Review of operative considerations in spinal cord stem cell therapy
2021-03-11PavanUpadhyayulaJoelMartinRobertRennertJosephCiacci
Pavan S Upadhyayula, Joel R Martin, Robert C Rennert, Joseph D Ciacci
Pavan S Upadhyayula, Joel R Martin, Robert C Rennert, Joseph D Ciacci, Department of Neurological Surgery, University of California, San Diego, La Jolla, CA 92037, United States
Abstract Spinal cord injury (SCI) can permanently impair motor and sensory function and has a devastating cost to patients and the United States healthcare system.Stem cell transplantation for treatment of SCI is a new technique aimed at creating biological functional recovery.Operative techniques in stem cell transplantation for SCI are varied.We review various clinical treatment paradigms, surgical techniques and technical considerations important in SCI treatment.The NCBI PubMed database was queried for “SCI” and “stem cell” with a filter placed for “clinical trials”.Thirty-nine articles resulted from the search and 29 were included and evaluated by study authors.A total of 10 articles were excluded (9 not SCI focused or transplantation focused, 1 canine model).Key considerations for stem cell transplantation include method of delivery (intravenous, intrathecal, intramedullary, or excision and engraftment), time course of treatment, number of treatments and time from injury until treatment.There are no phase III clinical trials yet, but decreased time from injury to treatment and a greater number of stem cell injections both seem to increase the chance of functional recovery.
Key Words: Stem cell; Spinal cord injury; Operative techniques; Stem cell transplantation; Intramedullary
INTRODUCTION
Spinal cord injury (SCI) is an acute traumatic event that impairs patients’ motor and sensory function; SCI is both debilitating for the individual patient as well as for the healthcare system as a whole.Although inconsistently reported, the global prevalence of SCI ranges between 236 to 1009permillion[1].In the United States this corresponds to an incidence of between 12000 and 20000 cases with an annual total cost of almost 10 billion dollars[2].The pathophysiology of SCI involves a primary traumatic insult followed by a secondary cascade characterized by immune activation, proinflammatory mediators, edema, ischemia, reactive oxygen species generation and loss of membrane integrity[3].The primary and secondary cascade combine to create profound neurological deficits that impair normal function.
Accordingly, SCI treatment is aimed at functional and neurological recovery.Given that the functional deficits stem from neuronal damage, a major focus in this field is the regeneration of nerve tissue.To achieve this end many preclinical and clinical trials using stem cell-based therapies have begun[4].All clinical trials to date have delivered stem cellsvia3 routes: (1) Intrathecal/Intradural; (2) Intramedullary; or (3) Intravenous.
In this review we will highlight the operative considerations associated with SCI stem cell transplantation to identify common elements that may underlie the success of any given intervention.Special attention will be given to the injection site, method of delivery and treatment algorithms.
THE CURRENT LANDSCAPE OF CLINICAL INVESTIGATION INTO STEM CELL THERAPIES FOR SCI
Stem cell-based treatment for SCI is a topic of increasing clinical investigation.Currently, 18 clinical trials are registered as completed on clinicaltrials.gov with as many as 37 others recruiting patients.These span the gamut between Phase I and Phase III clinical trials.To date, most data show that stem cell injection into the spinal cord is safe with minimal side effects.While the first human trial in 2010 used human epithelial serous cystadenocarcinoma oligodendrocyte progenitor cells injected at the lesion site[5], current clinical investigations use a host of different stem cell types.These types generally fall into three broad groups: Embryonic stem cells, mesenchymal stem cells, or neural-derived stem cells.The most common group, mesenchymal stem cells, can be harvested from many sites including bone marrow-mesenchymal stem cells (BM-MSC), umbilical cord-MSC or adipose tissue-MSC[6].The pros and cons of these different stem cells have been greatly debated[4].
To provide an accurate summary, the database of clinical trials, clinicaltrials.gov was examined for any SCI studies using stem cells.The search criteria: “SCI and stem cell” was used and completed studies were examined.Furthermore, the NCBI PubMed database was searched for “SCI” and “stem cell” with a filter placed for “clinical trials”.A total of 39 articles resulted, with 29 articles being relevant to the question at hand (9 articles were not focused on SCI/stem cell transplantation, 1 nonhuman model).The relevant data are summarized below with the articles summarized in Table 1.Where motor improvement is reported, this references changes in key muscle group function based on a five point grading scale as standard in SCI literature.Follow-up across all clinical trials reporting functional outcomes,vssafety profile, was a minimum of 10 mo.
TECHNICAL CONSIDERATIONS IN STEM CELL DELIVERY
Stem cell transplant techniques
Across the many clinical trials examining the use of stem cells (SCs) in the treatment of SCI, there are a few key technical considerations.The first is the method of injection.Intravenous and intrathecal injection have relatively few technical considerations since IV injection and lumbar puncture are routine procedures.Intramedullary injection of stem cells, however, poses a greater challenge.The spinal cord generally oscillates with respiration and cardiac pulsation.Studies in animals show that the pulsatile nature of the cord is increased following increases in blood pressure and that it is not due to cerebrospinal fluid based wave transmission.While respiratory oscillation causes greatest spinal cord movement in the thoracic spine, the genuine spinal cord pulsation is thought to be driven by the blood flow through radicular arteries[7].This spinal cord pulsation may pose an issue with intramedullary injection techniques.In general there are two canonical ways of achieving intramedullary spinal cord injections: The free hand technique, and a stereotactic technique utilizing a bed mounted frame.It is important to note that the stereotactic technique, as it is mounted to the bed, does not necessarily improve stability in relation to spinal cord pulsation.A recent study by Leviet al[8]described the use of a free hand technique.Specifically, to achieve an injection depth of 3-5 mm they marked the injection needle using a rongeur or silicone tip and had the surgeon stabilize the needle using two hands anchored at the edge of the surgical field.The injection time, which was a maximum of 3 min and 30 sperinjection, required the use of two hands for stabilization[8].
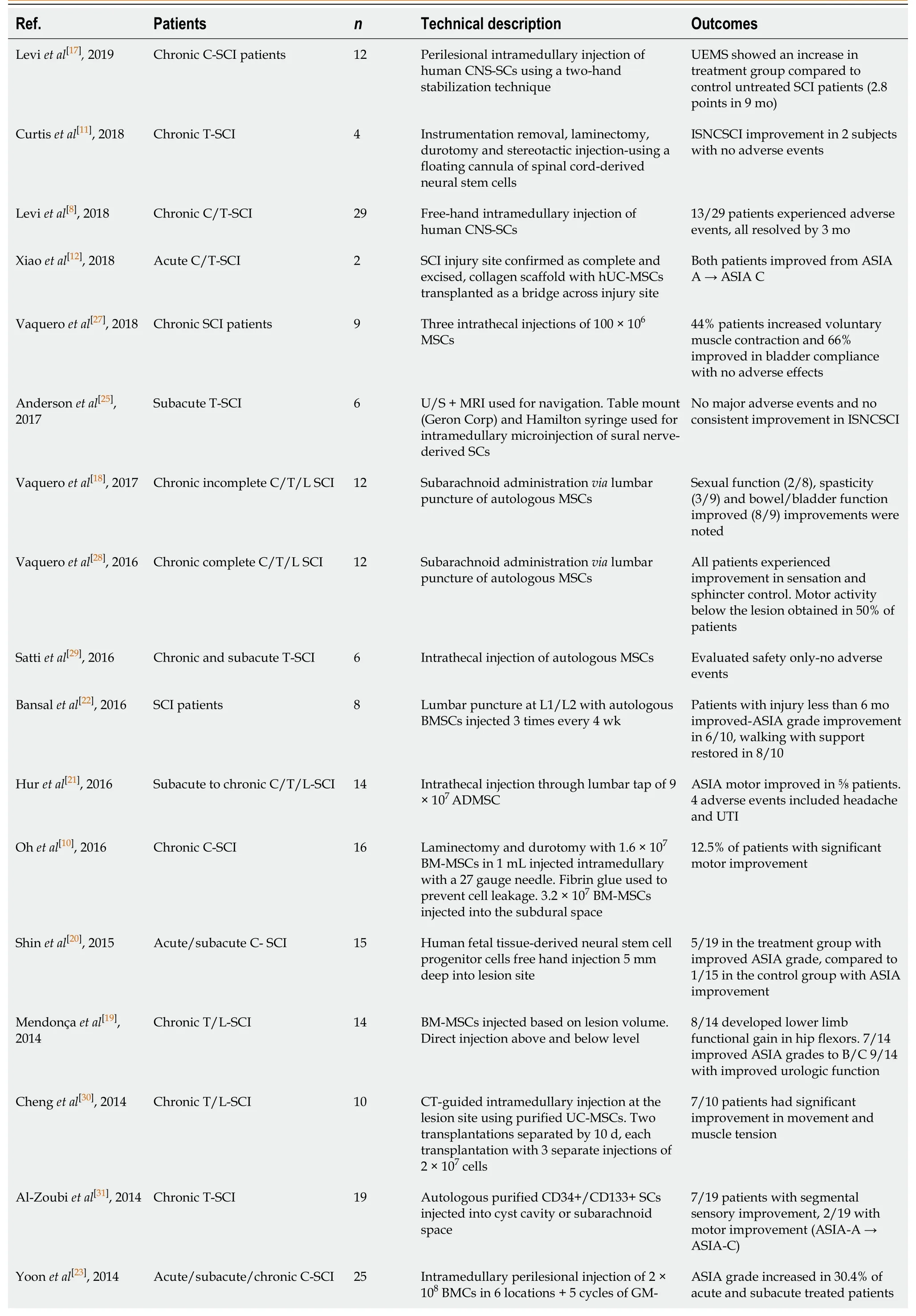
Table 1 Summary of clinical trials using stem cell therapy in spinal cord injury

C: Cervical; T: Thoracic; L: Lumbar; ISNCSCI: International Standards for the Neurological Classification of Spinal Cord Injury; ASIA: American Spinal Injury Association Impairment Scale; MSC: Mesenchymal stem cells; ADMSC: Adipose derived mesenchymal stem cells; BMC: Bone marrow cells; BMMSCs: Bone marrow-derived mesenchymal stem cells; SEP: Somatosensory evoked potentials; MEP: Motor evoked potentials; HSC: Hematopoietic stem cell; GM-CSF: Granulocyte macrophage colony stimulating factor; Subq: Subcutaneous; LT/PP: Light touch/pinprick; VAC: Voluntary anal contraction; SCI: Spinal cord injury; UEMS: The European Union of Medical Specialists; CT: Computed tomography; EMG: Electromyography; OESC: Ovarian epithelial serous cystadenocarcinoma; UIT: U-shaped skin incision technique; CNS: Central nervous system; UC: Ulcerative colitis; UE: Upper-extremity; MRI: Magnetic resonance imaging.
Although no serious complications occurred in this trial or others that used a freehand technique[9,10], the damage to the spinal cord may be obfuscated by the presence of preexisting pathology.Moreover, the need for stabilization may limit the maximum injection time and thereby the amount of stem cells able to be transplanted.Curtiset al[11]described a novel technique using a floating cannula that is able to address these theoretical issues.This cannula is attached to an XYZ manipulator mounted directly on a patient's vertebral column.The cannula is able to move with pulsation of the spinal cord making long term injections feasible with minimal damage to the existing spinal cord tissue[11].
Whether the preservation of existing spinal cord tissue is technically necessary is up for debate.While the previously described studies focused on chronic SCI, a study by Xiaoet al[12]in acute SCI patients completely excised the necrotic spinal cord around the SCI lesion.The authors placed a collagen scaffold impregnated with human umbilical cord MSCs in this area and showed nerve conduction across the SCI lesion plus functional improvement from American Spinal Injury Association Impairment Scale (ASIA) A to ASIA C in two patients[12].This study was built on previous work by Limaet al[13], where necrotic scar tissue was excised and an olfactory mucosal autograft was placed.Importantly, this radical technique of laminectomy, scar excision and mucosal autograft replacement in chronic cervical and thoracic SCI led to marked functional improvement.In the earlier study by Limaet al[13], 28% of patients had recovery of bladder sensation or voluntary anal contraction[13], while in the more recent study 55% of patients had ASIA improvement of at least 1 grade[14].Importantly, this second study involved intensive rehabilitation following autograft transplantation.It is important to note that olfactory mucosa grafts have been associated with spinal masses pointing to their increased regenerative potential but also to their increased side effect profile[15,16].As a technical consideration, excision of scar tissue may allow for increased regeneration of neural white matter tracts and may be an important technical consideration even with other injection techniques that do not use a graft substrate or scaffold.
Number of treatments
Although some studies of SCI show improvement following a single stem cell injection[11,17], studies with multiple injections spaced out over time seem to have greater improvements in outcomes[8,18].A comparison of two trials conducted by the same group, Ohet al[10]and Parket al[9], further illustrated this point.The original study, by Parket al[9], was a Phase I single-arm study and included the injection of BMMSCs derived from iliac crest grafts into both the intramedullary and subdural space with additional injections into the thecal space using lumbar puncture at 4 wk and 8 wk following the initial operation.Six of 10 patients showed motor improvement and 3 showed gradual improvement in activities of daily living[9].The follow-up study, by Ohet al[10], was a Phase III clinical trial and the study authors used the same initial injection treatment paradigm with no follow-up injections.The one time injection yielded poor functional improvement (12.5% with improved motor outcomes) compared to the multiple injection protocol (60% with motor improvement or improvement on Activities of Daily Living).Although limited, these data point to the importance of optimizing chronicity of SC injection in SCI patients.Importantly, while 5/16 patients had diffusion tensor imaging magnetic resonance imaging changes showing tracts spanning the SCI level in the single injection Phase III clinical trial compared to 7/10 showing such changes in the pilot study[10].It is difficult to associate the differences solely due to the presence of multiple treatments.Multiple other studies have shown improvements in ASIA scores, motor function and urodynamics with only a single stem-cell treatment[19,20].Furthermore, understanding the number of cells transplanted and its effect on outcomes is more difficult.Various trials have transplanted cell numbers ranging from 1 × 105to 40 × 107.There was no consistent relationship between cell number and outcome over the trials reported.It remains to be seen whether a multiple treatmentvssingle treatment protocol could improve functional recovery in a large-scale clinical trial.
Time from injury to treatment
Generally, SCI is characterized based on chronicity into acute, subacute and chronic phases.While the time course for each period is highly variable, the acute phase generally spans days post-injury, the subacute phase weeks post-injury and the chronic phase months post-injury.Although some studies on chronic SCI show improvement following stem cell injection[8,11], studies in the acute or subacute SCI population seem to show a more dramatic return of function[10,21,22].A study by Yoonet al[23]stratified patients into acute (< 13 d), subacute (14 d to 8 wk) and chronic (> 8 wk) groups with all patients receiving intramedullary injection of BM-MSCs with systemic granulocyte macrophage colony stimulating factor treatment.Notably, 30.4% of the acute and subacute treated patients had improved ASIA grade (ASIA A to Asia B/C), while none of the chronic patients showed any improvement[23].This was not limited to intramedullary cell transplantation.Bansalet al[22]demonstrated that lumbar puncture for delivery of BM-MSCs led to ASIA grade improvement in 6/10 patients who were less than 6 mo from injury with no patients over 6 mo from injury achieving functional improvement[22].
A study by Shinet al[20], used human fetal tissue-derived neural stem cell progenitor cells and included both a control group and an intramedullary cell transplantation group.Notably, while 26% of patients in the transplantation group had ASIA grade improvement, only 6.6% had improvement in the control group[20].This disparity between transplantation and control group patients was also seen by Karamouzianet al[24].These authors injected purified BM-MSCsvialumbar puncture and noted that 45 patients in the cell transplantation group had ASIA improvement compared to 15% in the control group (P= 0.09)[24].This helps to answer the major critique that some degree of functional improvement occurs in the acute/subacute period naturally and may explain the functional improvements seen in the acute to subacute cell transplantation studies.Also, the fact that both studies with control groups used different stem cell types and different methods of injection (lumbar puncturevsintramedullary injection) also highlights the importance of the treatment windowvsmechanism of treatment.This is further supported by the studies carried out by Bansalet al[22]and Yoonet al[23].These studies had patients in multiple treatment windows, with intramedullary or lumbar puncture delivered BM-MSCs and noted that patients in the acute to subacute period from injury achieved greater functional improvement[22,23].In fact, of the studies that focused on the acute/subacute SCI population, only one out of seven studies showed no motor or functional improvement with the rest having a subset of patients that had ASIA grade improvement.The single study with no functional improvement notably used a novel form of stem cells derived from peripheral Schwann cells and included subjects from 4-7 wk following injury-generally on the upper limit as compared to other acute/subacute studies[25].
FUTURE DIRECTIONS
In summary, clinical studies to date have highlighted a few key findings.First, that stem cell injection is generally well tolerated with a minimal side effect profile when appropriately dosed.The use of stem cell treatment in SCI may lead to functional improvement when intervention is performed in the acute to subacute treatment window and when multiple treatment injections are utilized.A few pre-clinical studies are examining devices that could facilitate intramedullary stem cell injection.One such device tested in rodents and pigs by Kutikovet al[26]injects cells in a trail creating longitudinal tracts of neural stem cellsvsisolated injection sites.In doing so they demonstrated a novel technique for stem cell injections able to create new tracts that span multiple spinal cord levels[26].Continued technological development could help facilitate intramedullary stem cell injection over longer periods of time, thereby obviating the greatest risk to this treatment, the need for surgical delivery.The aim of this manuscript is to help optimize clinical trial parameters, patient selection and cell transplantation techniques.Comparative clinical trials using different types of stem cells are necessary to determine what type of cells are most efficacious in improving functional outcomes in patients.
CONCLUSION
Stem cell treatment for SCI is a burgeoning field.While numerous studies have focused on the biological aspect of this treatment, technical challenges remain.Time from injury to treatment, the duration and chronicity of treatment and the actual delivery of cells are important considerations.Currently, the lack of phase III clinical trials directly studying these factors makes it difficult to draw conclusions.Early evidence seems to suggest that longer treatment paradigms soon after injury may be most beneficial.Finally, operative techniques and devices that can effectively target the intramedullary space could help with stem cell delivery and functional recovery following treatment.
杂志排行
World Journal of Stem Cells的其它文章
- Prior transfusion of umbilical cord mesenchymal stem cells can effectively alleviate symptoms of motion sickness in mice through interleukin 10 secretion
- Platelet-rich plasma vs bone marrow aspirate concentrate: An overview of mechanisms of action and orthobiologic synergistic effects
- Targeting mesenchymal stem cell therapy for severe pneumonia patients
