Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review
2021-03-11MarianaMirandaSampaioMariaLusaCordeiroSantosHannaSantosMarquesVinciusLimadeSouzaGonalvesGlauberRochaLimaArajoLuanaWeberLopesJonathanSantosApolonioCamiloSantanaSilvaLuanaKauanydeSantosBeatrizRochaCuzzuolQuziaEstfan
Mariana Miranda Sampaio, Maria Luísa Cordeiro Santos, Hanna Santos Marques, Vinícius Lima de Souza Gonçalves, Glauber Rocha Lima Araújo, Luana Weber Lopes, Jonathan Santos Apolonio, Camilo Santana Silva, Luana Kauany de Sá Santos, Beatriz Rocha Cuzzuol, Quézia Estéfani Silva Guimarães, Mariana Novaes Santos, Breno Bittencourt de Brito, Filipe Antônio França da Silva, Márcio Vasconcelos Oliveira, Cláudio Lima Souza, Fabrício Freire de Melo
Mariana Miranda Sampaio, Maria Luísa Cordeiro Santos, Glauber Rocha Lima Araújo, Luana Weber Lopes, Jonathan Santos Apolonio, Camilo Santana Silva, Luana Kauany de Sá Santos,Beatriz Rocha Cuzzuol, Quézia Estéfani Silva Guimarães, Mariana Novaes Santos, Breno Bittencourt de Brito, Filipe Antônio França da Silva, Márcio Vasconcelos Oliveira, Cláudio Lima Souza, Fabrício Freire de Melo, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil
Hanna Santos Marques, Vinícius Lima de Souza Gonçalves, Campus Vitória da Conquista,Universidade Estadual do Sudoeste da Bahia, Vitória da Conquista 45083-900, Bahia, Brazil
Abstract Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm and was the first neoplastic disease associated with a well-defined genotypic anomaly - the presence of the Philadelphia chromosome. The advances in cytogenetic and molecular assays are of great importance to the diagnosis, prognosis, treatment,and monitoring of CML. The discovery of the breakpoint cluster region (BCR)-Abelson murine leukemia (ABL) 1 fusion oncogene has revolutionized the treatment of CML patients by allowing the development of targeted drugs that inhibit the tyrosine kinase activity of the BCR-ABL oncoprotein. Tyrosine kinase inhibitors(known as TKIs) are the standard therapy for CML and greatly increase the survival rates, despite adverse effects and the odds of residual disease after discontinuation of treatment. As therapeutic alternatives, the subsequent TKIs lead to faster and deeper molecular remissions; however, with the emergence of resistance to these drugs, immunotherapy appears as an alternative, which may have a cure potential in these patients. Against this background, this article aims at providing an overview on CML clinical management and a summary on the main targeted drugs available in that context.
Key Words: Chronic myeloid leukemia; Breakpoint cluster region-Abelson murine leukemia; Immunotherapy; Tyrosine kinase inhibitors; Philadelphia chromosome;Diagnosis
INTRODUCTION
Chronic myeloid leukemia (CML) is a malignant myeloproliferative disorder characterized by a clonal hematopoietic stem cell proliferation. CML was the first malignant disease related to a cytogenetic abnormality[1]and its pathogenesis has been extensively studied. The advances in this field allowed the development of targeted therapies with tyrosine kinase inhibitors (TKIs) with high rates of therapeutic success,increasing substantially patient survival and disease prevalence[2]. As for its incidence,CML affects about 0.7-1.0/100000 individuals per year and this rate has been stable over the last few years. With regard to the sociodemographic profile of CML patients,there is a slightly higher predominance among men, and the diagnosis usually occurs around the sixth or seventh decade of life[3].
CML is characterized by the presence of the Philadelphia chromosome, which is the result of a balanced reciprocal translocation between the long arms of 9 and 22 chromosomes [t (9; 22) (q34; q11)]. The fusion of theAbelson murine leukemia(ABL) gene on chromosome 9 with thebreakpoint cluster region (BCR)gene on chromosome 22 results in theBCR-ABL1fusion gene, which encodes the BCR-ABL oncoprotein[4]. This protein is a persistently active tyrosine kinase that promotes unrestricted replication,inadequate differentiation, and resistance to apoptosis[4,5]. The continuous proliferation of these stem cells with a high capacity for differentiation favors the appearance of additional mutations that can provide resistance to standard treatment representing a negative impact on prognosis[6].
Despite the high success rate in treatments with TKIs, the emergence of resistance to TKIs has led to the development of new drugs and immunotherapy has been considered as an alternative to these patients, aiming to reduce disease recurrence and chronic use of medication[7].
CML: A BRIEF HISTORIC REVIEW
CML was the first leukemia discovered, being described around 1840 by David Craigie, John Hughes Bennet, and Rudolph Virchow through autopsies on individuals who had presented with very similar findings, such as hepatosplenomegaly, fever,leukocytosis[8], and an unusual blood appearance and consistency, described by Alfred Velpeau in the 19thcentury as “thick blood”[9]. There were several interpretations concerning such blood aspects throughout the years. Initially, some specialists attributed the thick blood to the presence of pus due to some infectious process[9,10];however, such condition was not diagnosable even with autopsy. The explanation that refuted the purulent blood theory came from Alfred Donné. He detected a large amount of white blood cells, resulting from an interruption in the hematopoietic cells maturation[9]. In 1845, John Hughes Bennett hypothesized that an infection generates what he called leukocytemia (white cell blood), due to the large amount of white blood cells found during the autopsy, and Virchow, in his turn, inferred that the disease is caused by hematopoietic changes, coining the term “weisses blut”-white blood(Leukemia - leukamie in German)[8,11].
In 1960, David Hungerford and Peter Nowell, two cytogenetics scholars, joined to determine if leukemia was linked to specific chromosomal abnormalities[12]. It was the first time that an association between an oncological disease and a chromosomal abnormality was established[13]. They identified the presence of a minute chromosome in two CML patients, which they called the “Philadelphia chromosome” (Ph) and,later, Janet Rowley refined this discovery by proving in 1973 that it was a balanced reciprocal translocation between the long arms on chromosomes 9 and 22: t (9; 22)(q34; q11)[14]. Currently, it is known that the Ph chromosome is not restricted to CML,and it can be found, especially in its p190 isoform, in individuals with acute lymphoblastic leukemia (ALL)[15]. However, the identification of this chromosome remains as an important cytogenetic marker of CML and its detection has implications for the diagnosis, prognosis, and treatment of the disease[16].
In addition to the genetic cause, in 1970, the possibility of leukemia also having a viral etiology was investigated based on the research by Herbert Abelson and Louise Rabstein, who identified theABLgene in a murine virus and its physiological counterpart found in normal human cells[15,11]. From a retroviral infection of hematopoietic stem cells withBCR-ABL1P210 in mice, it was discovered that the fusion of a region of theBCRgene on chromosome 22 with another part of theABL1gene on chromosome 9 results in balanced reciprocal translocation t (9; 22),demonstrating a disease induction similar to CML[17]. This fusion leads to the translation of proteins with high tyrosine kinase activity, culminating in uncontrolled signaling pathways and cell cycles of primitive leukocyte lines, which increases cell proliferation and inhibits apoptotic mechanisms[9,11,13,18].
PATHOGENESIS
Ph chromosome-related CML
The aforementioned mutation results in the production of oncoproteins[19]. In addition,epidemiological data describe that about 90%-98% of CML patients have this mutation[t (9;22) (q34; q11.2)], making the Ph chromosome the main pathological theory for the disease[20,21].
Notably, different proportions of that translocation may be reflected in different clinical presentations. These different phenotypes are caused by a variation in the BCRs, a process that can occur with the different proteins of theABLgene: P210, P190,and P230. In this sense, it is noticed that the phenotypeBCR-ABL1P210 is more related to CML (95%) than the phenotypeBCR-ABL1P190, which is better associated with Blymphoblastic leukemia/lymphoma (B-ALL/LBL), although it is found in about 1% of CML cases[22]. As for the P230 protein, it has been rarely described in the literature[21,23].However, so far there is no clear elucidation of how these different phenotypes related to thePhgene reflect different processes of leukemogenesis.
Regarding BCR-ABL-related neoplastic effects, there is an influence of this protein on several cell growth pathways, including RAS-RAF1-MEK-ERK, PI3K-AKT, and SFKs-STAT1-STAT5[24]. The Ph chromosome product revokes the need to stimulate the activation of these pathways, leading to uncontrolled and exacerbated cell growth and division[21]. Among the cellular effects generated by this neoplastic process, are the increase in the number of reactive oxygen species, breakage and damage in the repair of the DNA strand, lack of control of the typical cell cycle, dysregulation of cell adhesion, and inhibition of apoptosis and autophagy[25]. In addition, these changes make the initially mutated site more susceptible to further mutations, facilitating the progression of the disease[21]. In this sense, studies show that patients with CML mostly start from a single mutation, represented by the Ph chromosome, and, as far as the disease progresses, the rates of additional chromosomal abnormalities become higher(30%-70%)[26]. In this scenario, the natural history of the disease is slow to evolve during the chronic phase (CP), which, after the aforementioned events associated with the mutation site, gives rise to a phase of accelerated progression and a blast crisis(BC), also called blast phase[22].
The mechanisms that trigger the BC are not fully understood. This phase is characterized by a marked proliferation of undifferentiated hematopoietic progenitors that can invade peripheral blood, and the role of theBCR-ABL1mutation appears to be less preponderant in this context. Moreover, higher rates of therapeutic failure with TKIs are observed among affected individuals and additional mutations are found in about 80% of these patients, such as double Ph chromosome, trisomy 8 or 19,isochromosome 17, and other mutations that may present alone or in combination. On the other hand, the presence of the activeBCR-ABL1mutation seems to be necessary to trigger the BC, since it is uncommon in patients with good therapeutic response to TKIs; moreover, changes in cell division and differentiation promoted by this mutation may contribute to that process[27,28]. The main genetic abnormalities present in the stem cells associated with the BC are the higher expression ofMYCproto-oncogene,inactivating mutations of p53, and increased β-catenin signaling, which play prominent roles on CML expansion and BC transformation[28].
Immunobiology of myeloid leukemia
The host immune system can be an important factor in disease progression and relapses by suppressing the residual leukemic cells that remain after molecular remission. In patients with CML, before treatment, an increased population of Treg cells and abnormal activation of the programmed death 1 (PD-1)/programmed death receptor ligand 1 (PD-L1) signaling is observed. The PD-L1 present in CML cells binds to PD-1 receptor on T cells, reducing its effector function[7,29]. The increased production of arginase I, common to several types of cancer and chronic diseases, by myeloidderived suppressor cells results in the accelerated metabolism of L-arginine, an essential amino acid to the normal function of T cells. These factors result in a downregulation of the immune response[29,30].
The important role of the immune system in the prevention and control of CML is corroborated by the fact that, after allogeneic transplantation of pluripotent hematopoietic stem cells and lymphocyte infusion, the T cells eliminate the tumor by interacting with the tissue-restricted minor histocompatibility complex and with the leukemia-associated antigens. Furthermore,BCR-ABL1-positive healthy individuals are protected by immunosurveillance, although the mutation does not occur in the stem cells of these patients[31].
DIAGNOSIS
The diagnosis of CML is based on anamnesis, physical examination, and laboratory data, including cytogenetic and molecular tests. Through the association of these information, it is possible to identify the disease stage and to direct the most appropriate monitoring and treatment[32,33].
The diagnosis of CML usually occurs during routine medical appointments or blood tests in asymptomatic individuals[34]. About 30% to 50% of patients diagnosed with CML in the United States are asymptomatic[5,35]. The absence of symptoms is more common in the first of the three phases of the disease, the CP. Most CML diagnoses occur during this period, approximately 85%, with 40% being asymptomatic[2]. Right after the CP, most patients can progress to an accelerated phase (AP), and then the socalled BC can occur. However, approximately 20% of individuals progress directly from the CP to a BC, without any typical manifestation of the AP[5].
During the CP, the individual's immune system, if competent, maintains an asymptomatic status over a long period[36]. However, the CP can also manifest with symptoms such as anemia, splenomegaly, fatigue, weight loss, malaise, easy satiety and fullness, or pain in the upper left quadrant can occur. The less common manifestations during a CP are priapism, bleeding, thrombosis, retinal hemorrhage,and hepatomegaly[37]. Splenomegaly is the most common physical condition associated with CML, found in 50%-60% of the cases, whereas hepatomegaly develops in only 10%-20% of the patients[35,38].
During AP, the patients present with more severe symptoms that can include bone pain, skin infiltrate, lymphadenopathy, and worsening of the anemia[5,36]. Moreover,fever, arthralgia, and abdominal pain may also occur as results of splenic infarction[35].The BC manifests as an acute leukemia with worsening of the symptoms present in the previous stages with bleeding, worsening fever, and secondary infections[5].
Atypical CML (aCML) affects about 5% of patients with CML, and includes absence of Ph chromosome and negativeBCR-ABL1rearrangement, leukocytosis with left shift,splenomegaly, and marked myeloid dysplasia. These patients usually have a more unfavorable prognosis with poor response to treatment[39,40].
Considering that most patients are asymptomatic and the need for differential diagnosis with other hematological and systemic conditions, some tests are essential for the diagnosis of CML, such as blood count, bone marrow (BM) aspirate,cytogenetics, karyotyping, and qualitative and quantitative PCR[37]. The diagnostic workup, according to the latest European Leukemia Net (ELN) recommendations,should include physical examination with emphasis on the presence of hepatomegaly or splenomegaly, electrocardiogram, biochemical profile, complete blood cell count and differential count, BM aspirate for morphology, and cytogenetics performed by chromosome banding analysis of Giemsa-stained metaphases as well as molecular study, preferably through reverse transcriptase polymerase chain reaction (RT-PCR) to detect, typify, and quantify theBCR-ABL1transcripts. The molecular testing is becoming widely available, replacing the cytogenetic monitoring[41].
Blood count
Blood count during the CP shows leukocytosis with left-shifted myeloid maturation,revealing immature basophilic and eosinophilic myelocytes and metamyelocytes[2,36].Also, the platelet count can vary, being high or low, and anemia can also occur[36]. The low phagocytic activity of granulocytes is typical of CML and differentiates the condition from other chronic myeloproliferative disorders[38].
To consider that a case of CML is in its AP, the blood count must present, according to the World Health Organization (WHO), at least one of the following criteria: 10%-19% of blasts, ≥ 20% of basophils, splenomegaly or an altered platelet count not attributable to treatment, persistence or increase of the white blood cell (WBC) count(> 10 × 109/L) during treatment, or even resistance-related parameters[42]. ELN defines such condition as the presence of 15%-29% of peripheral blood blasts or more promising peripheral blood blocks > 30% with blasts < 30%, or a ≥ 20% growth in basophils and platelet counts that is not attributed to treatment[38].
According to WHO criteria, BC is defined by extramedullary accumulation of blasts or a ≥ 20% proportion of blasts in peripheral blood or BM[42], or ≥ 30% of blasts,according to ELN criteria[38].
Molecular findings in aCML can include anemia, thrombocytopenia, and low incidence of basophilia[39]. The WHO criteria for aCML includes leukocytosis (WBC count ≥ 13 × 109/L) due to increase of neutrophils and their precursors with dysgranulopoiesis, neutrophil precursors ≥ 10% of leukocytes, minimal absolute basophilia (< 2% of leukocytes), no or minimal absolute monocytosis (usually < 10% of leukocytes), and blasts < 20% in the blood and BM[42]. The criteria also includes hypercellular BM with granulocytic proliferation and dysplasia, which can also occur in erythroid or megakaryocytic lineages, and the differential diagnosis with primary myelofibrosis, polycythemia vera, or essential thrombocythemia[42-44].
BM aspirate
BM aspirate is mandatory in all cases of suspected CML. The test can provide very important information, which allow for confirmation of the diagnosis and to determine the disease staging based on the percentages of blasts and basophils[5].Besides, BM aspirate also allows analyses for the percentages of promyelocytes,myelocytes, and eosinophils[2]. According to the WHO criteria, the presence of 10%-19% of blasts in the BM is characteristic of CML in an AP[42]. The ELN criteria, on the other hand, characterizes an AP based on the presence of 15%-29% of blasts in BM or blasts plus promyelocytes > 30%, with blasts < 30%[43].
Cytogenetics
Regarding cytogenetics, an important finding is the hybrid geneBCR-ABL1and/or the t (9; 22) (q34.1; q11.21), which is pathognomonic for CML. The Ph chromosome is present in 95% of the CML patients and variant Ph chromosome translocations can involve three or more chromosomes[45]. Moreover, cytogenetic is useful to detect additional genetic abnormalities, being important to monitor the clonal evolution and CML progression. Secondary abnormalities usually include trisomy 8, isochromosome 17, and duplicate Ph chromosome, besides other abnormalities in small frequency,such as trisomy 19, trisomy 21, trisomy 17, and deletion 7[37,46].
Morriset al[47]described variant Ph translocations, involvingBCR, ABL1, and additional chromosome sites, but the prognosis ofBCR-ABL1variants is still controversial. The trial informs that patients with apparently normal karyotype can have theBCR-ABL1fusion gene only detected by more sensitive molecular techniques[47,48]. The presence of t (9; 22) (q34; q11.2) confirms the diagnosis, whereas additional abnormalities indicate CML progression. Possible variant Ph translocation and complex BCR-ABL1 rearrangements require fluorescence in situ hybridization(FISH) analysis to confirm the clinical condition[47]. Cytogenetics is the gold standard for Ph chromosome detection, despite its low sensitivity (about 1%-5% of Ph-positive cells), the need for BM, and cell culture, which may result in delay of diagnosis[49]. This process is used in most medical centers and clinical trials, especially to detect additional chromosome abnormalities and clonal evolution. Unfortunately,karyotyping is still laborious, expensive and time consuming[50,51].
BM analysis by cytogenetics can also be used in the diagnosis of a BC, being an important predictor of the transformation of blasts. Besides, flow cytometry or cytochemistry can be used to define if the BC is of the lymphoid or myeloid type[52].
Cytogenetics in aCML can also show abnormalities, even though there is no specific molecular abnormality for that condition and multiple mutations are often present in various combinations[53]. The cytogenetics assists in the differential diagnosis with chronic neutrophilic leukemia and other myelodysplastic conditions, but clinical and laboratory findings, as mentioned above, are preponderant[54].
FISH
The FISH analysis relies on the colocalization of large genomic probes specific toBCRABL1genes and rapidly identifies specific genomic abnormalities, detectingBCR-ABL1rearrangements when cytogenetics is negative or when metaphase cells are not obtained[51,55]. The FISH technique is very useful in cases that lack cytogenetic evidence,revealingBCR-ABL1rearrangements and undetected t (9; 22) in an apparently normal karyotype, documenting Ph-negative rearrangements[55]. Among these genomic abnormalities, the prognosis and treatment seem to be similar, and the impact on survival is still unclear[56].
When combined with cytogenetics, the FISH can provide relevant information, such as deletions,BCR-ABL1fusion gene, and other translocations. Nevertheless, it is not able to monitor clonal evolution as cytogenetics does and its results depend on high quality commercial probes. The FISH is commonly used in medical centers and presents a low false-positive rate[49]. Nonetheless, FISH test is expensive and not widely available, requiring equipment and trained staff able to perform and interpret the test result[57,58].
Huntlyet al[59], in their study, divided the FISH technique into two available types.Extra-signal BCR-ABL FISH probes flank the breakpoint ofBCR-ABL1gene and cover 5´ABL genes. It promotes the detection ofBCR-ABL1on the derivative chromosome 22 and reveals deletions on der(9) chromosome. Double-color Double FusionBCR-ABL1FISH probes detect the fusion genes on chromosomes 9 and 22, revealing in 5’ABLand 3´BCRdeletion on der(9)[59]. In this method, only larger deletions are found. FISH analysis is faster than using karyotype due to its independence of cell culture[51].
RT-PCR
RT-PCR technology is mainly used to determine the frequency ofBCR-ABL1fusion transcript variants, detecting the M-bcr transcripts[49]. The RT-PCR is a technique that amplifies the region around the splice junction betweenBCRandABL, detecting minimal residual disease[5], which is fundamental for CML diagnosis and monitoring.RT-PCR can be divided into qualitative, useful for diagnosis, and quantitative, ideal for monitoring residual disease[55]. Baccaraniet al[60]showed that residual leukemia cells are only detected through RT-PCR[60], which emphasizes the importance of the method in these scenarios.
This technique is ideal for Ph-negative CML, detectingBCR-ABL1transcripts, and when the karyotype tests cannot be done. The RT-PCR does not face certain cytogenetic limitations, such as the dependence on BM metaphases and cell proliferation. Multiplex RT-PCR is also able to identify the molecular configuration profile. Furthermore, diagnosing Ph-negative patients withBCR-ABL1rearrangements is indispensable since the treatment options in that context are very similar to the ones available for Ph-positive CML. Multiplex RT-PCR uses peripheral blood, avoiding BM punctures, and it is faster than cytogenetics and conventional PCR, which favors the use of this technique[49,61]. However, RT-PCR standardization requires appropriate equipment, reagents, calibrators, and staff training, increasing the test-related costs. In Brazil, few laboratories are able to perform the RT-PCR test, and its costs are not covered by the public national health system[62].
Next-generation screening
Next-generation screening (NGS) is a promising technique expected to improve CML diagnosis. NGS is able to detectBCR-ABL1transcripts variants down to 1% abundance and TKI-resistant mutations.BCR-ABL1kinase domain (KD) mutation is a mechanism of resistance in CML, especially to TKIs, observed in 50% of the patients in which imatinib treatment is unsuccessful. However, NGS faces obstacles for its implementation, such as high costs and prolonged runtime. The NGS testing can be useful in cases that do not respond to TKI treatment, allowing therapy optimization before CML transforms into BC[63,64].
In the Soveriniet al[65]trial, 236 CML patients with failure and warning TKI response were analyzed by conventional cytogenetics (Sanger Screening, SS) and NGS. Fifty-one patients who were negative for mutations by SS showed low-level mutations on NGS.Furthermore, NGS identified additional low-mutations that were not detected by conventional cytogenetics in 29 out of 60 patients positive for mutations by SS. Hence,NGS was able to identify mutations that were undetectable by SS in 34% of the patients, being crucial for prognosis and clinical decisions[65]. With cost reduction,additional studies, and its implementation in clinical practice, NGS is expected to improve CML diagnosis and therapy, besides advances in drug development for patients with treatment failure[66].
Differential diagnosis
CML can be difficult to differentiate from other myeloproliferative or myelodysplastic syndromes. Polycythemia vera can manifest with leukocytosis and thrombocytosis.Individuals with primary osteomyelofibrosis may present with splenomegaly,neutrophilia, and thrombocytosis, for example[5,35].
Other conditions can be distinguished through specific laboratory findings(including negative Ph chromosome). Chronic myelomonocytic leukemia has dysplastic characteristics, cytopenia, and more intense monocytosis than CML as well as absence of basophilia[35,38].
CML with the P230 BCR-ABL transcript is associated with predominant neutrophilia, which can lead to a misdiagnosis of chronic neutrophilic leukemia.Therefore, cytogenetic evaluation is very important in all patients. Some CML patients may have isolated thrombocytosis without leukocytosis. This finding can be due to an essential thrombocytosis, but basophilia, which is usually present in CML, as well as cytogenetic and molecular tests may aid in the differential diagnosis[5,38].
Molecular monitoring
Cytogenetic analysis is important for diagnosis, prognosis, and monitoring of therapeutic response[67]. All patients should undergo a BM examination to establish the diagnosis, assess the percentage of blasts and basophils, and perform cytogenetic analysis to confirm the presence of the Ph chromosome and to exclude clonal evolution, particularly i (17) (q10) -7/del7q, and 3q26.2 rearrangements, which are associated with a relatively poor prognosis[5,35,55,68].
The presence of the Ph chromosome should be monitored by conventional cytogenetic analysis to obtain complete cytogenetic response (CCR) or molecular response (MR) by analysis of transcription levels ofBCR-ABL1by RT-PCR and can correlate with prognosis[32,69,70]. The FISH is recommended for diagnosis in cases where the Ph chromosome is not detected by classical cytogenetics. FISH and RT-PCR are valuable tools in the identification of individuals with Ph-negative BCR-ABL-positive CML. In around 5%-10% of CML patients the Ph chromosome is not detectable by conventional cytogenetics and, among them, some may have submicroscopicBCRABL1aberrations, or more complex translocations in addition to the classic breakpoints of chromosomes 9 and 22[71].
The cytogenetics control can be used for frequent monitoring of a patient at risk of needing a change in therapy in case of treatment failure. Considering that FISH can quantify proliferating neoplastic cells in metaphase and non-proliferating cells in interphase, it used to be used for diagnosis and to analyze response to therapy using either peripheral blood or BM[72]. However, this technique has been replaced and it can only substitute chromosomal analysis in CML monitoring if BM cells are not obtained and/or for the definition of complete cytogenetic remission (CCyR)[69]. In addition, in patients with atypical transcripts, its use may be necessary for monitoring disease progression[41].
According to ELN, the monitoring of CML after the diagnosis is carried out with blood cell counts and differential cell counts as well as RT-PCR at least every 3 mo. A good sensitivity in RT-PCR is essential to adequately quantify theBCR-ABL1transcripts, guiding the therapeutic decision by achieving the milestones. The evolution of RT-PCR for CML diagnosis and monitoring has enhanced its costeffectiveness and reduced invasive procedures[41].
Monitoring the cytogenetics and MR to treatment emerged as a success factor to deal with a long-term disease. Patients with rapid cytogenetic or molecular remission(before 3 mo) and CCyR have a favorable prognosis[73]; more than 70% of them remain alive after 10 years. Karyotype is crucial in post-remission therapy decisions and molecular factors will determine treatment in individuals with normal karyotype[69].
CURRENT TREATMENT OF CML
The CML treatment has changed over time, as new medications are developed,especially those that are able to provide a balanced riskvsbenefit ratio. Some of the theories that support the use of immunotherapy in the treatment of CML are related to the fact that this leukemia has a slow development as well because the leukemic cells are accessible since they are found in the blood and in the lymphatic system.Moreover, these cells have a very specific tumor antigen - the aforementioned BCRABL oncoprotein[74]. CML is considered one of the most sensitive neoplasms to immune manipulation, in addition to having well-established therapies that allow an important reduction in tumor mass. Given this, the last 20 years deserve to be highlighted for an important progress in the understanding of tumor immunology,whether in the area of passive or active immunotherapy. With regard to passive immunotherapy, hematopoietic stem cell transplantation (HSCT) and the donor leukocyte infusion can be mentioned, while vaccines are part of active immunotherapy[75].
TKIs
TKIs are the current standard treatment for patients with CML. After all, they have been able to effectively prolong patient survival, along with their rates of cytogenetic and molecular responses[74]. The main TKIs used are imatinib, desatinib, nilotinib,bosotinib, and ponatinib. Thus, some of their main aspects will be discussed here.
Imatinib is considered a first-generation TKI as it is a pioneer in the activity of inhibiting platelet-derived growth factor (PDGFR), KIT, and ABL, with successful clinical development, being approved for the treatment of CML in 2001. This is the gold-standard treatment, as it results in more expressive cytogenetic and molecular responses and has fewer adverse effects than interferon-alpha (IFN-a)[75]. The half-life of imatinib is approximately 14 h in the human body. Comparing healthy individuals with CML patients of equivalent ages, this drug revolutionized the treatment and prognosis of CML, promoting similar survival rates between these populations[76].Recent reports suggest that almost half of CML patients treated with imatinib who obtained a lasting complete MR are able to discontinue treatment without relapse[77].The number of natural killer (NK) cells appears to be an interesting parameter to be used as a prognostic factor for treatment with imatinib. After all, some studies have shown an increase in NK cell count along with the successful interruption of this immunotherapeutic[78,79]. In addition, some studies report that imatinib induces a complete hematological and cytogenetic response in approximately 83% of patients with CML for 10 years[80,81]. Some common adverse effects include edema (due to changes in the permeability of small vessels), weight gain, conjunctival irritation,bleeding from mucous membranes, diarrhea, and even skin rash. Less frequently, it can cause changes in liver enzymes, anemia, thrombocytopenia, and neutropenia[82,83].Despite the increasing number of patients who obtain cytogenetic responses with this drug, about 30% of patients experience resistance to therapy, half of them showing the development of a point mutation in the ATP binding domain of the oncoprotein[84,85].
Dasatinib is a second-generation TKI and has become a central issue for research in the treatment of CML. So far, the number of CML patients who have benefited from treatment with this TKI remains unknown. Moreover, it has a shorter time on the market compared to imatinib[86]. Dasatinib is known as a “double inhibitor” because it inhibits, in addition to PDGFR and KIT, several members of the Scr family of tyrosine kinase. In addition, it has a half-life of 3-6 h and has a potency around 325 times greater than that of imatinib[87,88]. This medication is recommended for use when there is resistance to imatinib as well as other second- and third-generation TKIs[89,90]. The DASISION study demonstrated that the CCyR and Major MR (MMR) rates for the use of dasatinib at the dose of 100 mg, once daily, were higher and resulted in a faster and more effective response than that observed with the use of imatinib at a dose of 400 mg, once daily[91]. However, an important factor that can become an obstacle in the treatment of CML with this medication is the presence of pleural effusion as the main adverse effect in 30% to 40% of patients[92], which can lead to a discontinuation rate of 29%[93]. Other adverse reactions may include thrombocytopenia in higher rates when compared with imatinib, which can lead to gastrointestinal bleeding[88]. Joint pain may be present and, rarely, pulmonary hypertension, which appears to be reversible with discontinuation of medication[94]. With regard to the follow-up of patients using this medication, the dose of dasatinib can be reduced to 50 mg per day, or even be administered every other day, if the disease is optimally controlled[92].
Nilotinib is a highly specific derivative and inhibits BCR-ABL with a potency approximately 25 times greater than that observed with imatinib, whether administered at a dose of 150 mg twice daily or 200 mg twice daily[95]. In addition, its use is approved for the treatment of patients with newly diagnosed CML in CP and patients with CML in CP or AP who are resistant or intolerant to prior therapies[96]. In the same review by Tianet al[96], they reported that this medication has a peak serum concentration about 3 h after administration, that its bioavailability is increased by about 82% when ingested with a high-fat meal compared to the fasting state, and that its metabolism is mainlyviacytochrome P450 3A4. With regard to adverse effects related to this medication, studies have reported elevations in total bilirubin, acute pancreatitis, and, mainly, development of a kind of "metabolic syndrome"[97]. Valentet al[98]demonstrated in their study that, over a period of 5 years, nilotinib was associated with a prevalence of diabetes and cardiovascular events of 15% and 20%, respectively.These effects are of extreme concern, especially when this medication is used as a first line therapy, because of its potential lethality. However, another publication by Hochhauset al[99]reported that even with these possible effects, the use of nilotinib 300 mg twice daily has a positive risk-benefit ratio. Therefore, continuous monitoring and evaluation of cardiovascular risk factors is required for patients selected for treatment.
Bosutinib is an oral SRC/ABL TKI licensed since 2012, which has shown to be effective in the treatment of resistant CML that does not harbor mutations in the T315l or V299L ABL KD, in all disease phases[100]. The main factor that distinguishes it from the inhibitory potential from other TKIs is that it does not block PDGFR or KIT[101].Unlike previous TKIs, it has a relatively lower toxicity and its side effects are usually gastrointestinal disorders, such as diarrhea, which usually resolves spontaneously after 1 wk to 2 wk, cutaneous manifestations such as rash, in up to 20% of patients, and joint pain. In addition, bosutinib has been shown to have a safe cardiovascular profile,similar to imatinib, with a dose of 300 to 500 mg per day being recommended[102,103]. In addition, Tiribelliet al[104]provided data that demonstrate that bosutinib is a good therapeutic choice in patients who develop pleural effusion when using dasatinib[104].
With the use of TKIs, some patients may develop resistance due to the T315l mutation in theBCR-ABL1gene. Thus, third-generation TKIs like ponatinib have been developed. This medication is considered a pan-BCR-ABL1inhibitor that potently inhibits the T315I mutant and overcomes resistance based on mutations[105,106].However, several studies have shown that ponatinib is associated with a high risk of developing hypertension, which can be severe, and even thromboembolic events. In fact, a first-line study comparing ponatinib, at a dose of 45 mg per day, with imatinib had to be suspended because of the high prevalence of cardiovascular events in the group using ponatinib[107,108]. Therefore, other studies using a reduced dosage of ponatinib, from 15 to 30 mg per day, have found a reduced incidence of cardiovascular effects. At the moment, the combination of ponatinib with antithrombotic medications is being tested[109]. Corteset al[110]demonstrated in the PACE study that the estimated overall 5-year survival among patients using this medication was 73%, and evidenced the occurrence of treatment-associated rash (47%), abdominal pain (46%), and thrombocytopenia (46%)[110].
In view of the presence of the BC as a final phase in the evolution of CML and the high morbidity and mortality of patients affected by that condition, it is essential to establish an appropriate treatment for the crisis. The BC is the result of continuous BCR-ABL activity and oxidative stress[27,111,112]and its incidence has considerably decreased after the acquisition of TKI therapy. According to Saußele and Silver[28],studies have indicated an 8-year cumulative incidence of BC of 5.6% with the use of TKI compared to 12%-65% before the TKI era. Also in this work, there is a recommendation for treatment with a second- or third-generation TKI and use of chemotherapy,if necessary. The choice of chemotherapy should be based on the type of BC: If lymphoid, it can be used in an ALL-regimen with vincristine and prednisone, and in case of myeloid BC, an acute myeloid leukemia-regimen with anthracyclines and cytarabine is suggested. However, the BC response to TKI may be transient, which indicates that most cells are still sensitive to BCR-ABL inhibition. Thus, the most effective treatment for BC would be the early reduction of tumor burden and the elimination of BCR-ABL, which is still a challenge[113].
Resistance to TKIs
Although remarkable progress has been achieved in increasing the effectiveness of TKIs directed to BCR-ABL, resistance to these drugs is still being observed in CML patients. In such cases, stem cell transplantation is the only method that has proven to be effective in curing patients; however, this alternative is limited by donor availability[114]. Resistance to TKIs occurs in less than 10% of patients. On the other hand, persistence of residual quiescent cells affects the majority of patients, leading to chronic use of medication. Although only a part of patients become resistant to the treatment, there is a range of heterogeneous factors leading to persistence and resistance[115]. Patients who do not respond to drugs at the beginning of treatment have a primary resistance, whereas CMLs that initially respond to therapies and subsequently become resistant to them are classified as secondarily resistant[116].Moreover, the resistance mechanisms are divided into two broad groups, namely BCRABL-dependent and BCR-ABL-independent mechanisms[117].
BCR-ABL-dependent resistance
Concerning the BCR-ABL-dependent drug resistance, mutations in theBCR-ABL1KD correspond to the most common type of resistance to TKIs. Such mutations promote steric modification of the protein structure and can lead to a stabilization of the active conformation of BCR-ABL, impairing drug ligation and, therefore, causing resistance.Among these mutations, stand out the ATP-binding P-loop between the amino acids 244 and 255, the C-loop between the amino acids 350 and 363, and the A-loop between the amino acids 381 and 402[118].
Among the countless mutation possibilities in theBCR-ABL1KD, the T315I is the most frequent when considering imatinib resistance, since it affects from 4% to 15% of CML patients who are resistant to that medicine[119]. It is characterized by the replacement of a threonine in the 315thposition with an isoleucine, leading to a significant resistance to TKIs. Also known as “gatekeeper” T315I because it is a homologous mutation of the “gatekeeper” threonine residue, this mutation can cause a steric shock, impairing not only imatinib action, but also impeding nilotinib, dasatinib,and bosutinib to make up a hydrogen bonding with the protein. Therefore, this is an even more concerning mutation since it affects other generations of TKIs[120].
The sequential use of TKIs may contribute to the emergence of resistance-related mutations that can be classified as polyclonal, when two or more mutations separately affect the KD of distinct BCR-ABL proteins, or compound, when mutations simultaneously affect a single protein. It has to be emphasized that the mainBCRABL1multiple mutations involve T315I and loop-P mutations[121].
Finally, some resistance mechanisms are associated with DNA repair pathways and genomic instability. In CML cells, BCR-ABL stimulates an excessive production of reactive oxygen species, which leads to genomic instability, predisposing the occurrence of potential mutations that can even take place inBCR-ABL1and cause TKI resistance[122,123]. In addition, the BCR-ABL kinase promotes the degradation of uracil DNA glycosylase, which is an important component of the DNA base excision repair pathway, an important mechanism for preventing the accumulation of mutations[124].
BCR-ABL-independent resistance
Among the several and heterogeneous BCR-ABL-independent resistance mechanisms,evidence suggests the existence of a BCR-ABL potential to activate an autocrine mechanism that confers a partial or complete growth factor autonomy. CD34+ cells of CML produce interleukin (IL)-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF), which promotes STAT5 phosphorylation. As a result, these proliferating cells are protected by GM-CSF against imatinib and nilotinib through the JAK2/STAT5 pathway. Furthermore, the CML resistance has been related to the overexpression of various other proteins, namely forkhead box protein O1, protein kinase C Eta (PRKCH), the SRC kinase family, 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 3 (PFKFB3), B-catenin nuclear protein, akirin-2, tumor progression locus 2, nuclear factor-kappa B (NF-kB and NF-kB-p65), and MEK/ERK[118].
The BCR-ABL-independent mechanisms can even occur through membrane transporters. Since these components are essential for the availability and consequent effectiveness of the drug, the CML therapeutic process can be negatively influenced if they are affected[118]. The organic cation transporter-1 (OCT-1) works as a cell influx pump for imatinib. Therefore, adequate OCT-1 functioning is associated with better MMR rates, event-free survival, and global survival in CML patients in imatinib use[125]. On the other hand, the ineffectiveness of this transporter can lead to imatinib resistance. Regarding asciminib resistance, the efflux of this drug involves mainly the ABCG2, which, if overactivated, can reduce the intracellular levels of this drug to undetectable levels[126].
If leukemic stem cells in CML are TKI-resistant, such resistance tends to persist in long-term therapy. Despite the limited knowledge about the mechanisms through which the leukemic stem cells become resistant to the current therapies, it is believed that it may be associated with the quiescence of these cells, which is controlled by intrinsic regulatory mechanisms and extrinsic signals from the microenvironment.Evidence has suggested that a stop in the cell cycle of such stem cells induced by specific signals from stromal cells may lead to resistance[127].
The frequency of chromosomal abnormalities in Ph chromosome-positive cells is positively associated with the severity of the disease. The most common chromosomal aberrations are an additional Ph chromosome, chromosome 8 trisomy, trisomy 19, and anomalies in the chromosome 17, and all of them are associated with imatinib resistance[116]. In addition, alterations in gene expression as a result of genetic modifications contribute to the resistance in CML. Among these alterations, stand out DNA methylation, histone modification, and noncoding RNAs[118].
New drug generations
Given the growing prevalence of TKI resistance in CML, various clinical trials have been conducted in order to make new drug options available for the patients affected by this disease. The following subtopics highlight the most promising alternatives in this context.
Asciminib
Asciminib is one of the most promising drugs among those aiming to be alternatives in the TKI resistance scenario. This medicine is an allosteric inhibitor that binds to the myristoylation site and alters the conformation of the KD to keep it inactive, and has the negative and mutedBCR-ABL1as its target; that is,ABL1mutations such as gatekeeper T315I are not able to promote resistance to this drug[128]. A recently published phase 1 study observed an asciminib activity in 150 individuals, from which 141 manifested CML and 9 had accelerated CML with resistance or severe side effects with the use of at least two ATP-competitive TKIs. A complete hematological response was achieved in 92% of patients with a hematologic relapse, whereas 54% of those who did not initially present a CCR obtained a CCR. Interestingly, 28% of T315I carriers had a considerable MR during 12 mo of follow up[129]. In addition, the combinations between asciminib and other competitive TKIs may be useful in preventing the emergence of other mutations since the asciminib target site differs from the target sites of the other drugs. In that context, another study showed that the ponatinibasciminib combination may be useful since asciminib is a substrate of the ABCG2 ATPbinding cassette transporter, whereas ponatinib is an ABCG2 inhibitor that can, partly,neutralize the positive regulation of ABCG2 as a resistance mechanism[130].
Considering asciminib as a promising drug to deal with the resistance and side effects of TKIs and aiming to increase the speed of response and the rates of deep molecular remission, there are currently phase 1[131]and phase 2[132,133]studies evaluating therapy with asciminib alone or combined with nilotinib, imatinib, and dasatinib. These studies aim to validate its safety, tolerability, and potential as firstline therapy. The preliminary results of the phase 1 study[131]published showed that asciminib achieved response in patients heavily pretreated, with unacceptable TKIs’side effects, failure in ponatinib treatment or T315I mutation, presenting as a promising alternative in these cases[129].
Radotinib
Radotinib is a BCR-ABL oral inhibitor indicated for newly diagnosed CML patients and for those with resistance to at least one TKI. Besides inhibiting the salvage-type BCR-ABL kinase, radotinib also inhibits other kinases, such as PDGFRα, PDGFRβ, ckit, and SRC, when used in higher concentrations. However, this drug presents no consistent effectiveness against T215I[134]. A phase 3 study carried out in various Asian countries compared radotinibvsimatinib in newly-diagnosed CML. The results showed significantly higher and faster MMR rates in the radotinib group after 12 mo and 48 mo of follow-up[135].
Danusertib
Aurora kinases are serine/threonine kinases that are crucial for coordinated cell division. They participate in the maturation of centrosomes, in the formation of mitotic fuse, in chromosomal segregation, and in cytokinesis. In that context, danusertib promotes the inhibition of the catalytic domain of aurora kinases as well as inhibits ABL and its mutated variations, including T315I[136]. This drug takes advantage of the crystal structure of the T315I KD and binds to the ATP-binding pocket of the enzyme active conformation, neutralizing the steric hindrance resultant from the substitution of threonine for isoleucine[137]. A phase 1 study about danusertib, that included 37 patients, showed subtle positive responses in patients with TKI-resistant CML in accelerated and blastic phases who carry T315I as well as in Ph chromosome-positive ALL. Among CML patients, 20% achieved a complete hematologic response and a CCR was observed in one individual[136].
HQP1351
HQP1351 is currently being clinically analyzed as a new generation multikinase inhibitor and it is expected to be useful in the treatment of TKI-resistant CML. Its main targets are the mutated wild-type BCR-ABL and KIT, including T315I. A phase 1 clinical trial administered HQP1351 in different doses in 101 patients, among which 87 had CP CML and 14 had AP CML. All individuals manifested resistance to other TKIs and 63% of them were T35I carriers. The results showed a complete hematologic response in 95% of individuals. Moreover, 69% of participants had a major cytogenetic response, 61% had a CCyR, and 37% had a MMR during a follow-up of 12 mo[138].
Immunotherapy
Vaccines are important immunotherapy components, being considered active immunotherapies. The advance in the knowledge on the BCR-ABL protein and its biological aspects has led to the development of a considerable diversity of studies based on the evaluation of vaccines based on peptides such as Pr-3, WT-1, and HSP70[139]. One of the major disadvantages of peptide-based vaccines is that they have the potential to induce an immune response to a few epitopes or just one, which increases the likelihood of an immune escape from CML cells[7,140]. On the other hand,studies on DNA vaccines have also been developed; however, their results are not yet available[141]. At the moment, therapeutic tumor cell vaccines modified by genes and halogenic tumor cell vaccines have also been tested. The former are advantageous because they carry a wide spectrum of antigens related to leukemia, promoting a wide and safe response. The latter have their cost-effectiveness as a positive point, and these cell lines seem to represent an unlimited source of immunizing antigens[31]. In addition,the appropriate time to use the vaccine is the period that follows normalization of the immune system activity with other therapeutic interventions against known immunosuppressive pathways. Thus, it is necessary to remember that immunotherapy should not be limited to the use of only one tool[142].
IFN-α was widely used in the treatment of CML in the 1980s, due to its disrupting role in the expression of theBCR-ABL1gene, in the activation of cell apoptosis factors,and in the recognition and elimination of CML-characteristic cells, in addition to promoting normal quiescent hematopoietic stem cell cycling[143-145]. The average rate of CCyR with IFN is 13%, ranging from 5% to 33%. This component targets highly quiescent leukemic stem cells, which have a high self-renewal capacity. Esserset al[145]demonstrated that INF-α was able to sensitize these stem cells. However, some adverse effects, such as flu symptoms, arthralgia, myalgia, neutropenia, and depression, contributed to the discontinuation of its use[145]. Currently, the discussion about the use of INF-α has returned, but in a combined use with TKIs. For example,Hjorth-Hansenet al[90]reported the results of their NordCML007 phase 2 study,combining dasatinib and pegylated IFN-α2b (PegIFN). A good tolerance of the combination with manageable toxicity and a marked increase in response rates after the introduction of PegIFN were observed.
Tumor cells have great efficiency in finding mechanisms that allow them to evade immunosurveillance[146]. As mentioned, one of these mechanisms occurs through PDL1 present in CML cells, which, when attached to the PD-1 receptor in T cells, reduces their function[7,29]. Thus, some therapies seek to block the action of PD-1 and PD-L1 by anti-PD-1 and anti-PD-L1 antibodies, presenting, so far, promising results. Moreover,the adverse effects related to these drugs seem to not impair their use. Furthermore, a study that evaluated the use of anti PD-1/PD-L1 in 296 patients with solid tumors in advanced stages has found complete or partial treatment responses in these patients[147]. Some trials have shown that the use of immunomodulating antibodies are able to act by controlling the action of regulatory molecules in the immune system that compromise the functioning of T cells, being considered checkpoint inhibitors[148,149].One of these inhibitors, nivolumab, has been undergoing phase 1 tests for use in CML[150]. A meta-analysis that included 6800 patients concluded that the use of drugs with anti-PD-1/PD-L1 action in cases of refractory or advanced cancers was highly effective, further demonstrating that patients with hematological cancer had better results than those with solid cancer, in addition to performing allogeneic (allo) HSCT before using anti-PD-1/PD-L1 may be more effective in immune responses[151].Moreover, studies performed in animal models demonstrated an increase in the survival rate of mice with CML, which were treated with αPD-L1 monoclonal antibody. This drug would block the PD-1/PD-L1 interaction and contribute positively to the regulation and action of immune system cells such as leukemia-specific cytotoxic T lymphocytes, CD8+ T lymphocytes, CD4+T cells, and, possibly, B lymphocytes, stimulating the immune response to the tumor[152]. Corroborating the previous research, the inhibitory pathway of the PD-1/PD-L1 interaction was able to stimulate anin vitroproduction of the IL-2, a Th1 profile cytokine, in an environment with CML cells, suggesting a possible positive effect on the activation and action of T lymphocytes[153].
Allogeneic transplantation
During the 1970s, Donnall Thomas’ work showed the efficacy of the allo HSCT as curative therapy for CML, becoming the first-line treatment in patients in CP younger than 55-years-old[154,155]. With the emergence of TKIs, allo HSCT is no longer the firstline therapy, since 95% of those affected by CML are diagnosed in the CP, in which TKIs perform better[156-158]. The allo HSCT, as well as the TKIs used in the main therapy,has an excellent response against clonal cells, and the procedure-related morbidity and mortality has dropped sharply in recent years, with a consequent reduction in complications, such as graft versus host disease (commonly known as GVHD)[159]. The procedure is performed in patients of all ages, mainly in the sixth decade of life, but elderly patients less often meet the inclusion criteria for transplants. However, the age is not enough to determine the risks and benefits involved in the transplantation procedure[160-162].
With the improvement of treatment with TKIs and its effectiveness, only 6% of patients progress to AP or BC. However, in addition to the original mutation, other cytogenetic changes may occur, which limits the action of some first-line therapies[163].As a rescue therapy, allo HSCT should be reserved for patients who are newly diagnosed in AP and BC phases or who are resistant to TKIs in these phases (including to ponatinib)[164]. This resistance may occur due to the T315I mutation in the coding domain of theBCR-ABL1gene[10]. Although this demonstrates a worse prognosis, it also raises the possibility of performing an allo HSCT, which has a healing potential[27].
In the first-line treatment, if there is resistance, it is acceptable to consider using the second-line treatment, and in case of subsequent resistance, the allo HSCT should be considered[165]. In general, regardless the line of treatment, if the molecular or cytogenetic response to two or more TKIs is suboptimal (that is,BCR-ABL1transcription > 1% or an in CCR with Ph+> 0%), an allo HSCT should be quickly considered, checking the compatibility of the human leukocyte antigen between patient and siblings, as well as searching for other possible donors in BM banks[41,51].
TKI therapy before and after transplant
Many patients undergo TKI therapy prior to allo HSCT[166]. Some studies demonstrate that it is related to a higher overall survival (measured from the transplant) if compared to patients who had not previously used TKI (in this case, imatinib) during first CP[167].
Another study (which analyzed 449 patients in AP, in second CP, and in BP of CML)found that the previous results do not apply to the advanced stage of the disease, so the use of these drugs before the allo HSCT did not alter the overall survival, nor leukemia-free survival, recurrence, treatment-related mortality or GVHD when compared to the group that had not previously used a TKI[168]. However, the indicators already known for performing allo HSCT should still be the main factors in determining prognosis[169].
After performing allo HSCT, BCR-ABL may still be detected for a few months,considering that allograft adaptation is not immediate[170]. However, checking for the presence of BCR-ABL (which can be negative, fluctuating or positive) during the next 6 mo can predict a prognosis for cure or relapse[171]. In this sense, the administration of a standard dose of imatinib as a prophylactic therapy against possible relapses after allo HSCT can be performed, since it can potentiate anti-tumor effects by dendritic cells[172].Compared to the previous standard, this practice is more effective if performed in CP of CML, being less useful if performed in advanced phases[170-174].
Indications and risks of performing the allo HSCT
In the era of TKIs, the indication for HSCT varies according to the stage at diagnosis and should never be dissociated from the risks related to the disease[175,176]. In this way,the European Blood and Marrow Transplant Group (EBMT) developed a risk and chance score for performing the allo HSCT based on five criteria, including the stage of the disease, the age of the recipient, the type of donor, the donor sex, and the interval between diagnosis and transplantation. The sum of the scores varies from 0 to 7 in each item (Table 1)[177,178].
At CP, allo HSCT should be considered based on resistance to second-generation TKIs, being it first- or second-line, as well as on the ponatinib failure after 3 mo of treatment[89]. CP patients who fail in the second line of treatment with TKIs, regardless the EBMT risk score, should be immediately referred for an allo HSCT and the ABL mutational analysis must be performed to better choose the therapy before transplantation[179].
The CML advanced phase is present in the risk analysis carried out by EBMT,ranging from 1-2 points and, therefore, having an impact on the transplant result[180].For cases in which there is progression or an initial diagnosis in AP, the chance of TKI failures is enormous, and allo HSCT should be done as soon as the condition stabilizes[181]. Patients in AP CML tend to benefit better from allo HSCT compared to treatment with imatinib, under the influence of two criteria: The diagnosis was over 12 mo ago or the amount of blasts in the peripheral blood is greater than 5%[182]. For those patients in BC with a frank explosion, allo HSCT is not recommended, and healing through transplantation occurs in a small number of cases in this phase of the disease(< 10%)[41,183]. Thus, the decision to perform a transplant must consider numerous factors such as the risks of the procedure and the disease stage/progression. A study that analyzed a Swedish database showed a greater post-transplant overall survival at 5 years for individuals in the first CP (96.2%) and lower for those in AP or BP (36.9%)[184].
NEW PERSPECTIVES IN THE CML TREATMENT
Faced with the possibility of therapy-resistant cells to conventional drugs[185]or other treatment problems, whether by non-remission or progression of the disease and possible adverse effects, new research is looking for alternatives that treat the CML more effectively and improve the life quality of the patients[186]. The following topics gather some of the most relevant therapeutic strategies in this context.
Peroxisome proliferator-activated receptor gamma inhibitors
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) is a transcription factor that may interfere with the action of the protein OCT-1, which, as previously explained, is related to the transport of imatinib intoBCR-ABL1+ cells, among other functions[187]. The ligands of PPAR-γ, such as pioglitazone, are drugs currently used to treat diabetes that have demonstrated utility in the therapy of CML[188]. Studies have reported the use of these medicines as therapies for some neoplasms, acting as an inhibitor of tumor cells growth or increasing apoptotic capacity, decreasing cell proliferation[189].
Once this therapeutic class is connected to its receptor, a negative interference in the transcription of the RNA STAT5, an oncogenic mediator related to the stability of the CML cells, occurs and an increase in the OCT-1 expression is observed, making the cell more sensitive to the imatinib action[190]. Moreover, it was reported that pioglitazone combined with imatinib increases the apoptotic capacity of tumor cells by a positive regulation of caspase 3, which may mean that a combined therapy with these two drugs can be an alternative for resistant CML treatment[186]. Telmisartan, a partial agonist of the PPAR-γ, has shown to be effective in circumventing the resistance to imatinib, possibly being more effective than pioglitazone[185].
There are reports of patients who had improvements in clinical conditions with the combined use of TKIs and PPAR-γ inhibitors, getting a more favorable MR than the patients who used only imatinib. However, more studies that assessed the effectiveness of this combination in the treatment of CML still are necessary[188].
Immune modulation
WT1 peptide vaccine: TheWT1gene was initially isolated from the Wilms’ tumor,being responsible for its suppression. However, another form of this molecule, the wild-type WT1, showed to be highly expressed in hematopoietic neoplasms, such asCML[191]. Therefore, due to its high levels and role in the oncogenesis process, this gene may become a possible therapeutic target, contributing to a better patient prognosis[192].
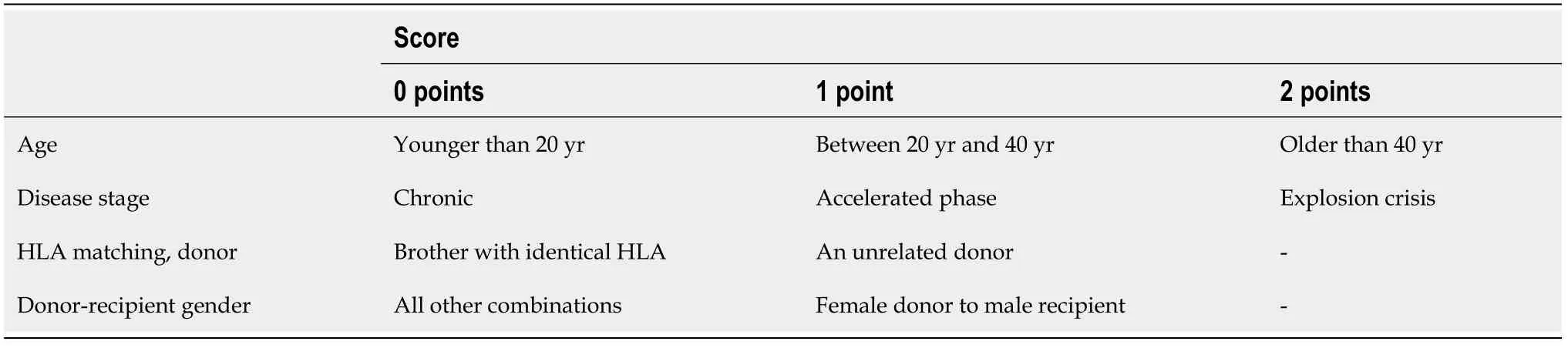
Table 1 European Group for Blood and Marrow Transplant risk score for allo hematopoietic stem cell transplantation
The WT1 peptide vaccine was able to stimulate the emergence of CD8+ cytotoxic T cells with combat specificity to the leukemic cells, suggesting a positive immunomodulation and specific antigen response[193]. Corroborating that, a phase 2 study also reported a favorable MR of the peptide vaccine use, which interfered in theBCR-ABL1gene transcription, evidencing a possible ability to assist in the CML treatment, and has also shown to be safe[194]. In addition, the combined use of the peptide vaccine and the imatinib made possible the BCR-ABL mRNA reduction, a marker of residual disease in the peripheral blood. Furthermore, it contributed to decreasing the imatinib doses necessary for the treatment, reducing the chances of side effects without interfering with the therapy effectiveness[195].
Peptides derived from the BCR-ABL gene
The peptides generated from the fusion geneBCR-ABLhave been studied as possible therapeutic targets, because the use of oncogenic antigens in the treatment of some neoplasms has shown less ability to generate side effects once they act directly in abnormal cells, besides presenting a cure potential[196,197].
The use of the peptide vaccines proved to be secure and able to stimulate a favorable antigen-specific immune response, also improving CD4+ T cell responses,indicating possible efficacy in the CML treatment[198]. Moreover, a significant decline of BCR-ABL mRNA level in peripheral blood was detected after vaccine use[199]. Lastly,the combined therapy among the vaccine and imatinib may also reduce the doses of the TKIs necessary for the treatment, which may reduce the chances of possible side effects[141].
Dipeptidyl peptidase IV inhibitors
Dipeptidyl peptidase IV (DPPIV) is a membrane protein that, in leukemia cells, is able to degrade and interfere in the stroma-derived factor 1 action, a chemokine that is expressed in normal myeloid stem cells related to the interaction and stability of the hematopoietic niche[200]. DPPIV proved to be efficient in the differentiation of normal cells from leukemia cells, which may contribute to either diagnosis or prognosis of this disease, besides being a possible therapeutic target[201].
Patients who used gliptins, DPPIV inhibitors used to treat type 2 diabetes mellitus,along with CML treatment significantly reduced the BCR-ABL mRNA levels,indicating possible synergistic effects between the medicines. Furthermore, the gliptins were also able to inhibit the expansion and proliferation of CML cells, which may contribute to neoplasm control[200]. However, due to the scarce amount of research in this area, further studies need to be done aiming to evaluate the appropriate therapeutic doses[201].
Concluding, the new therapeutic approaches in the CML treatment emerge as an alternative to circumvent possible problems of conventional therapy. Although there are few studies in this area, the combined use of the TKIs with these new therapeutic options has already been related to improvements in the patients’ clinical conditions and prognosis[202].
CONCLUSION
Although the knowledge about CML cytogenetics has been well elucidated, the role of innate and adaptive immunity in the prevention as well as in the development of this disease and the role of additional mutations should be understood to a broader extent,allowing the advancement of new therapies, aiming to improve the quality of life and survival of these patients. New drugs and the advancement of immunotherapy are essential to tackling TKI resistance and maintaining low residual levels of cancer cells.
杂志排行
World Journal of Clinical Oncology的其它文章
- Metastatic hormone-sensitive prostate cancer: How should it be treated?
- New Year’s greeting and overview of World Journal of Clinical Oncology in 2021
- Pancreatic cancer in the era of COVID-19 pandemic: Which one is the lesser of two evils?
- New frontiers in focal therapy for prostate cancer: Prostate-specific membrane antigen positron emission tomography/magnetic resonance imaging
- Autosegmentation of cardiac substructures in respiratory-gated,non-contrasted computed tomography images
- Racial disparities in immune-related adverse events of immune checkpoint inhibitors and association with survival based on clinical and biochemical responses
