of 15 (Fig.S214) while several unidentifi ed isomers were produced in the sonicated solutions of 21 (Fig.S222).Compound 15 was readily converted into 21 by sonicating in H2O, MeOH, and EtOH (Fig.S215), whereas the reverse conversion from 21 into 15 was detectable after the MeOH and EtOH solutions were refluxed for 2 h and after the sonicated H2O solution was refluxed for 4 h (Figs.S228-S230).Compounds 17,18, and 25 were consistently detectable in the sonicated H2O, MeOH, and EtOH solutions of both 15 and 21 (Figs.S217, S220, S221, S226, S231, and S232); meanwhile,13a was detectable after the sonicated solutions of 15 were refluxed for 2 h (Fig.S219), but abundantly appeared in the sonicated solutions of 21 (Fig.S228).In addition, the ethyl ethers 7,9,10, and 13 were observable in the sonicated EtOH solution of 15 (Figs.S216 and S218), and production of 7,9, and 10 from 21 was secured after the sonicated EtOH solution was further refluxed for 1.0 h (Figs.S224 and S225).Moreover, generation of 22 was detectable in the sonicated solutions of 21 (Figs.S223).Particularly the transformation from 21 to the ethyl ethers 7 and 13 in the H2O and MeOH solutions (Figs.S223, S224, and S227) was unexpected and of interesting, which was confi rmed by the paralleled and repeated experiments.Because the ethyl unit to form 7 and 13 was highly suspected to be producible in the reaction system, its ambient ethanol origin should not be excluded.
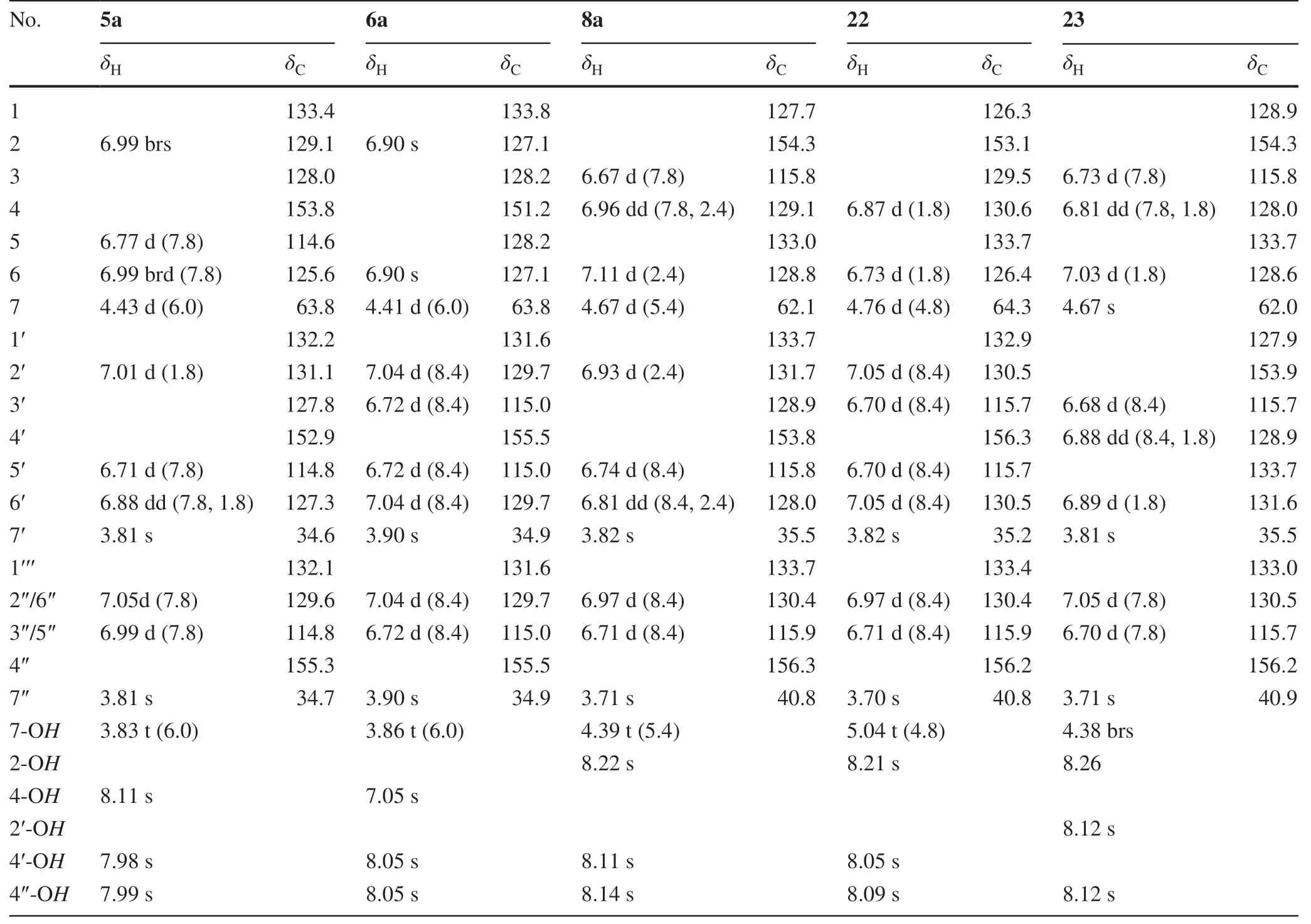
Table 3 The NMR spectroscopic data of compounds 5a,6a,8a,22, and 23

Fig.3 Overlaid chromatograms of the extracted negative ion at m/z 349.145 [M - H] - : a, b compounds 5 and 6 in CH 3 CN, respectively; c, d MeOH solutions of 5a and 6a were refluxed for 1 h, respectively; e MeOH solution of p-hydroxybenzyl alcohol was sonicated for 0.5 h then refluxed for 1.0 h
The above experiments demonstrated that the composition of the refluxed solutions ofp-hydroxybenzyl alcohol are highly dependent upon the solvent and refluxing time.Because of the abundant occurrence ofp-hydroxybenzyl alcohol inG.elata[50— 58] (Fig.S233), thep-hydroxybenzyl-derived dimers and trimers from the “tian ma” extracts must be formed at least partially by refluxing of the drug materials in H2O, MeOH, or EtOH.Meanwhile, some dimers and trimers can be converted and/or transformed each other.This supports that indeed the chemical reactions take place during the processing and decocting of “tian ma” to produce the compounds and to modify the chemical composition.
2.3 UPLC/HRESIMS Analysis of H 2 O, MeOH,and EtOH Extracts of the G.elata rhizomes
To confi rm the chemical reactions during processing and decocting of the herbal medicine [80], the freshG.elatarhizomes were collected at the same field of “tian ma” (the steamed and dried rhizomes) and the extracts were prepared by sonicating of the freshG.elatarhizomes and “tian ma” in H2O, MeOH, and EtOH for 0.5 h, respectively, followed by refluxing (sampling time: 0.5 h, 1.0 h, 1.5 h, 2.0 h, 4.0 h, and 6.0 h).The extract samples were analyzed by UPLCHRESIMS using the aforementioned pure compounds as the references, showed that the composition and relative content of the extracts were varied with the extracting solvent and refluxing time.
The precursorp-hydroxybenzyl alcohol and compounds 5a/ 6a,13a,15,17,18,21, and 25 were detectable in the extracts obtained by sonicating of the freshG.elatarhizomes and “tian ma” in H2O, MeOH, and EtOH, respectively (Supporting Information Figs.S233-S239), except that the trimers 5a/ 6a,18, and 21 were undetectable in the H2O extract (Figs.S234 and S235).In addition, the relative content of the isomers 13a and 15 in the fresh rhizome extracts were reversed in the “tian ma” extracts (Fig.S236), suggesting that 13a was generated at least partially during processing of the drug material.

Fig.4 Overlaid chromatograms of the extracted negative ion at m/z 363.161 [M - H] - : a- e compounds 7- 11 in CH 3 CN, respectively; (f, g) EtOH solutions of 6a and 8a were refluxed for 1.0 h, respectively; h EtOH solution of p-hydroxybenzyl alcohol was sonicated for 0.5 h then refluxed for 1.0 h
For the freshG.elatarhizomes (Figs.S240-S261), the trimeric isomers 5a/ 6a,8a, and 21- 23 were detectable in the H2O, MeOH, and EtOH extracts after refluxed for 2 h, 4 h, and 6 h (Fig.6 and Supporting Information Figs.S240, S241, S247, S248, S254, and S255).The dimers 14a and 24 were detectable in the MeOH and EtOH extracts after refluxed for 1 h and 2 h (Figs.S250, S251, S258, and S259).The dimer 16 and trimer 18 were detectable in all the H2O, MeOH, and EtOH extracts after refluxed for more than 1 h (Fig.7 and Supporting Information Figs.S242, S244, S249, S251, S256, and S259).Interestingly compound 18 disappeared in the MeOH extract after refluxed for 6 h (Fig.S249).Among the ethers, compound 13 was detectable only in the EtOH extracts of the freshG.elatarhizomes (Fig.S257), indicating that this compound was formed from contacting with EtOH in the experimental procedure.When compared with the refluxed H2O extracts (Figs.S240 and S243), with increase of the refluxing time, the relative contents of 5a/ 6a and 13a were decreased in the MeOH and EtOH extracts (Figs.S247, S250, S254, and S258) while 13 was relatively increased in the EtOH extracts (Fig.S257).This demonstrated that 5a/ 6a and 13a were reacted with the solvents to be transformed into the corresponding methyl and ethyl ethers during refluxing, though compounds 5- 7 were undetectable in the freshG.elatarhizome extracts possibly due to low content.
As compared with extracts of the freshG.elatarhizomes, more compounds were detectable in the “tian ma” extracts (Figs.S262-S289).Compounds 2 and 3 appeared in all the refluxed “tian ma” extracts (Figs.S262, S270, and S280) and 1 in the EtOH extracts after refluxed for 2 h and 6 h (Fig.S279).Particularly the methyl ethers 5 and 6 appeared only in the refluxed MeOH extracts (Fig.8 and Supporting Information Fig.S271) and the ethyl ethers 7,9, and 14 in the refluxed EtOH extracts (Figs.8, S284, and S285).This further supports that the methyl and ethyl ethers were produced from reaction of the corresponding dimeric and trimeric benzyl alcohols (such as 5a/ 6a,13a, and 14a) with the solvents.
The isolated minor compounds 4,8,9- 12,19, and 20 were undetectable in the extracts of either the freshG.elatarhizomes or “tian ma”, this may be explained by their low contents, since 4,10- 12, and 19 were also undetectable in the refluxed EtOH solutions ofp-hydroxybenzyl alcohol and since relative low peak intensities of 8,9, and 20 were observed in chromatograms of the refluxed EtOH and/or H2O solutions ofp-hydroxybenzyl alcohol.The detectable main compounds in the extracts were completely identical to the main products from the refluxed solutions ofp-hydroxybenzyl alcohol.Particularly the dimeric analogues 13a,15,17,21, and 25 were readily detectable in all the sonicated extracts of the freshG.elatarhizomes and “tian ma” as well as the refluxed solutions ofp-hydroxybenzyl alcohol.

Fig.5 Overlaid chromatograms of the extracted negative ion at m/z 257.119 [M—H] — : a— c compounds 12— 14 in CH 3 CN, respectively; d, e EtOH solutions of 13a and 14a were refluxed for 1.0 h, respectively; f EtOH solution of p-hydroxybenzyl alcohol was sonicated for 0.5 h then refluxed for 1.0 h
Because the high abundance and reactivity ofp-hydroxybenzyl alcohol in the freshG.elatarhizomes and “tian ma” were repeatedly confi rmed [50— 58,86— 89], the above results unraveled that the constituents of the extracts were modifi ed at least partially by the chemical reactions ofp-hydroxybenzyl alcohol when the drug materials were refluxed with H2O, MeOH, and EtOH.Nevertheless, the natural occurrence and production of thep-hydroxybenzyl alcohol-derived dimers and trimers should not be excluded since there is no evidence for exception of the reactions under physiological environments.
2.4 Activities of the Purifi ed Compounds
Because previous studies revealed neuronal protection, anti-inflammatory, and antioxidant played important roles in the neurological eff ects of the extracts and chemical constituents ofG.elata[92], the purifi ed compounds from the aqueous extract were assayed preliminarily on the corresponding cell-based models [72,79].At a concentration of 10 μmol/L, as compared with the blank control, compounds 5,7,17- 19 attenuated rotenone-induced PC12 cell damage by increasing the cell viability from 56.08 ± 4.4% to 71.28 ± 9.4%, 82.43 ± 0.08%, 77.91 ± 0.07%, 62.25 ± 7.89%, and 76.26 ± 11.3%, respectively.At the same concentration, as compared with the model group,17 and the positive control bicyclol protected DL-galactosamine (GalN)-induced hepatocyte (WB-F344 cell) damage by increasing cell survive rates from 16 to 21% and 19%, respectively.In addition, compounds 5,7,8,17, and 19 and the positive control dexamethasone inhibited LPS-induced NO production in mouse peritoneal macrophages with inhibition rates of 78.1%, 74.7%, 91.4%, 92.1% and 83.4%, respectively, while 5,7,8,12,15,17- 19, and the positive control glutathione inhibited Fe 2+ -cystine-induced rat liver microsomal lipid peroxidation with inhibition rates of 74%, 59%, 82%, 62%, 93%, 67%, 89%, 78%, and 49%.The results indicated that compound 17 was active in all the four assays while 5,7, and 19 were active in the three assays.The remaining compounds including gastrodin andp-hydroxybenzyl alcohol were inactive at the same concentration.
The previous study on the isolated guinea-pig ileum smooth muscle showed that 17 and the alcohol form of 11 had inhibitory eff ects on neurotransmitter release induced by stimulation of nicotine, serotonin, and vanilloid receptors, while 18 and 21 aff ected acetylcholine-induced contraction more directly [90].Compounds 13,14,17,18, and 21 were activators of melatonin receptors [7].In addition,15,17, and the methyl ether analogue of 11 exhibited signifi cant inhibitory eff ects on collagen, epinephrine, arachidonic acid, U46619 induced platelet aggregation [93],17 had vasodilatory eff ect [94], and 18 was found to be a heat shock factor 1 (HSF1) inhibitor [95].The studies also demonstrated that the main components gastrodin andp-hydroxybenzyl alcohol were less active or inactive as compared with the “tian ma” extracts as well as the dimers and trimers [90,93,94].Thus, thep-hydroxybenzyl alcohol-derived dimers and trimers, which are the modifi ed and recombined components during processing and decocting of the drug material, have important contributions to the clinic eff ects of the “tian ma” decoction.
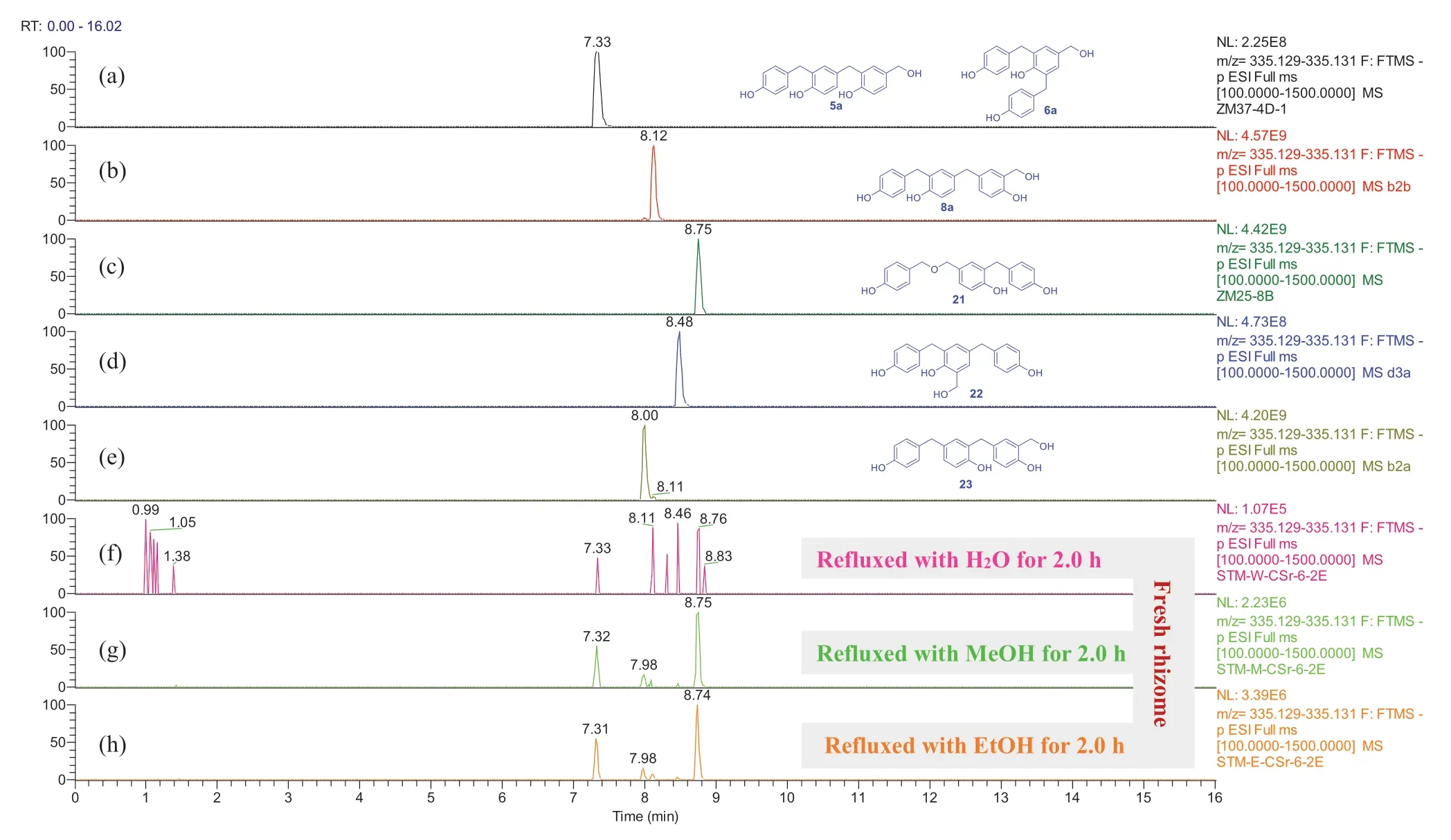
Fig.6 Overlaid chromatograms of the extracted negative ion at m/z 335.130 [M - H] - : (a-e) compounds 5a/ 6a,8a, and 21- 23 in CH 3 CN, respectively; (f-h) extracts obtained by sonicating of fresh G.elata rhizomes with H 2 O, MeOH, and EtOH for 0.5 h then refluxing for 2.0 h, respectively
3 Conclusions
Ten newp-hydroxybenzyl-derived dimers and trimers, gastrodibenzins A-D and gastrotribenzins A-F, together with ten known analogues, were isolated from an aqueous extract of “tian ma”.Compounds 2 and 3 represents the first examples ofp-hydroxybenzyl-coupled protocatechualdehydes.From the refluxed aqueous solution ofp-hydroxybenzyl alcohol, isolation and identification of 5a,6a,8a,13a,14a,15,17- 19,21,24- 25, andp-hydroxybenzaldehyde, in combination with UPLC-HRESIMS analysis, unraveled that: (a) thep-hydroxybenzyl unit in the “tian ma” chemical constituents were originated fromp-hydroxybenzyl alcohol through self-condensation (4- 15 and 17- 20) and inter-condensation with other molecules (1- 3 and 16), which could be produced, modifi ed, and recombined during processing and decocting of the drug material; (b) thep-hydroxybenzylderived methyl and ethyl ethers (such as 4- 14) could readily be formed by contacting to the solvents MeOH and EtOH in the experimental procedure, respectively; (c) the chemical constituents of ‘tian ma” extracts were highly dependent upon processing and extracting protocols including the solvents and refluxing time.This study, together with our previous results 71-80 , provides valuable insights into medicinal chemistry behind the processing and decocting protocols of TCM.This unravels that the composition and content of the diversep-hydroxybenzyl-derived constituents of “tian ma” and their contributions to the pharmacological eff ects are modifi ed and recombined by the processing and decocting.The processing and decocting protocols of TCM indeed enhance the medicinal values of the herbal medicine and deserve to be further investigated and to be in deep validated for more complex formulations.
4 Experimental
4.1 General experimental procedures
See Supporting Information.
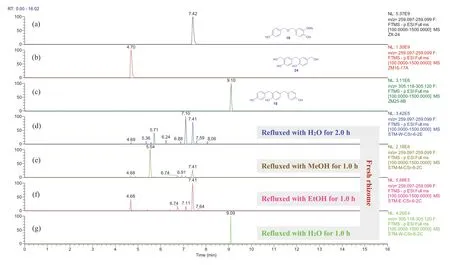
Fig.7 Overlaid chromatograms of the extracted negative ions at m/z 259.098 [M - H] - for (a, b, d-f) and at 305.119 [M - H] - for (c, g), respectively: (a-c) compounds 16,24, and 18 in CH 3 CN, respec-tively; (d-g) extracts obtained by sonicating of fresh G.elata rhizomes with H 2 O, MeOH, and EtOH for 0.5 h then refluxing for 1.0 h or 2.0 h, respectively
4.2 Plant material
See Supporting Information.
4.3 Extraction and isolation
For preliminary extraction and isolation, see Refs.71-75 and Supporting Information.Fraction C1 (66 g) was separated by CC over Sephadex LH-20, successively eluting with H2O, 30% EtOH, 50% EtOH, and 95% EtOH, to give subfractions C1-1—C1-4.Further fractionation of C1-2 (36 g) by RP-MPLC (C18silica gel, 50 μm, YMC), eluting with a gradient of increasing MeOH (0—100%) in H2O, yielded subfractions C1-2-1—C1-2-4.Subfraction C1-2-3 (9.3 g) was chromatographed over silica gel, eluting with a gradient of increasing MeOH (0—100%) in CHCl3, to yield C1-2-3-1-C1-2-3-5.Fraction C1-2-3-2 (3.98 g) was separated by CC over Sephadex LH-20 (MeOH) yielded C1-2-3-2-1-C1-2-3-2-3, of which C1-2-3-2-2 (1.31 g) and C1-2-3-2-3 (1.26 g) were individually isolated by RP-HPLC (60% MeOH in H2O,C 18 column, 2.0 mL/min) to aff ord 6 (8.6 mg,tR= 26.5 min) from the former and 11 (19.3 mg,tR= 27.6 min) from the latter.Fraction C1-2-3-3 (0.61 g) was chromatographed over Sephadex LH-20 (50% MeOH) to give a mixture, which was successively isolated by CC over silica gel (CHCl 3 -MeOH, 15:1) and RP-HPLC (70% MeOH in H2O,C18column, 2.0 mL/min) to obtain 14 (13.8 mg,tR= 11.9 min).Further fractionation of C1-3 (36 g) by CC over Sephadex LH-20 (50% MeOH) gave C1-3-1-C1-3-4.Fraction C1-3-2 (1.2 g) was isolated by CC over silica gel, eluting with a gradient of increasing MeOH (0-100%) in CHCl3, to yield C1-3-2-1 (0.48 g), which was further separated by RP-HPLC (65% MeOH in H2O,C18column, 2.0 mL/min) to aff ord 4 (56.2 mg,tR= 31.2 min).
Fraction C2 (302 g) was subjected CC over silica gel (ethyl acetate—EtOH—H2O, 16:2:1-2:2:1) to give subfractions C2-1-C2-5.Subfraction C2-1 (52.5 g) was chromatographed over silica gel, eluting with a gradient of increasing MeOH (0-100%) in CHCl3, to yield C2-1-1-C2-1-6.Fraction C2-1-2 (7.2 g) was re-separated by CC (CHCl3—MeOH, 100:0-0:10) to yield C2-1-2-1-C2-1-2-5, of which C2-1-2-3 (1.8 g) C2-1-2-5 (1.5 g) was further fractionated by CC over Sephadex LH-20 (MeOH) to yield C2-1-2-5-1-C2-1-2-5—3.Fraction C2-1-2-5—2 (180 mg) was separated by CC over silica gel (CHCl3—MeOH, 10:1) to give C2-1-2-5-2-1-C2-1-2-5-2-3, of which C2-1-2-5-2-1 (28 mg) was isolated by preparative TLC (CHCl3-MeOH, 10:1), followed by purifi cation with RP-HPLC (52% MeOH in H2O, Ph column, 2.0 mL/min) to obtain 2 (2.3 mg,tR= 12.5 min) and 3 (2.1 mg,tR= 13.6 min).
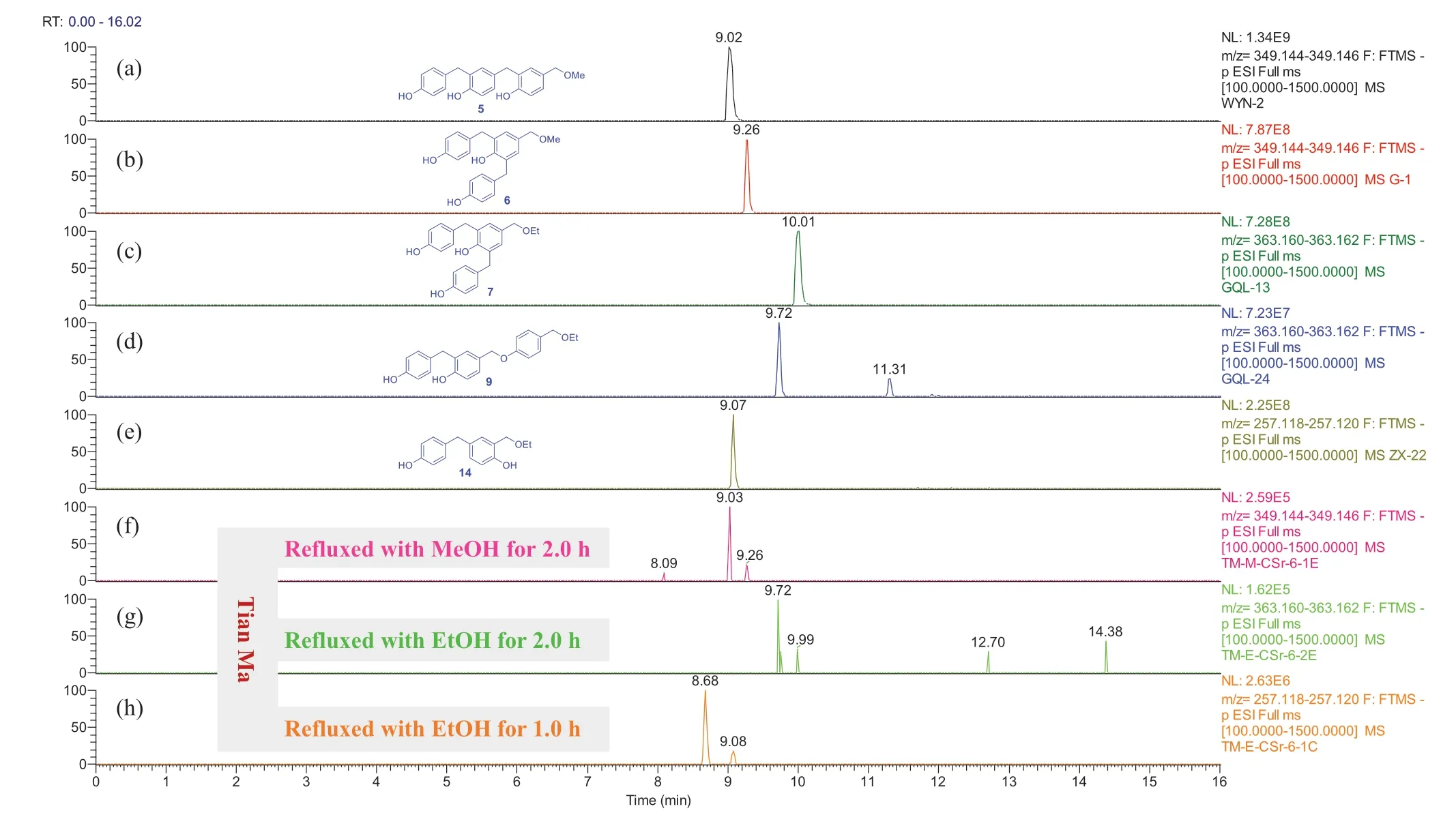
Fig.8 Overlaid chromatograms of the extracted negative ions at m/z 349.145 [M - H] - for (a, b, f); and at 363.161 [M - H] - for (c, d, g); and at 257.119 [M - H] - for (e, h), respectively: (a-e) compounds 5,6,7,9, and 14 in CH 3 CN, respectively; (f-h) extracts obtained by sonicating of “tian ma” with MeOH and EtOH for 0.5 h then refluxing for 1.0 h or 2.0 h, respectively
Fraction C3 (237 g) was subjected to CC over silica gel, eluting with a gradient of increasing MeOH (0-100%) in EtOAc followed by 30% EtOH, to yield fractions C3-1-C3-5 based on TLC analysis.Fraction C3-1 (27.3 g) was separated by silica gel CC (petroleum ether-ethyl acetate, 50:1—1:1) to give C3-1-1-C3-1-6, of which C3-1-1 (780 mg) was further fractionated by Flash CC over reversed phase silica gel (C18) (10—70% MeOH in H2O) to yield C3-1-1-1-C3-1-1-8.Purifi cation of C3-1-1-2 (34 mg) by RP HPLC (50% MeOH in H2O,C18column, 2.0 mL/min) obtained 17 (22.3 mg,tR= 47.5 min).Fraction C3-1-2 (2.15 g) was further separated by MPLC over reversed phase silica gel (C 18 ), eluting with a gradient of increasing MeOH (0-80%) in H2O, to aff ord C3-1-2-1-C3-1-2-6, of which C3-1-2-1 (840 mg) was chromatographed over Sephadex LH-20 (MeOH) to yield C3-1-2-1-1 and C3-1-2-1-2.Isolation of C3-1-2-1-2 (402 mg) by preparative TLC (CHCl3—MeOH, 20:1) aff orded 15 (350 mg).Fraction C3-1-2-2 (100 mg) was separated by preparative TLC (CHCl3—MeOH, 10:1) to give C3-1-2-2-1-C3-1-2-2-3.Purifi cation of C3-1-2-2-1 (32 mg) by RP HPLC (55% MeOH in H2O,C18column, 2.0 mL/min) to aff ord 13 (24 mg,tR= 38.2 min).Isolation of C3-1-2-3 (5.3 g) by silica gel CC (CHCl3-MeOH, 25:1) aff orded 18 (400 mg).Fraction C3-1-3 (900 mg) was separated by silica gel CC (CHCl3—MeOH, 15:1) to give C3-1-3-1-C3-1-3-7, of which C3-1-3-5 (240 mg) was further isolated by silica gel CC, eluting with a gradient of increasing acetone in petroleum ether (20:1-5:1), to yielded C3-1-3-5-1-C3-1-3-5-4.Fraction C3-1-3-5-3 (8.5 mg) was successively separated by RP-HPLC (60% MeCN in H2O,C18column, 2.0 mL/min) and chiral HPLC (hexane—isopropanol, 3:1, AD-H column, 2.0 mL/min) to obtain 9 (1.2 mg,tR= 29.0 min) and 10 (1.5 mg,tR= 27.0 min).Fraction C3-1-3-6 (180 mg) was separated by silica gel CC (petroleum ether-acetone, 10:1—5:1) to aff ord C3-1-3-6-1-C3-1-3-6-4, of which C3-1-3-6-2 (16.5 mg) isolated by preparative TLC (petroleum ether-acetone, 2:1) and purifi ed by RP-HPLC (50% MeCN in H2O, Ph column, 2.0 mL/min) to yield 19 (11.5 mg,tR= 48.2 min).Isolation of C3-1—4 (1.25 g) by silica gel CC (CHCl3—MeOH, 10:1—1:1) yielded C3-1-4-1-C3-1-4-4, of which C3-1-4-1 (68 mg) was separated by preparative TLC (petroleum ether-acetone, 2:1) and further purifi ed by RP-HPLC (45% MeCN in H2O,C18column, 2.0 mL/min) to obtain 5 (33.2 mg,tR= 41.5 min).Separation of C3-1-4-3 (230 mg) by CC over Sephadex LH-20 (CHCl3—MeOH, 1:1) gave C3-1-4-3-1-C3-1-4-3-3, of which C3-1-4-3-1 (46 mg) was further separated by RP-HPLC (59% MeOH in H2O,C18column, 2.0 mL/min) to aff ord 7 (22 mg,tR= 60.5 min) and 8 (12.7 mg,tR= 42 min).
Fraction C4 (7 g) was isolated by silica gel CC, eluting with a gradient of increasing acetone (0-100%) in petroleum ether, to yield fractions C4-1-C4-18.Fractionation of C4-3 (29 mg) by CC over Sephadex LH-20 (petroleum ether—CH2Cl2—MeOH, 5:5:1) gave C4-3-1-C4-3-2, of which C4-3-1 (11.1 mg) was purifi ed by RP HPLC (80% MeOH in H2O,C18column, 2.0 mL/min) to yield 12 (4.2 mg,tR= 12.3 min).Separation of C4-8 by CC over Sephadex LH-20 (petroleum ether—CH2Cl2—MeOH, 5:5:1) yielded C4-8-1-C4-8-3, of which C4-8-1 (13 mg) was isolated by RP HPLC (70% MeOH in H2O,C18column, 2.0 mL/min) to give C4-8-1-1 and C4-8-1-2.Further purifi cation of C4-8-1-1 (7 mg) by RP HPLC (50% MeOH in H2O,C18column, 2.0 mL/min) obtained 1 (3.4 mg,tR= 12.1 min).Isolation of C4-9 (597 mg) by CC over Sephadex LH-20 (petroleum ether—CH2Cl2—MeOH, 5:5:1) gave C4-9-1-C4-9-4, of which C4-9-2 (20.0 mg) was purifi ed by RP HPLC (56% MeOH in H2O,C18column, 2.0 mL/min) to obtain 16 (16.6 mg,tR= 24.1 min).Separation of C4-10 (95 mg) by CC over Sephadex LH-20 (petroleum ether—CH2Cl2—MeOH, 5:5:1) yielded C4-10-1 and C4-10-2, of which C4-10-1 (7 mg) was isolated by preparative TLC over silica gel (petroleum ether-acetone, 1:1) then purifi ed by RP HPLC (70% MeOH in H2O,C 18 column, 2.0 mL/min) to aff ord 20 (3.2 mg,tR = 24.2 min).
Gastrodibenzin A (1): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 205 (3.03), 233 (2.51), 284 (2.00) nm; IRνmax3392, 2975, 1681, 1614, 1514, 1444, 1376, 1206, 1143, 1101, 845, 803, 725 cm —1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 1; (+)-ESIMS:m/z311 [M + Na] + ; (-)-ESIMS:m/z288 [M - H] - ; (+)-HRESIMS:m/z311.1254 [M + Na] + (calcd.for C17H20O4Na, 311.1254).
Gastrodibenzin B (2): yellowish amorphous powder (MeOH); UV (MeOH)λmax (logε) 210 (3.58), 238 (3.57), 283 (3.30), 322 (3.10); IRνmax 3218, 2730, 1675, 1597, 1514, 1450, 1417, 1372, 1299, 1241, 1202, 1144, 1100, 1046, 1023, 991, 888, 825, 803, 762, 724, 623 cm -1 ; 1 H NMR (DMSO-d6, 600 MHz) and 13 C NMR (DMSO-d6, 150 MHz) data, see Table 1; (-)-ESIMS:m/z243 [M - H] - , 487 [2 M - H] - ; (+)-HRESIMS:m/z245.0807 [M + H] + (calcd.for C14H13O4, 245.0808), 267.0625 [M + Na] + (calcd.for C14H12O4Na, 267.0628), 283.0362 [M + K] + (calcd.for C14H12O4K, 283.0367).
Gastrodibenzin C (3): Brownish amorphous powder (MeOH); UV (MeOH)λmax(logε) 205 (3.85), 229 (3.83), 286 (3.53), 315 (3.37); IRνmax 3354, 2844, 2731, 1722, 1663, 1593, 1513, 1446, 1370, 1303, 1222, 1142, 1102, 1016, 980, 917, 868, 834, 783, 745, 699, 627, 587 cm -1 ; 1 H NMR (acetone-d6, 500 MHz) and 13 CNMR (acetone-d6, 125 MHz) data, see Table 1; (-)-ESIMS:m/z243 [M - H] - , 487 [2 M - H] - ; (+)-HRESIMS:m/z245.0805 [M + H] + (calcd.for C14H13O 4 , 245.0808), 267.0620 [M + Na] + (calcd.for C14H12O4Na, 267.0628).
Gastrodibenzin D (4): white amorphous powder (MeOH); UV(MeOH)λmax(logε) 207 (4.03), 227 (3.58), 276 (3.21) nm; IRνmax3036, 2975, 2930, 2867, 1729, 1613, 1586, 1513, 1466, 1378, 1353, 1301, 1227, 1172, 1106, 1002, 937, 872, 825, 748, 709, 659, 598, 519 cm -1 ; 1 H NMR (DMSO-d6, 500 MHz) and 13 C NMR (DMSO-d6, 125 MHz) data, see Table 1; (+)-ESIMS:m/z339 [M + Na] + ; (+)-HRESIMS:m/z339.1572 [M + Na] + (calcd.for C19H24O4Na, 339.1567).
Gastrotribenzin A (5): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 205 (3.93), 226 (3.48), 281 (3.04) nm; IRνmax3282, 3018, 2927, 2828, 2728, 2607, 2257, 2127, 1891, 1611, 1511, 1440, 1376, 1264, 1173, 1107, 1076, 1022, 1001, 949, 911, 879, 825, 780, 707, 648, 620, 532 cm -1 ; 1 H NMR (DMSO-d6, 500 MHz) and 13 C NMR (DMSO-d6, 125 MHz) data, see Table 2; (+)-ESIMS:m/z373 [M + Na] + , 389 [M + K] + ; (+)-HRESIMS:m/z373.1397 [M + Na] + (calcd.for C22H22O4Na, 373.1410).
Gastrotribenzin B (6): White amorphous powder (MeOH); UV (MeOH)λmax(logε) 206 (4.09), 229 (3.51), 279 (3.12) nm; IRνmax3385, 3013, 2918, 2851, 1612, 1512, 1476, 1441, 1381, 1346, 1257, 1216, 1174, 1139, 1074, 1018, 995, 952, 911, 877, 830, 788, 770, 720, 596, 543, 514 cm -1 ; 1 H NMR (DMSO-d6 , 500 MHz) and 13 C NMR (DMSO-d6, 125 MHz) data, see Table 2; (+)-ESIMS:m/z389 [M + K] + ; (+)-HRESIMS:m/z373.1410 [M + Na] + (calcd.for C22H22O4Na, 373.1410).
Gastrotribenzin C (7): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 206 (4.13), 225 (3.59), 280 (3.19); IRνmax3357, 2977, 1612, 1598, 1513, 1477, 1445, 1373, 1355, 1228, 1172, 1140, 1098, 1070, 1013, 980, 911, 868, 833, 789, 736 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 2; (+)-ESIMS:m/z387 [M + Na] + , 403 [M + K] + , (-)-ESIMS:m/z727 [2 M - H] - ; (+)-HRESIMS:m/z387.1566 [M + Na] + (calcd.for C23H24O4Na, 387.1567), 403.1313 [M + K] + (calcd.for C23H24O4K, 403.1306).
Gastrotribenzin D (8): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 205 (4.42), 227 (4.12), 282 (3.57); IRνmax3379, 3020, 2975, 2926, 1673, 1510, 1440, 1354, 1244, 1173, 1108, 1072, 1013, 893, 819, 781 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 2; (+)-ESIMS:m/z387 [M + Na] + , 403 [M + K] + , (-)-ESIMS:m/z363 [M - H] - ; (+)-HRESIMS:m/z387.1572 [M + Na] + (calcd.for C23H24O4Na, 387.1567), 403.1314 [M + K] + (calcd.for C23H24O4K, 403.1306).
Gastrotribenzin E (9): white amorphous powder (MeOH); UV (MeOH)λmax (logε) 204 (4.19), 228 (3.98), 279 (3.19); IRνmax3374, 3264, 3020, 2924, 2852, 1704, 1647, 1612, 1512, 1442, 1375, 1301, 1231, 1173, 1108, 1071, 1006, 941, 914, 894, 823, 781 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) spectroscopic data (Table 2); 13 C NMR (acetone-d6, 150 MHz) spectroscopic data (Table 2); (+)-ESIMS:m/z387 [M + Na] + , 403 [M + K] + ; (+)-HRESIMS:m/z387.1563 [M + Na] + (Calcd.for C23H24O4Na, 387.1567).
Gastrotribenzin F (10): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 204 (4.19), 228 (3.98), 279 (3.19); IRνmax3361, 3228, 3028, 2926, 2855, 1704, 1660, 1612, 1511, 1443, 1375, 1230, 1172, 1109, 1071, 1008, 941, 913, 873, 822, 776 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 2; (+)-ESIMS:m/z387 [M + Na] + , 403 [M + K] + ; (+)-HRESIMS:m/z387.1564 [M + Na] + (calcd.for C23H24O4Na, 387.1567).
4-[4′-(4″-Hydroxybenzyloxy) benzyloxy]benzyl ethyl ether (11): white amorphous powder (MeOH); UV(MeOH)λmax(logε) 205 (4.13), 224 (3.55), 280 (3.32) nm; IRνmax3348, 3070, 2976, 2898, 2860, 2811, 1894, 1614, 1584, 1518, 1460, 1418, 1390, 1354, 1309, 1257, 1121, 1080, 1045, 1012, 955, 897, 872, 825, 764, 708, 616, 568, 518 cm -1 ; 1 H NMR (DMSO-d6, 500 MHz) and 13 C NMR (DMSO-d6, 125 MHz) data, see Table 2; (+)-ESIMS:m/z403 [M + K] + ; (+)-HRESIMS:m/z387.1566 [M + Na] + (calcd.for C23H24O4Na, 387.1567), 403.1308 [M + K] + (calcd.for C23H24O4K, 403.1306).
4.4 Isolation of Products from the Refluxed Aqueous Solution of p-Hydroxybenzyl Alcohol
p-Hydroxybenzyl alcohol (10 g) were refluxed in water (250 mL) for 40 h, then the solution was concentrated under reduced pressure to yield a residue.The residue was chromatographed over reversed phase silica gel (C18, 300 g) with a gradient elution increasing MeCN in H2O (5-100%) to aff ord subfractions Fr.1-1-Fr.1-25 based on TLC and UPLC analysis.Fraction Fr.1-2 (60 mg) was further separated by PTLC (petroleum ether—EtOAc, 3:1) to yield 26 (2.5 mg).Isolation of Fr.1-3 (1.1 g) by CC over silica gel (MeOH-CHCl 2 , 20:1) aff orded 13a (1.0 g) and a mixture.The mixture was separated by PTLC (CH2Cl2—MeOH, 15:1) to give 4-hydroxybenzaldehyde (10.2 mg),14a (63.6 mg),24 (2.0 mg), and 25 (3.4 mg).Fr.1-4 (112 mg) was chromatographed over silica gel (MeOH—CHCl2, 30:1-10:1) to yield Fr.1-4-1 and Fr.1-4-2, which were separately isolated by PTLC (CH2Cl2—MeOH, 10:1) to aff ord 5a (2.6 mg),6a (2.8 mg),15 (2.6 mg), and Fr.1-4-1-1 from the former and 18 (26 mg) and 21 (22 mg) from the latter.Fr.1-4-1-1 was further separated by RP-HPLC (ph column, 60% MeCN in H2O, 2.0 mL/min) gave 17 (32 mg) and 19 (3.4 mg).Reversed-phase (C 18 ) flash chromatography of Fr.1-5 (156 mg) yielded subfractions Fr.1-5-1-Fr.1-5-6, of which of which Fr.1-5-1 (15 mg) and Fr.1-5-2 (20.0 mg) were separately isolated by RP-HPLC (PBT column, 64% MeCN in H2O, 2.0 mL/min) to yield 22 (6.1 mg) from the former and 8a (10.3 mg) and 23 (7.1 mg) from the latter.The measured spectroscopic data of the isolated compounds were identical to those reported for 4-hydroxybenzaldehyde [82], 4-hydroxy-3-(4′-hydroxybenzyl)benzyl alcohol (13a) [90], 4-(4′-hydroxybenzyl)-2-hydroxymethylphenol (14a) [91], bis(4-hydroxybenzyl)ether (15) [82], 4,4′-methylenediphenol (17) [82], 2,4-bis(4-hydroxybenzyl)phenol (18) [84], gastropolybenzylol A (19) [8], gastrol (21) [85], 4-hydroxy-3-(4′-hydroxy-3′-hydroxymethylbenzyl)benzyl alcohol (24) [91], and 4-hydroxy-3-(4′-hydroxybenzyl)benzaldehyde (25) [71], respectively.The structures of the new compounds 5a,6a,8a,22, and 23 were determined by analysis of spectroscopic data (see below) including 2D NMR spectroscopic data (Fig.2 and Supporting Information Figs.133-177).
4-Hydroxy-3-[4′-hydroxy-3′-(4″-hydroxybenzyl)benzyl] benzyl alcohol (5a): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 204 (3.94), 223 (3.58), 281 (3.00); IRνmax3351, 3020, 2960, 2920, 2850, 1611, 1539, 1510, 1440, 1365, 1259, 1174, 1106, 1033, 821, 706 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 3; (-)-HRESIMS:m/z335.1295 [M - H] - (calcd.for C21H19O4, 335.1278), 371.1061 [M + Cl] - (calcd.for C21H20O4Cl, 371.1045).
在医学生的本科学习过程中,对胃镜知识涉及的章节有限,且不作为常规教学内容,因此,研究生阶段学习胃镜的知识及操作可以帮助消化内科专业的研究生加深对消化系统疾病的认识并提高诊治能力。就目前我科室内镜室对研究生胃镜教学的现状,有以下几点思考。
4-Hydroxy-3,5-di-(4-hydroxybenzyl)benzyl alcohol (6a): white amorphous powder (MeOH); UV (MeOH)λmax (logε) 205 (4.09), 224 (3.84), 279 (3.12); IRνmax 3490, 3436, 3352, 3209, 2917, 2885, 2858, 1610, 1601, 1512, 1472, 1445, 1380, 1350, 1311, 1293, 1271, 1248, 1217, 1171, 1135, 1106, 1017, 975, 955, 914, 899, 875, 824, 793, 779, 726 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 3; (-)-HRESIMS:m/z335.1293 [M - H] - (calcd.for C21H19O4, 335.1278), 371.1058 [M + Cl] - (calcd.for C21H20O4Cl, 371.1045).
2-Hydroxy-5-[4′-hydroxy-3′-(4″-hydroxybenzyl)benzyl]benzyl alcohol (8a): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 203 (4.07), 236 (3.37), 282 (3.10), 290 (3.17); IRνmax 3320, 3230, 3022, 2956, 2911, 2842, 2710, 2593, 2484, 1644, 1613, 1511, 1437, 1361, 1264, 1243, 1201, 1155, 1140, 1120, 1103, 987, 976, 937, 913, 842, 815, 800, 772, 645 cm -1 ; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 3; (-)-HRESIMS:m/z335.1294 [M - H] - (calcd.for C21H19O4, 335.1278), 371.1058 [M + Cl] - (calcd.for C21H20O4Cl, 371.1045).
2-Hydroxy-3,5-di-[4-hydroxybenzyl]benzyl alcohol (22): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 204 (4.22), 236 (3.24), 291 (2.96); IRνmax3330, 3021, 2913, 2842, 2709, 2608, 2494, 1673, 1612, 1600, 1513, 1481, 1448, 1366, 1227, 1173, 1143, 1102, 1043, 1013, 961, 883, 831, 775 cm -1 ; 1 H NMR (acetone-d6 , 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 3; (-)-HRESIMS:m/z335.1294 [M - H] - (calcd.for C21H19O4, 335.1278), 371.1060 [M + Cl] - (calcd.for C21H20O4Cl, 371.1045).
2-Hydroxy-5-[2′-hydroxy-5′-(4″-hydroxybenzyl)benzyl]benzyl alcohol (23): white amorphous powder (MeOH); UV (MeOH)λmax(logε) 203 (4.18), 236 (3.64), 282 (3.40), 290 (3.40); IRνmax3516, 3452, 3372, 3179, 3019, 2928, 2898, 2841, 2711, 2605, 1650, 1608, 1506, 1444, 1428, 1370, 1297, 1253, 1205, 1157, 1143, 1124, 1106, 978, 961, 934, 917, 880, 841, 818, 773 cm-1; 1 H NMR (acetone-d6, 600 MHz) and 13 C NMR (acetone-d6, 150 MHz) data, see Table 3; (-)-HRESIMS:m/z335.1293 [M - H] - (calcd.for C21H19O4, 335.1278), 371.1059 [M + Cl] - (calcd.for C21H20O4Cl, 371.1045).
4.5 Preparation and UPLC-HRESIMS Analysis of the p-Hydroxybenzyl Alcohol,15 and 21 Solutions
p-Hydroxybenzyl alcohol (each 25.0 mg, purchased from Beijing Ouhe Technology CO., LTD), was dissolved in round-bottom flasks with 25.0 mL of H2O, MeOH, EtOH, respectively, and compound 15 (each 1.0 mg) or 21 (each 1.0 mg) was dissolved with 1.0 mL of the solvents.Two parallel experiments were set for each compound and solvent.The solutions were ultrasonicated (280 W) for 0.5 h, then heated in a liquid alloy bath to reflux.Two parallel experiments were set for each solvent.The solutions (each 50 μL) were sampled after ultrasonicated and at refluxing times of 0.5 h, 1.0 h, 1.5 h, 2.0 h, 4.0 h, and 6.0 h, respectively.Each the sampled H2O solution was diluted with MeCN to 1.0 mL.The sampled MeOH and EtOH extracts were diluted with MeOH and EtOH to 1.0 mL, respectively.The diluted samples were individually filtrated and the filtrates were analyzed by UPLC-HRESIMS under following conditions: Q Exactive Focus LC—MS/MS spectrometer; ACQUITY UPLC BEH C18column (1.7 μm, 2.1 × 100 mm); temperature, 25 °C; flow rate, 0.4 mL/min; gradient elution of increasing CH3CN in H2O from 5 to 45% in 15.0 min then to 100% in 1.0 min.
4.6 Preparation and UPLC/HR-ESI—MS Analysis of the Rresh G.elata Rhizomes and “tian ma” Extracts
The freshG.elatarhizomes and “tian ma” were cut into small pieces, respectively.The pieces of plant materials (each 12.0 g) were ultrasonicated (280 W) in round-bottom flasks with 30 mL of H2O, MeOH, and EtOH for 0.5 h, respectively, followed by heating in a liquid alloy bath to reflux.Two parallel experiments were set for each the plant material and solvent.The extracts (each 400 μL) were sampled after ultrasonicated and at refluxing times of 0.5 h, 1.0 h, 1.5 h, 2.0 h, 4.0 h, and 6.0 h, respectively.Each the sampled H2O extract was diluted with 65% MeCN in H2O to 1.0 mL.The sampled MeOH and EtOH extracts were diluted with MeOH and EtOH to 1.0 mL, respectively.The diluted samples were individually filtrated and the filtrates were analyzed by UPLC-HRESIMS under the above described conditions, respectively.
4.7 Protective Assay Against Rotenone-induced PC12 Cell Damage
See Ref.[79].
4.8 Protective Assay Against DL-Galactosamine (GalN)-induced WB-F344 Cell Damage
See Ref.[96].
4.9 Inhibitory Assay Against LPS-induced NO Production in Mouse Peritoneal Macrophages
See Ref.[97].
See Refs.[72,73].
AcknowledgementsFinancial support from the National Natural Sciences Foundation of China (NNSFC; Grant Nos.81730093, 81630094, and 81502942), CAMS Innovation Fund for Medical Science of China (2017-I2M-3-010 and 2016-I2M-1-004), and the Drug Innovation Major Project (2018ZX09711001-001, China) is acknowledged is acknowledged.
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflicts of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
猜你喜欢
杂志排行
Natural Products and Bioprospecting的其它文章
- In Memory of the Late Professor Jun Zhou (1932-2020)
- In Mourning and Memory of Late Professor Zhou Jun
- Natural Products in Cancer Therapy: Past, Present and Future
- On the Famous Traditional Chinese Medicine “Fu Zi”: Discovery, Research, and Development of Cardioactive Constituent Mesaconine
- The Outlook of the Development of Innovative Products from Biocompatible Natural Spider Silk in the Beauty Thread-Lifting Industry
- Neolignans and Norlignans from Insect Medicine Polyphaga plancyi and Their Biological Activities
