Effects of alloying elements and thermomechanical process on the mechanical and corrosion properties of biodegradable Mg alloys
2021-03-10GungorIncesu
A.Gungor,A.Incesu
a Metallurgical and Materials Engineering,Karabuk University,78050 Karabuk,Turkey
b TOBB Tech.Sciences Vocational School,Karabuk University,78050 Karabuk,Turkey
Received 10 April 2020;received in revised form 28 August 2020;accepted 15 September 2020
Available online 6 October 2020
Abstract In this study,four different Mg-Zn-Ca-Mn alloys were produced using gravity die casting method.Mn content was kept constant in all alloys,while Zn and Ca were added in two different ratios.Homogenization heat treatment was applied to all cast alloys and a specimen from each homogenized alloy was hot rolled.Microstructure,mechanical properties,and corrosion behavior of the homogenized and hot rolled alloys were investigated as comparatively.Following results were obtained;grain size decreased significantly with increasing Zn content,Zn promoted Ca2Mg6Zn3 phase formation,corrosion resistance increased with increasing Zn/Ca atomic ratio,hot rolling resulted in much higher tensile strength but much lower ductility and toughness.Among the homogenized and hot rolled alloys,ZXM300-h alloy(hot rolled ZXM300 alloy)with its 146±2.5MPa yield strength,229±3.7MPa tensile strength,% 1.6±0.1 elongation and 0.029mm/yr.immersion corrosion rate exhibited the best mechanical properties and corrosion resistance to be used as a biodegradable alloy such as fracture bone plate material.
Keywords:Biodegradable;Mg alloys;Hot rolling.
1.Introduction
Developing new and better implant materials for orthopedic surgery is gaining more importance in recent years.Although metals,ceramics and polymers are used as implant materials,metals have advantages over ceramics and polymers due to their better combination of strength,toughness,and ductility.Metallic biomaterials such as stainless steel,Co-Cr alloys,and Ti alloys,etc.are corrosion resistant and widely used as permanent implant materials in orthopedic surgery.However,these metallic alloys are not preferred as temporary implant materials(screws,pins,bone plates,etc.)since removing these implants from the body after healing requires a second surgery operation which may have a potential risk of infection.In addition to its physical and mechanical properties similar to those of bone tissue,magnesium implants do not require a second surgery operation to be removed from the body due to its ability to disappear completely in the body fluid[1].
Magnesium as compared to other metals is easily dissolved in aqueous solutions particularly in the presence of Cl−ions[2].The easy degradation of magnesium in Cl−ion containing environments,which is considered to be a negative effect for many commercial applications,makes magnesium extremely attractive for the development of metal-based biodegradable material[3,4].Although easy degradation property provides an advantage to magnesium,the fast degradation rate of magnesium must be brought to acceptable levels for its clinical applications to ensure the healing time of defective part in the human body[5].For example,magnesium-based fracture bone plate should remain in place without losing mechanical properties before the full healing time of the bone is 12 months[6]and fully degradation of implant material should be within 12–24 months[7].Therefore,the implant material must be degraded in a controlled manner without causing unwanted side effects[8].It was suggested by Gui et al.that the rate of degradation of the magnesium based biomaterial to be used as a fracture bone plate must be lower than 0.5mm/yr.in simulated body fluid(SBF)at 37°C[9].Due to this degradation property,magnesium and its alloys have a wide range of applications in the medical field[10].
The selected base metal and alloying elements used in living tissue are required to be biocompatible and must not have any harmful/toxic effects on the body.In this perspective,magnesium is present in large amounts in bone tissue and the total amount in the human body is around 19g[11].Therefore,Mg is an essential and nutritious element for the human body and preferred as a base material.When considering nutritious,non-toxic and harmless alloying elements for the human body,a few elements such as Zn,Ca,and Mn,etc.come forward[12,13].Although Zn,Ca,and Mn elements are useful,considering the corrosion rate of the alloy,magnesium based degradable implant material should contain less than 6wt.% Zn,2wt.% Ca and 1.5wt.% Mn[13]and also its degradation rate must provide daily intake limit for each element or a better value.
Researches on pure magnesium show that the mechanical properties of pure magnesium are not sufficient to support fracture bone since the yield stress of pure magnesium(~20MPa)[14]is less than that of cortical bone(~120MPa)[15].Therefore,the mechanical properties of the magnesium must be improved too.Among the alloying elements,Zn,Ca,and Mn elements improve not only corrosion resistance of Mg but also the strength of Mg by solid solution strengthening,grain refining and precipitation hardening mechanisms.Further improvement in the mechanical properties of Mg alloys can be obtained with heat treatment and thermomechanical processes.
In recent years,many researchers have focused on the Mg based alloys and studied binary,ternary and quaternary Mg alloys using Zn,Ca,and Mn[16–21].Recent studies both focused on Mg-Zn-Ca ternary and Mg-Zn-Ca-Mn quaternary alloys[22–26].Among them,Wei et al.studied Mg-xZn(x=0.2;0.5;1;2)−0.1Mn-0.2Ca alloys[24]and determined that more grain refinement occurred and more secondary phases such as Ca2Mg6Zn3,Mg2Ca and Mg4Zn7formed with increasing Zn content.They also observed that mechanical strength and corrosion resistance of Mg-xZn-0.1Mn-0.2Ca alloys initially increased and then decreased with increasing Zn content of the alloy.Duley et al.investigated homogenization-induced age-hardening behavior and room temperature mechanical properties of Mg-4Zn-0.5Ca-0.16Mn(wt.%)alloy[25].They stated that alloys in as-cast form contained coarse networks of eutectic Ca2Mg6Zn3phase with the uniform grain size distribution of~79μm and that eutectic networks acted as crack initiation sites causing poor ductility.In addition,the homogenization treatment resulted in dissolution of the crack-initiating eutectic phases and a slight increase in grain size.Tong et al.produced ultra-fine grained Mg-5.0Zn-0.5Ca-0.3Mn(wt.%)alloy with simultaneously improved strength and ductility using equal channel angular processing(ECAP)[26].Zhang et al.studied Mg–Zn–Mn–Ca system,they determined that Ca was effective to reduce the grain size of the as-cast alloys up to 0.5wt.%and that the phase constitute was mainly controlled with Zn/Ca atomic ratio[27].They showed that divorced eutectic phase(α-Mg+Mg2Ca+Ca2Mg6Zn3)formed when Zn/Ca atomic ratio was less than 1.0–1.2 and that eutectic phase(α-Mg+Ca2Mg6Zn3)formed when Zn/Ca atomic ratio was more than 1.0–1.2.Du et al.listed the standard electrode potentials of the phases as Ca2Mg6Zn3>α-Mg>Mg2Ca[28].Since Mg2Ca phase has the lowest electrochemical potential among the phases,it preferentially degrades instead ofα-Mg phase by forming galvanic couple in the structure and accelerates corrosion rate.Therefore,it can be concluded from these studies that to increase the corrosion resistance of magnesium by controlling the microstructure,the Zn/Ca ratio of the alloy must be kept above the 1.0–1.2 range to avoid the formation of Mg2Ca phase.
As can be seen from the recent studies on quaternary alloy systems,it is not easy to obtain both acceptable corrosion resistance and required mechanical strength simultaneously with just alloying and heat treatment.Thus,further improvements in corrosion resistance and mechanical properties require the application of thermomechanical processes.As a result,nutritious and non-toxic elements for the human body(Zn,Ca,and Mn)were selected as alloying elements in this study.Percent of the alloying elements was determined based on their solubility in magnesium and formation of the preferred phases(α-Mg and Ca2Mg6Zn3phases according to Zhang et al.and Du et al.)in order to improve the corrosion resistance and mechanical properties of magnesium.In addition,hot rolling was applied to the homogenized alloys as a thermomechanical process for the further improvement of the mechanical properties.
2.Materials and method
Four different Mg-Zn-Ca-Mn magnesium alloys were produced using gravity die casting method.Alloys were prepared by using Mg(99.995% purity)ingots,Zn(99.95% purity)chips,Mg–wt.% 25Ca,and Mg–wt.% 1.2Mn master alloys.Alloys were melted in AISI 310 stainless steel crucible at 800°C under argon protective gas atmosphere in an electric resistance furnace.The melt was poured at 800°C into the permanent mold that was preheated to 400°C to assist the complete filling of the steel mold cavity and to minimize the casting defects like gas holes.CO2+0.8vol.% SF6gas mixture was used during casting to remove air from the environment.
Rigaku Primus II-WD-XRF spectrometer fitted with a 4kW Rhodium tube was used to determine the chemical compositions of the alloys.
Homogenization heat treatment was applied to all as-cast alloys at 400°C for 12hrs.and samples were cooled in the furnace.Then,all homogenized alloys were sliced and one sample from each homogenized alloy was taken and hot rolled.To separate the hot rolled alloys“-h”was added after the ASTM designation.For example,the designation of hot rolled ZXM300 alloy is ZXM300-h.
The hot rolling process was performed in a double roller rolling device with a 200mm roller diameter at a speed of 20rpm.All samples were subjected to a 10% deformation in each pass and a total of 30% deformation was applied in 3 stages.The initial thickness of the samples before hot rolling was set to 4.5mm.Before hot rolling,all samples were heated to 400°C and allowed to stand at this temperature for 30 min.Between the passages,samples were kept at 400°C for 10 min in the furnace and the next pass was applied.
Specimens for microstructural analyses were mounted in polymeric material and then polished using 180–2000 grit SiC papers in 90° parallel lines.After that,they were cleaned in distilled water,polished with 0.3μm alumina solution,and cleaned with ethanol.The samples were then etched with picral solution.The composition of picral solution is 5g of picric acid,6.5mL of acetic acid,20mL of distilled water,and 100mL of ethanol.
Optical microscope analyses were carried out using Nikon ECLIPSE MA200 reverse metallurgical microscope.Detailed microstructural investigations of alloys were carried out using Carl Zeiss ULTRA PLUS FESEM(Field Emission Scanning Electron Microscopy).In addition,Energy Dispersive Spectroscopy(EDS)was performed to assess the chemical compositions of the phases.
X-Ray Diffraction(XRD)analysis for phase identification was carried out using the Rigaku ULTIMA IV Diffractometer with Cu-KαX-Ray radiation under 40-kV acceleration voltage and 20mA current.The scanning speed of the measurements was 3deg/min and the scanning range was between 20° and 90° ICDD database was used to identify the phases of the X-ray diffraction pattern of the alloys.
Vickers microhardness test was performed via Q10 A+QNESS microhardness test machine using 1000g load and 15s dwell time.The average hardness values of the alloys were calculated from the measurements taken at 5 different indentations.
Tensile tests were conducted on a Zwick/Roell Z600 Universal Test Machine using standard bone shape flat specimens at 37±1°C[29]and 2mm/min test speed.
Potentiodynamic polarization tests were conducted in LONZA Hanks’BSS(Balanced Salt Solution),with Phenol Red at 37±1°C by using a PARSTAT 4000 Potentiostat Galvanostat.A standard three-electrode set up was used in electrochemical corrosion tests.A graphite rod was used as a counter electrode,a saturated calomel electrode was used as the reference electrode,and Mg alloys as the working electrode.Potentiodynamic polarization tests were performed at a scan rate of 0.5mV/s.Before the electrochemical corrosion tests,open circuit potentials were measured after stabilizing the system in 15 min.Since magnesium and its alloys do not fully comply with the Tafel theorem,a linear tangent line was drawn from the cathodic region and another line was drawn starting from−50mV below the OCP.Then,Icorrvalue was obtained from the intersection of these two lines.After that,the corrosion rate of the alloys was calculated using the formula derived from Faraday’s law.Details of electrochemical corrosion rate calculations can be reached from the ASTM G102–89 standard[30].
Electrochemical impedance tests were also carried in LONZA Hanks’BSS with Phenol Red at 37±1°C by using a PARSTAT 4000 Potentiostat Galvanostat.Electrochemical impedance spectra of the alloys were measured in the frequency range from 100MHz to 10MHz with a perturbation amplitude of 10mV.Test conditions of the experimental data were fitted using Z view software.
Immersion corrosion tests were conducted in LONZA Hanks’BSS,with Phenol Red at 37±1°C.A hot plate was used to keep the temperature constant at 37±1°C.Coupons in square prism shape(4mm in thickness,9mm in length,and 9mm in width)were used for immersion tests.The samples were kept in the solution for 4,8,12,24,and 48hrs.To avoid corrosion deposits,the samples were cleaned in the ultrasonic cleaner for 5 min at 180g/L chromic acid solution.Then,the final surface cleaning was applied in ethanol for 3 min in an ultrasonic cleaner and the samples were dried in blowing warm air.After drying,the final weights of the samples were measured with the aid of precision scales.Details of electrochemical corrosion rate calculations are given in the ASTM G31–72 standard[31].
3.Results and discussion
3.1.Chemical composition of the alloys
Nominal compositions and measured compositions of the alloys are given in Table 1.As it is seen from the table,the measured compositions are close to the nominal compositions of the alloys.
3.2.Phase analysis
Fig.1 shows XRD patterns of homogenization heat treated alloys before and after hot rolling.In the case of homogenized alloys,it can be seen that ZXM320 and ZXM510 alloys contain Ca2Mg6Zn3phase.However,the peaks belonging to Ca2Mg6Zn3phase were not observed in the XRD pattern of ZXM300 and ZXM500 alloys.As it can be seen from the SEM micrographs(Fig.3),Ca2Mg6Zn3phase is also present in these alloys,but it was not detected by XRD since its amount is out of the detection range of the XRD.An increase or decrease in the intensity of the peaks in the XRD patterns indicates an increase or decrease in the amount of crystals in particular orientations.It is seen that the observed phases(α-Mg+Ca2Mg6Zn3)in XRD analyses are consistent with the findings of Zhang et al.[27]since Zn/Ca atomic ratio of the alloys is more than 1.0–1.2.
XRD analysis results are supported by EDS point analyses results.Fig.2 shows the EDS point analyses of ZXM300-h alloy.In the spectrum,point 2 shows the chemical composition of the matrix(α-Mg).Other points show the composition of precipitates seen in the SEM micrograph of the focused region.All precipitates have a similar chemical composition ofCa2Mg6Zn3phase with slightly higher Mg,Zn,and Ca contents.Although Mn atoms are observed at some EDS points,it is thought that Mn is mainly distributed inα-Mg rather than Ca2Mg6Zn3phase.Higher percentages of Mg,Zn,and Ca elements and Mn observed in Ca2Mg6Zn3phase can be explained with the interference of incoming signals fromα-Mg phase of the eutectic structure(α-Mg+Ca2Mg6Zn3)and α-Mg matrix.
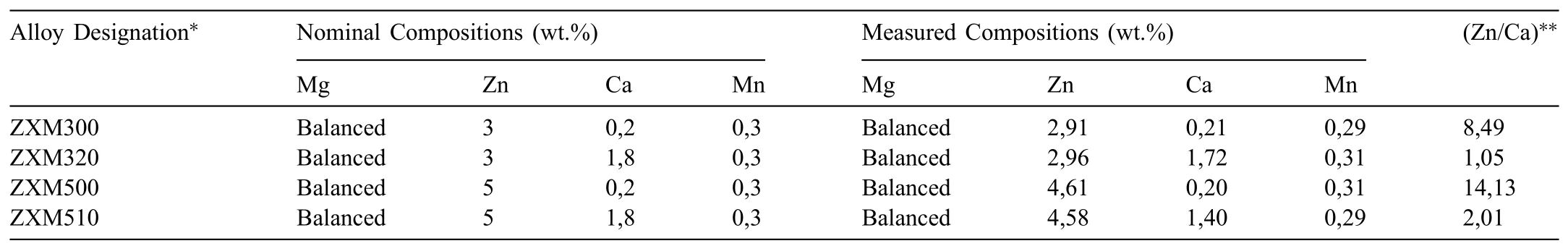
Table 1Nominal and measured compositions of the alloys after homogenization heat treatment.
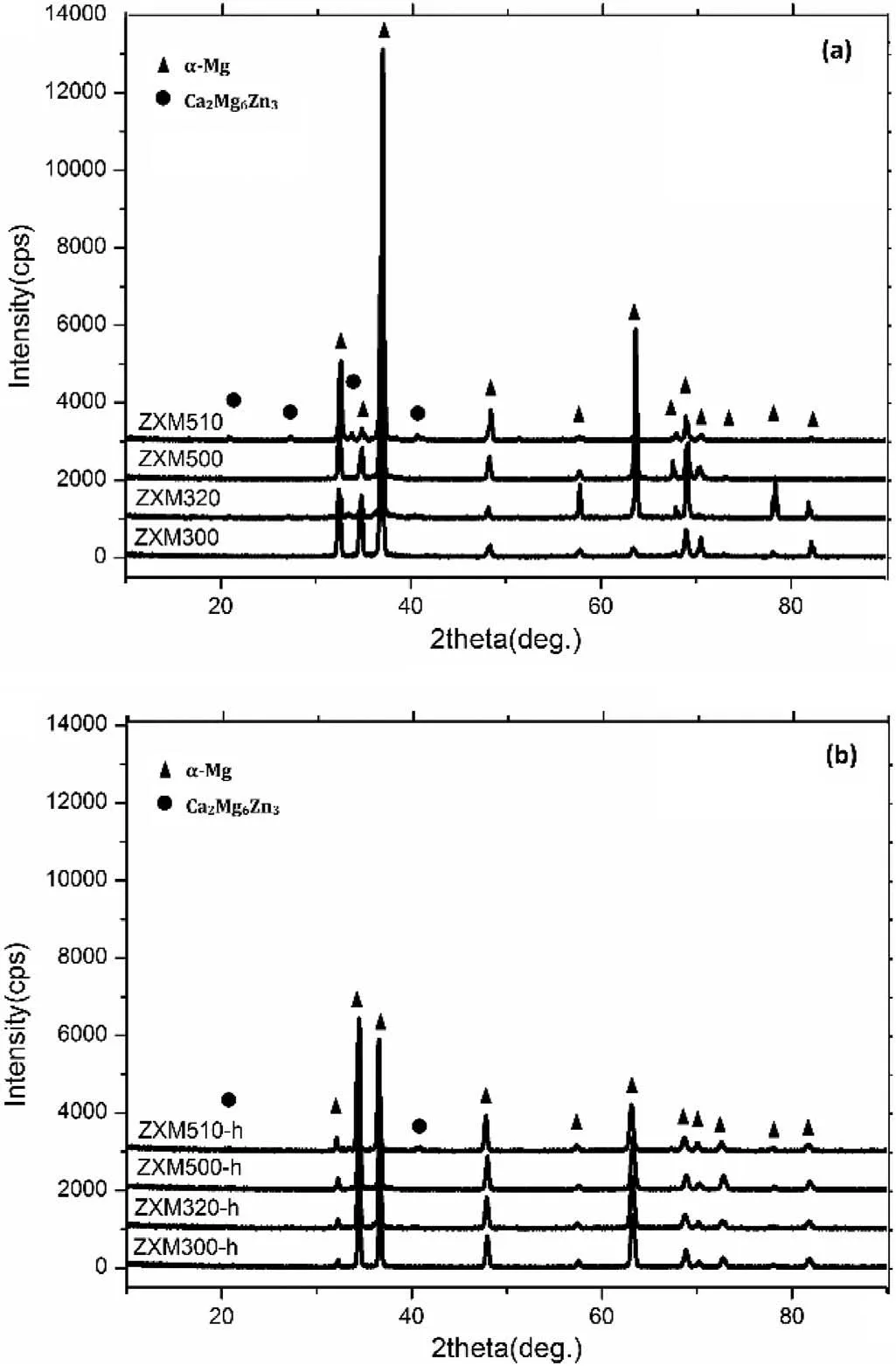
Fig.1.XRD patterns:a)homogenized alloys and b)hot rolled alloys.
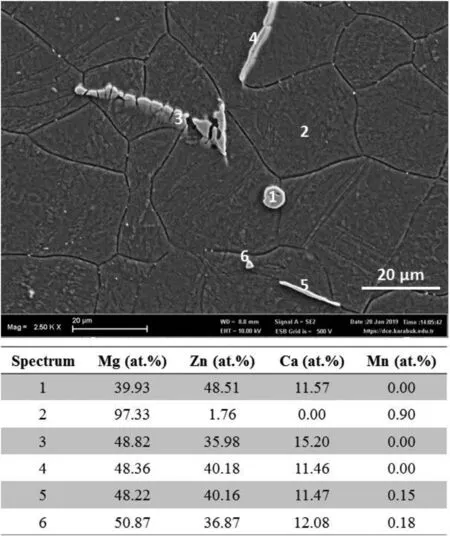
Fig.2.EDS point analyses of ZXM300-h alloy.
3.3.Microstructural analysis
SEM micrographs of all homogenized alloys(ZXM300,ZXM320,ZXM500 and ZXM510)are given in Fig.3.It can be clearly observed that all alloys have mainly coarse grain structure,twins,and secondary phases.The coarse grain structure of the alloys can be explained with grain growth and coarsening mechanisms during homogenization heat treatment.In alloys with low Ca content(ZXM300 and ZXM500),it is observed that grain size decreases with increasing Zn content of the alloy.When the micrographs of Ca rich alloys(ZXM320 and ZXM510)are analyzed,it is seen that grain size does not change much.Therefore,it can be inferred that Ca is not as an effective grain refiner as Zn.This can be supported by the study of Zhang and Yang that the grain refinement effect of Ca in Mg alloy is up to 0.5wt.% Ca[27].
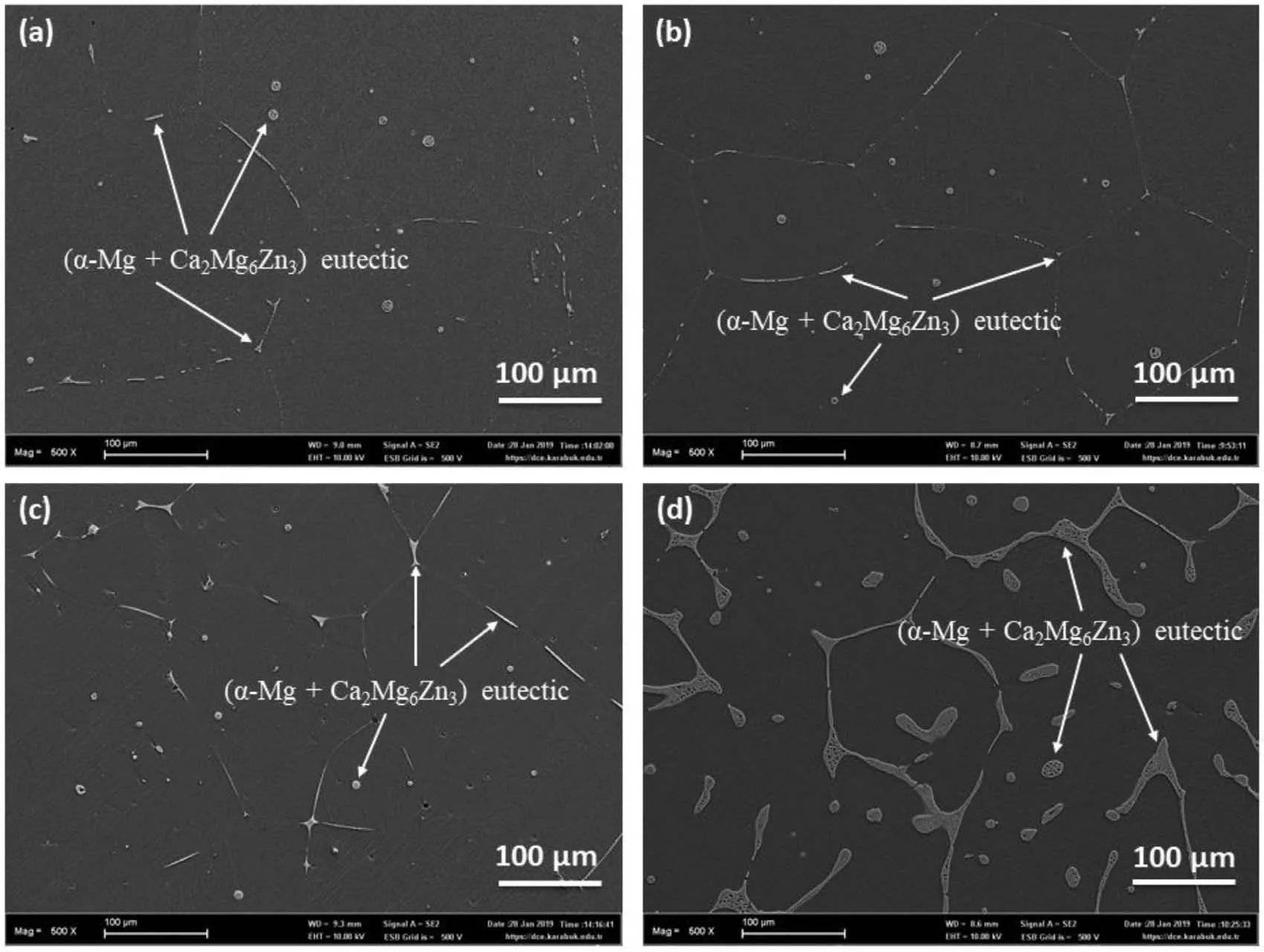
Fig.3.SEM micrographs of the homogenized alloys:(a)ZXM300,(b)ZXM320,(c)ZXM500,and(d)ZXM510 alloys.
Effect of Zn and Ca contents on grain refinement can be confirmed with optical microscope images as given in Fig.4.By comparing the grain structure and the average grain size of the alloys,it can be concluded that Zn is a better grain refiner than Ca.Strip-like secondary phase(Ca2Mg6Zn3)is mainly distributed along the grain boundaries and some of them are in a disk shape in the grains.In fact,Ca2Mg6Zn3phase is mainly part of the eutectic structure formed byα-Mg+Ca2Mg6Zn3phases.In addition,it is seen that amount of eutectic phase increases in micrographs from Fig.3a–d.This is more pronounced particularly in ZXM510 alloy.An increase in size and amount of eutectic phase can’t be explained only with either increase/decrease in Zn or Ca content of the alloy or Zn/Ca ratio,but it can be explained with an increase in the sum of the Zn and Ca content.Moreover,if Fig.3a(ZXM300)and 3c(ZXM500)are compared in terms of Ca2Mg6Zn3phase,it can be seen that Fig.3c has a higher volume fraction of Ca2Mg6Zn3phase.Although both alloys have same amount of Ca content,Zn content of ZMX500 is higher than that of ZXM300.As a result,it can be said that increasing Zn content of the alloys promotes the formation of Ca2Mg6Zn3phase.
Fig.5 shows SEM micrographs and Fig.6 shows optical microscope images of hot rolled alloys(ZXM300-h,ZXM320-h,ZXM500-h,and ZXM510-h).First of all,hot rolling performed above the recrystallization temperature results in finer grains and deformation twin formation.In addition,it is observed that some of the large grains are surrounded by fine grains.Most likely,these large grains correspond to pre-deformed primary grains and fine grains occur during dynamic recrystallization.Since twining is an important deformation mechanism in metals with low crystal symmetry such as hcp and bcc due to a low number of slip systems,twin formation occurs in hcpα-Mg matrix during hot rolling[32].In addition to twins,it is observed that fractures occur in the secondary phases during hot rolling.
3.4.Corrosion properties
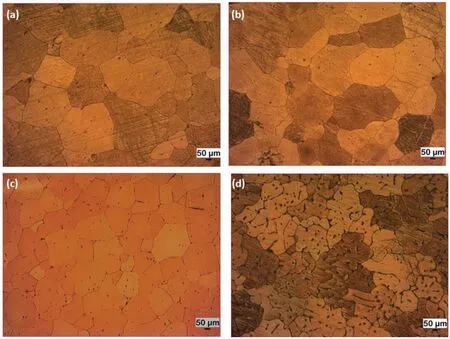
Fig.4.Optic microscope images of the homogenized alloys:(a)ZXM300,(b)ZXM320,(c)ZXM500,and(d)ZXM510 alloys.
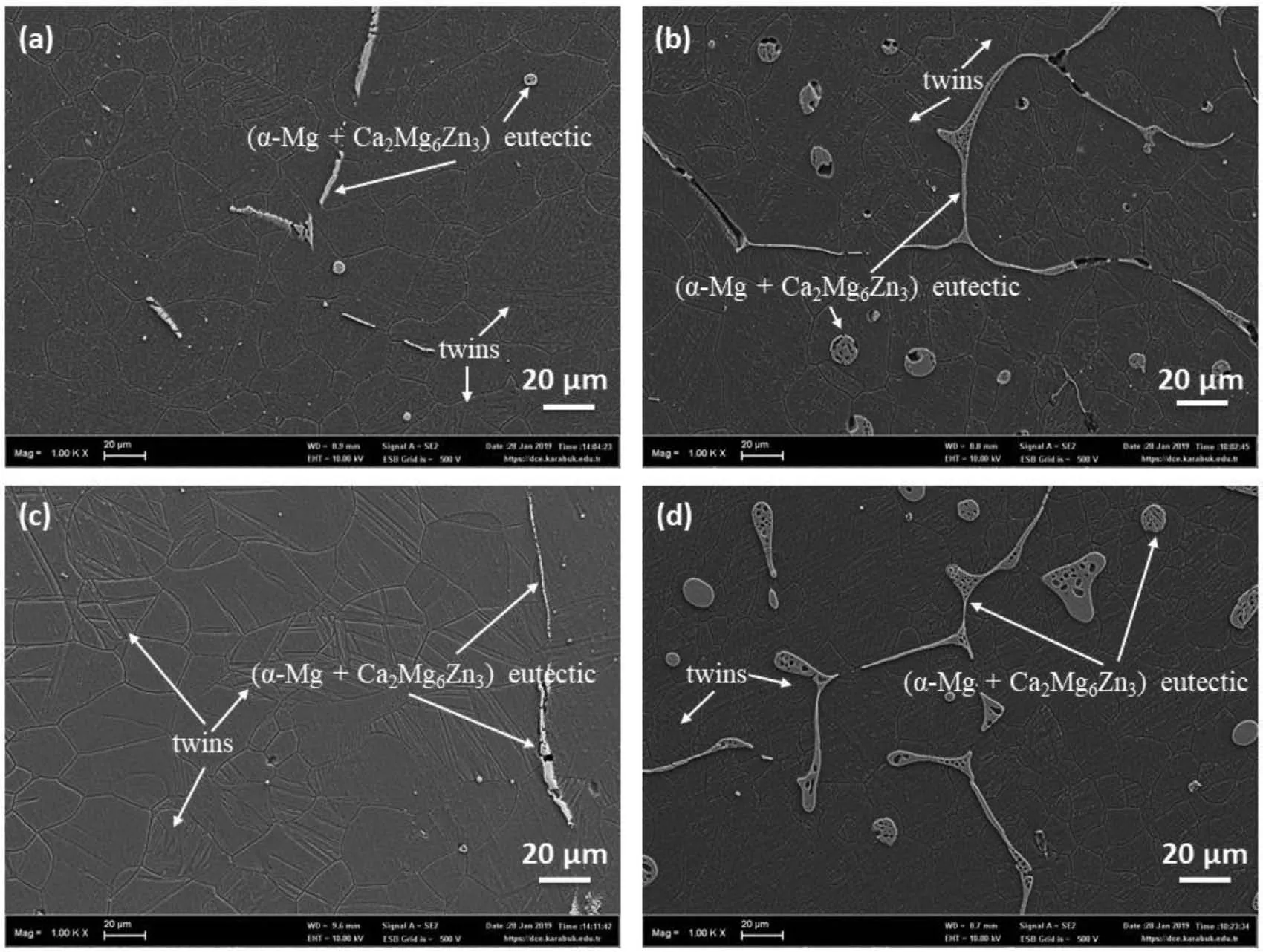
Fig.5.SEM micrographs of hot rolled alloys:(a)ZXM300,(b)ZXM320,(c)ZXM500,and(d)ZXM510 alloys.
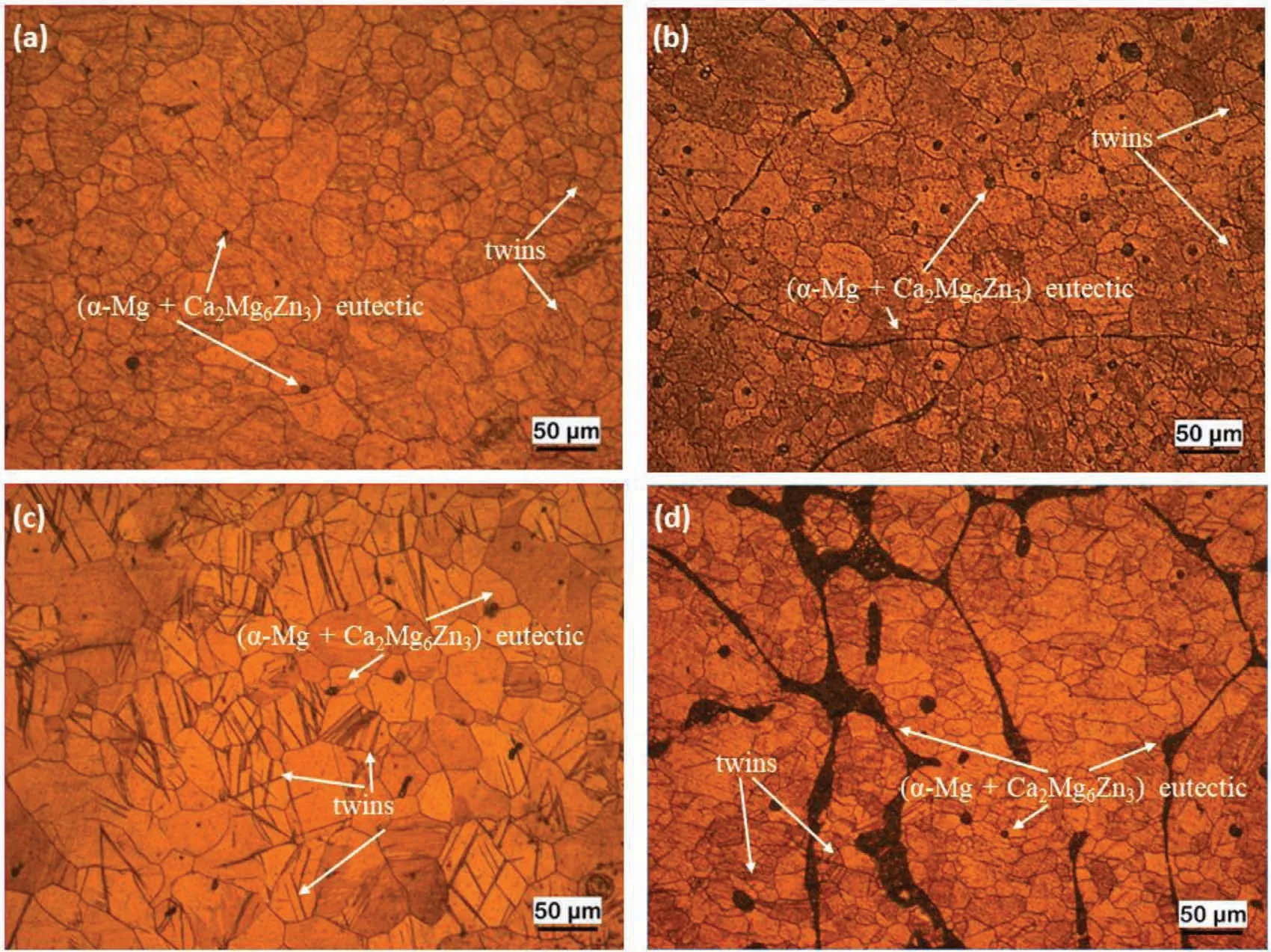
Fig.6.Optic microscope images of hot rolled alloys:(a)ZXM300-h,(b)ZXM320-h,(c)ZXM500-h,and(d)ZXM510-h.
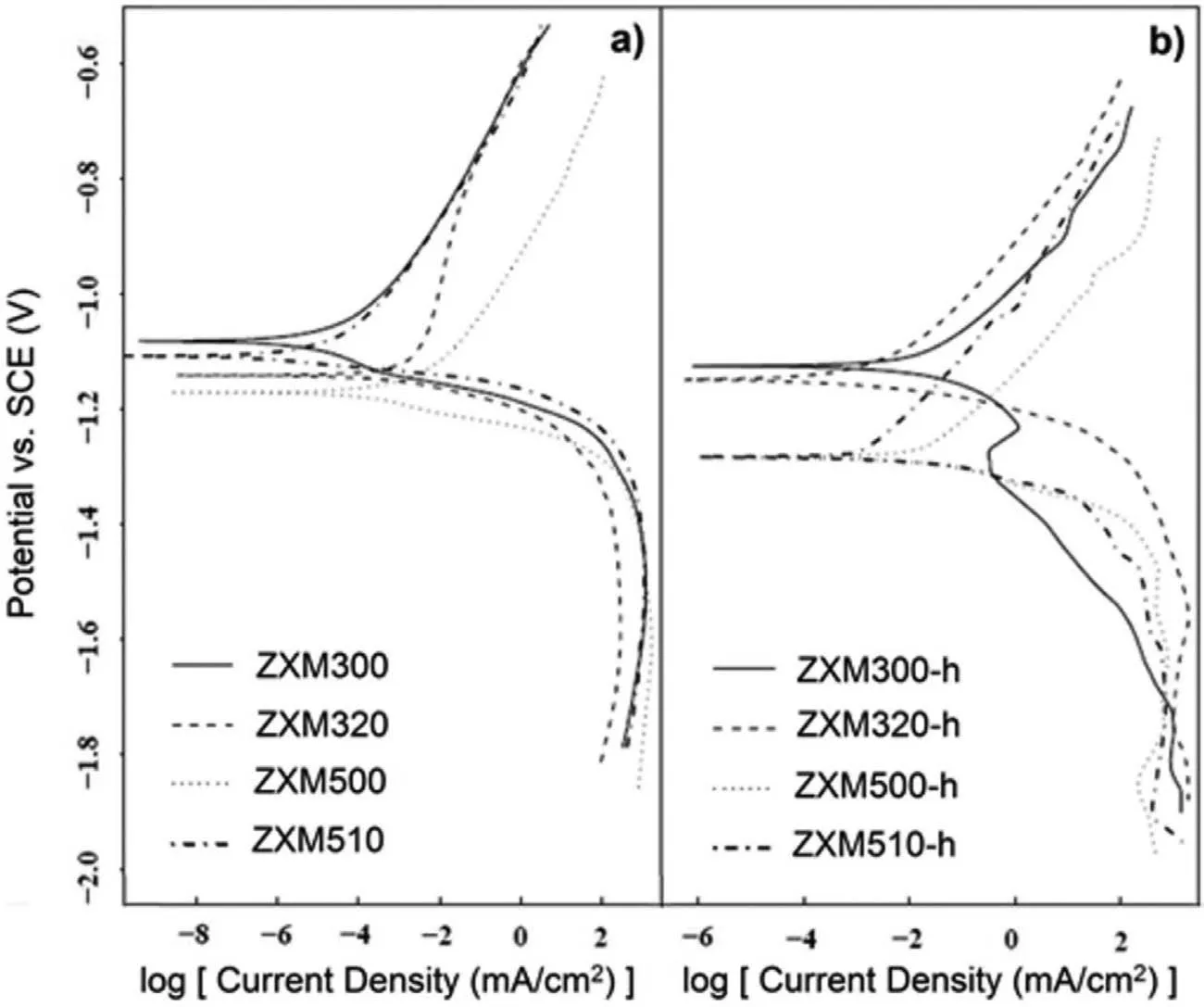
Fig.7.Potentiodynamic polarization curves of the alloys:a)before hot rolling and b)after hot rolling.
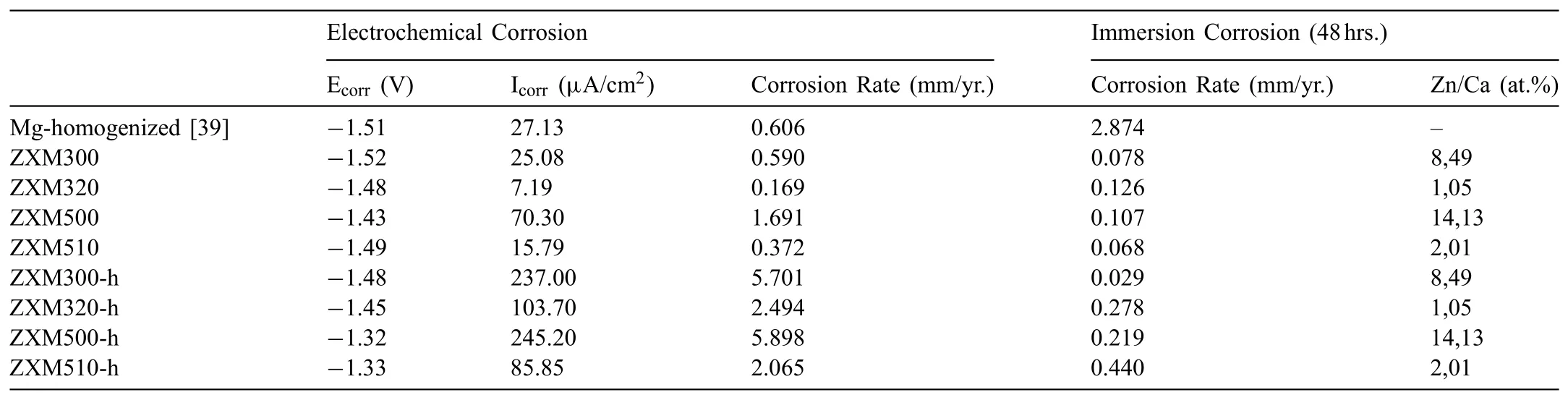
Table 2Comparison of corrosion rates of electrochemical corrosion test and immersion corrosion test results.
Corrosion behavior of the degradable Mg-Zn-Ca-Mn alloys was investigated by using electrochemical and immersion corrosion tests.Tafel curves of the alloys before and after hot rolling are given in Fig.7.When the electrochemical corrosion current densities and corrosion rates of homogenized alloys are compared,it is seen that corrosion current density and corrosion rate of the alloys increase with an increase in Zn/Ca atomic ratio(Table 2).Therefore,corrosion resistance of the alloys decreases with an increase in Zn/Ca atomic ratio.After hot rolling,corrosion potentials of all hot rolled alloys shift positively as compared to those of unrolled alloys.When the corrosion current density and corrosion rate of the hot rolled alloys are compared,trends similar to the change in the current densities and corrosion rates of unrolled alloys are observed except that corrosion current density and corrosion rate of ZXM320-h are higher than those of ZXM510-h.Lower corrosion current density of ZXM510-h can be explained with its higher amount of Ca2Mg6Zn3phase since the electrochemical potential of Ca2Mg6Zn3phase is higher than Mg phase and finer grain size[33–37].When the corrosion resistance of the alloys before and after hot rolling are compared,it is seen that unrolled alloys have much higher corrosion resistance than hot rolled alloys.Hot rolling increases the total stored energy of the alloy due to grain refinement,the formation of twins,and the occurrence of microcracks etc.because all of these are associated with surface energy.An increase in stored energy led by residual strains in deformed grains increases the sensitivity of the grains and grain boundaries in a corrosive environment.Thus,grain boundaries become more vulnerable and corrode easily in an aggressive environment.Decreasing grain size and increasing the total grain boundary area of the alloys during hot rolling provide more sites for the twin nucleation and growth.The presence of twins in the structure causes galvanic cell formation and an increase in twin density have an accelerating influence on the corrosion rate of the alloys.As it is going to be seen in Fig.12c and Fig.12d below,corrosion proceeds through the twinned structures.Aung and Zhou[38]state that the corrosion rate of the twinned structure is higher than the un-twinned structure.Kaviani et al.[22]indicate that micro cracks can be observed during hot deformation about 400 °C.These cracks form around the secondary phases because secondary phases create stress concentration regions and provide sites for the crack nucleation and progress.These cracks increase the corrosion rate by weakening the protective film layer formed on the surface during corrosion.Therefore,it is thought that fine grains,twin bands,and microcracks have negative effects on the corrosion resistance of the alloys.
The results obtained from electrochemical corrosion tests need to be supported by immersion corrosion test or hydrogen release tests since magnesium alloys do not fully comply with the Tafel theory due to the negative difference effect(NDE)[40].
In addition to Potentiodynamic polarization tests,electrochemical impedance spectroscopy(EIS)measurements were performed to understand the corrosion behavior of the alloys.To analyze the EIS spectra of specimens Nyquist plot equivalent electrical circuit model shown in Fig.8 was used.Rsrepresents the resistance of electrolyte between the working and reference electrodes,Rfilmis the resistance of the corrosion product film,Rctdescribes the charge transfer resistance at the interface of electrolyte solution and alloy substrate,CPEfilmis constant phase elements of the corrosion product film,and CPEdlis the capacitance of the charge transfer and double layer.
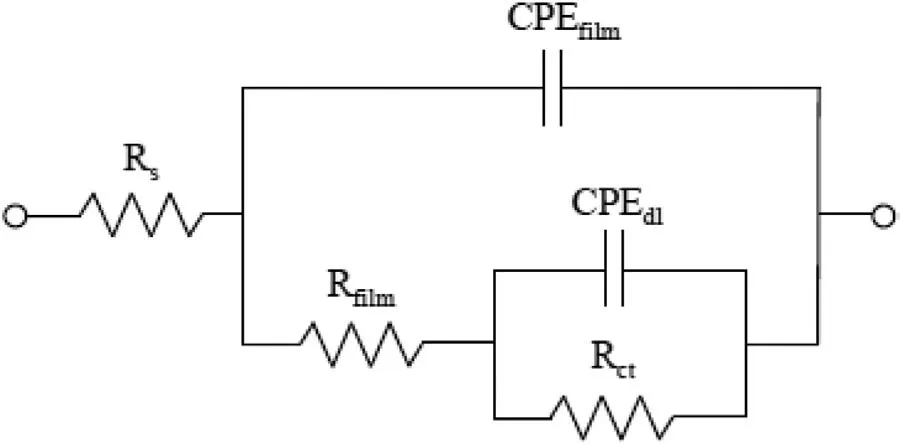
Fig.8.Nyquist plot.
The fitted data are listed in Table 3.The equivalent circuit for homogenization heat treated and hot rolled alloys is given in Fig.9.Theoretically,even if the compositions of the alloys are different,the Rsvalues obtained should be the same or very close to each other[20].It can be observed that the measured resistance of the electrolyte for all alloys is in the range of 10,983±0,980Ω·cm2.Generally,Rctis used as the factor evaluating the corrosion resistance of the alloys due to its essential effects on their corrosion current values[41].Large Rctand Rfilmvalues indicate that surface oxide film of alloys protects the alloy from attack by chloride ions in Hank’s solution[1,20,42,43].It can be seen in Table 3,ZXM300 and ZXM500 alloys have low charge transfer resistances 1256Ω·cm2and 785.5Ω·cm2,respectively.On the other hand,ZXM320 and ZXM510 alloys show higher charge transfer resistances than ZXM300 and ZXM500 alloys.It can be said that these differences occur due to the change in the secondary phase density in the structure because the secondary phase acts as a corrosion barrier to prevent alloy from chloride ion attacks.EIS results satisfy electrochemical corrosion tests in that corrosion resistances of ZXM320 and ZXM510 are higher than those of ZXM300 and ZXM500.Similar results were obtained for the hot rolled alloys.When electrochemical impedance spectra of the hot rolled alloys are compared,it is seen that ZXM300-h and ZXM500-h alloys have higher charge transfer resistancesthan ZXM320-h and ZXM510-h alloys.EIS results of the hot rolled alloys are found to be consistent with the corrosion test results in which ZXM300-h and ZXM500-h alloys have higher corrosion resistance.
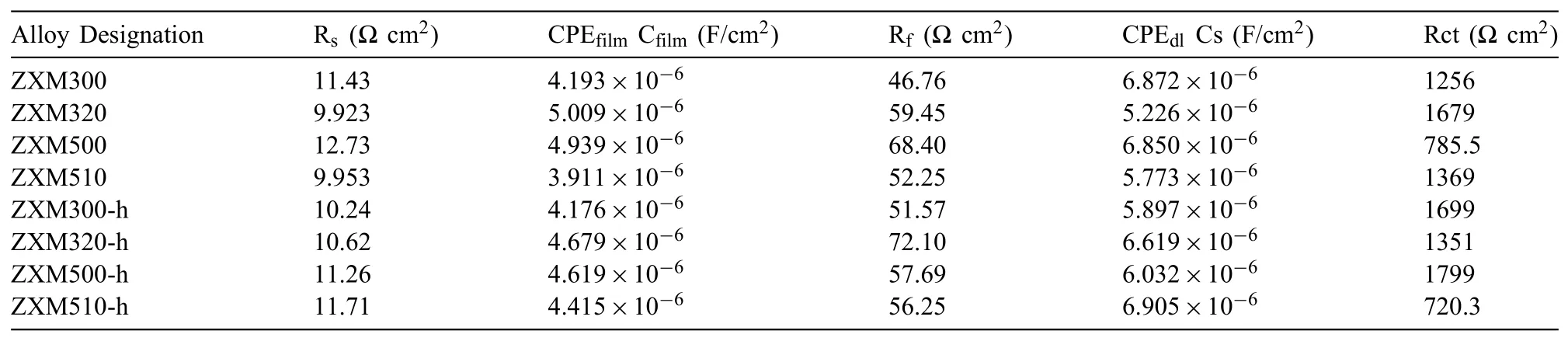
Table 3Fitted EIS results of specimens.
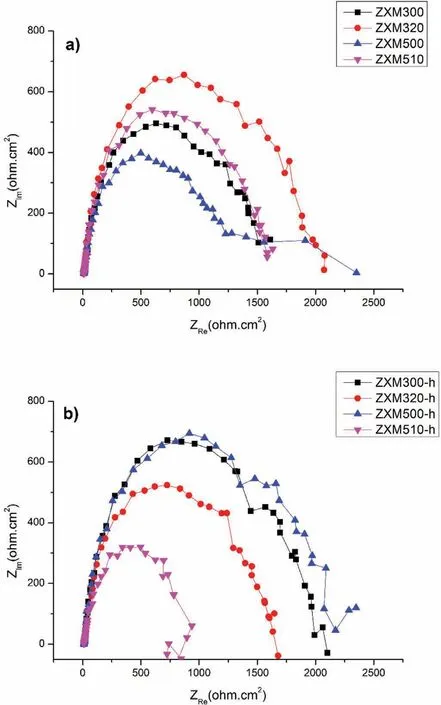
Fig.9.Equivalent circuit of the alloys:a)homogenization heat treated and b)hot rolled alloys.
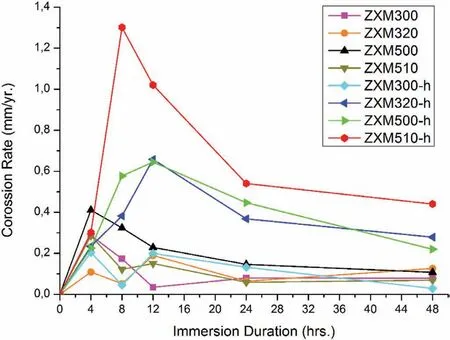
Fig.10.Immersion corrosion rates of homogenized and hot rolled alloys.
Immersion corrosion rates of all alloys(before and after hot rolling)are given in Fig.10,comparatively.First of all,irregular corrosion behavior is observed in all alloys up to an immersion time of 12hrs.Immersion corrosion rates of hot rolled alloys are higher for all immersion times than those of unrolled alloys.An increase in immersion corrosion rate of the alloys after hot rolling can be explained with the negative effects of increasing grain boundary area and twin density,breaking secondary phases and formation of micro-cracks,etc.on the corrosion resistance of the alloys.
The corrosion rates obtained from the electrochemical corrosion test and the immersion corrosion tests are given in Table 2,comparatively.As it can be seen from the results,the electrochemical corrosion rates of all alloys are much higher than the immersion corrosion rates.The reason is that the results obtained from the electrochemical corrosion test are measured in a short time and the aggressive period at the initial stage of corrosion.Since there is no passivation on the surface due to corrosion residues,the results of the electrochemical corrosion tests performed in a short time and clean surface are much higher.
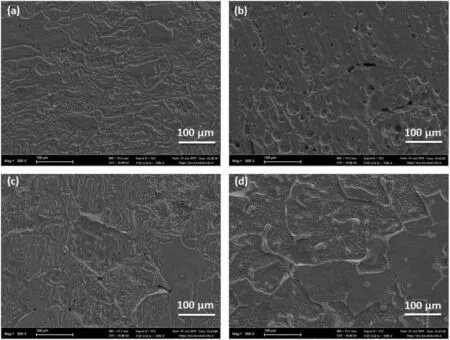
Fig.11.SEM micrographs of homogenized alloys after immersion corrosion test for 48hrs.:(a)ZXM300,(b)ZXM320,(c)ZXM500,and(d)ZXM510.
Fig.11 shows the SEM micrographs of homogenized alloys after the immersion corrosion test for 48hrs.duration.It is determined that the degradation is more superficial due to the homogeneous distribution of Mn in the structures since Mn has preventing effect of corrosion.It is seen that galvanic corrosion occurs betweenα-Mg acting as anode and Ca2Mg6Zn3phase acting as a cathode.The apparent presence of the secondary phase(Ca2Mg6Zn3)along the grain boundaries and inner grains indicates thatα-Mg is preferably corroded due to its lower electrochemical potential as compared to Ca2Mg6Zn3.Therefore,it can be concluded that the presence of Ca2Mg6Zn3secondary phase in the structure has a slowing effect on the corrosion rate of the alloys since Ca2Mg6Zn3phase acts as a barrier.Li et al.indicated that when a fine secondary phase precipitated as a network structure on the grain boundaries,it can act as a barrier to stop penetration of corrosion and as a result corrosion resistance of alloy improved[44].This is clearly seen especially in Fig.11c and d,corrosion proceeds directly within the matrix and secondary phases show a barrier effect against corrosion progression.In addition,galvanic cells betweenα-Mg matrix and Ca2Mg6Zn3initiates pitting corrosion in the surface of the alloys.As it is seen in Fig.11b,pitting corrosion is more pronounced in ZXM320 alloy in which corrosion proceeds deep into the grains.
Fig.12 shows the SEM micrographs of hot rolled alloys after the immersion corrosion test for 48hrs.It is observed that the specimens are more aggressively exposed to corrosion compared to unrolled alloys.The lower corrosion resistance of hot rolled alloys can be attributed to increase in grain boundary area and twin density,occurrence of micro-cracks,and formation of other defects in the alloys during hot rolling as it was explained above.
3.5.Mechanical properties
Tensile properties of the alloys before and after hot rolling are given in Fig.13 and Table 4,comparatively.It is seen that homogenized alloys have much higher ductility and toughness than hot rolled alloys,but lower tensile strength.
When the influence of the alloying elements on the mechanical properties of the homogenized alloys are investigated,it is found that tensile strength,ductility,and toughness of the homogenized alloys increase with the amount of Zn in the alloy.The reason for this increase is that Zn has a solution hardening effect on magnesium alloys[45].On the other hand,increasing the amount of Ca in the alloy results in much lower tensile strength,ductility,and toughness.This can be attributed to the higher volume fraction of Ca2Mg6Zn3phase that increases with increasing Zn and Ca content of the alloy and to the increasing brittleness.Therefore,ZXM500 alloy with the highest Zn and the lowest Ca content showed the highest tensile strength,ductility,and toughness among the homogenized alloys.
Hot rolling results in a significant increase in yield strength and tensile strength,but much lower ductility and toughness.Tensile strength and elongation of the hot rolled alloys having high Ca content(ZXM320-h and ZXM510-h)are lower than those of the alloys having low Ca content(ZXM300-h and ZXM500-h).It is thought that presence of higher volume fraction of Ca2Mg6Zn3phase in higher Ca containing alloys increases stress concentration sites and density of micro-cracks that have negative effects on the strength and ductility of the alloys.
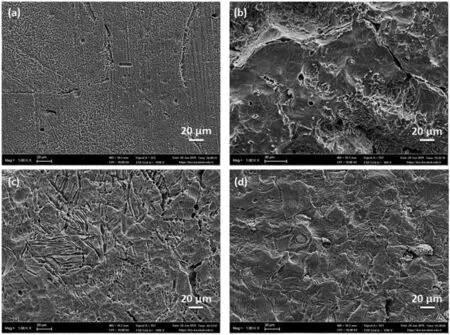
Fig.12.SEM micrographs of hot rolled alloys after immersion corrosion test for 48hrs.:(a)ZXM300-h,(b)ZXM320-h,(c)ZXM500-h,and(d)ZXM510-h.
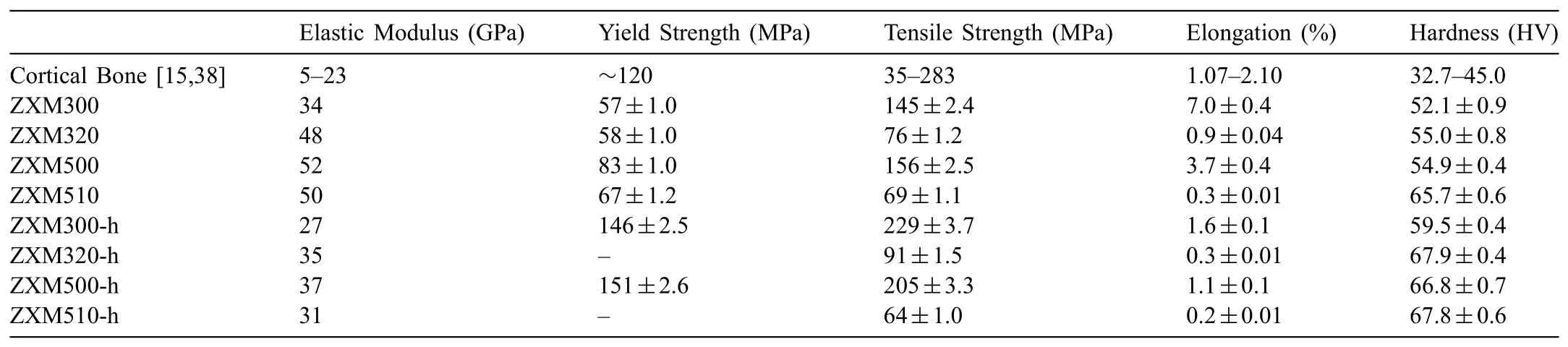
Table 4Mechanical test results of all samples and comparison of them with cortical bone.
A schematic comparison of hardness test results of all alloys is given in Fig.14.As it is seen from Fig.14,the hardness of the alloys increases with increasing Zn and Ca content of the alloy.As compared to Zn,Ca is more effective to increase the hardness.An increase in hardness values of the alloys can be attributed to an increase in volume percent of the secondary phase(Ca2Mg6Zn3).After hot rolling,the hardness of the alloys increases further.Increase in hardness is between 3.2% for the ZXM510 alloy and 23.5% for the ZXM320 alloy.The increase in the density of dislocation and the formation of twins during hot rolling causes an increase in hardness[38].
As a summary,when the mechanical properties of cortical bone are compared with the mechanical properties of the alloys studied here,it is seen that all homogenized alloys are much more ductile than cortical bone and they don’t have enough strength to carry loads.On the other hand,hot rolled ZXM300-h and ZXM500-h alloys satisfy all the properties of cortical bone because Young’s modulus,yield strength,tensile strength,elongation,and hardness values of these alloys are better than those of cortical bone(Table 4).When the corrosion rate and mechanical properties of the alloys are considered together,ZXM300-h comes to forward due to its much lower immersion corrosion rate than that of ZXM500-h.
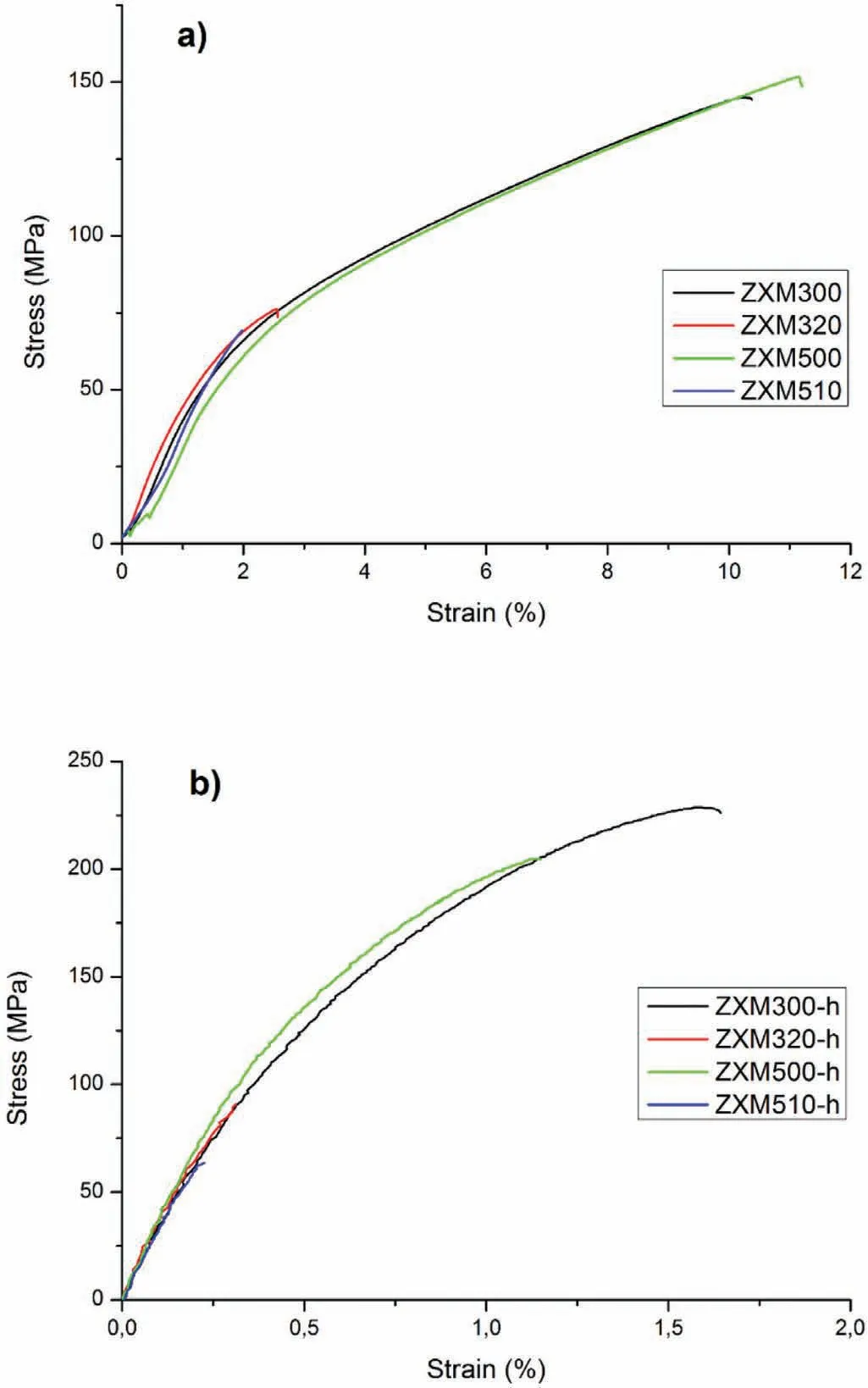
Fig.13.Stress vs.Strain curves of the alloys:a)homogenization heat treated and b)hot rolled.
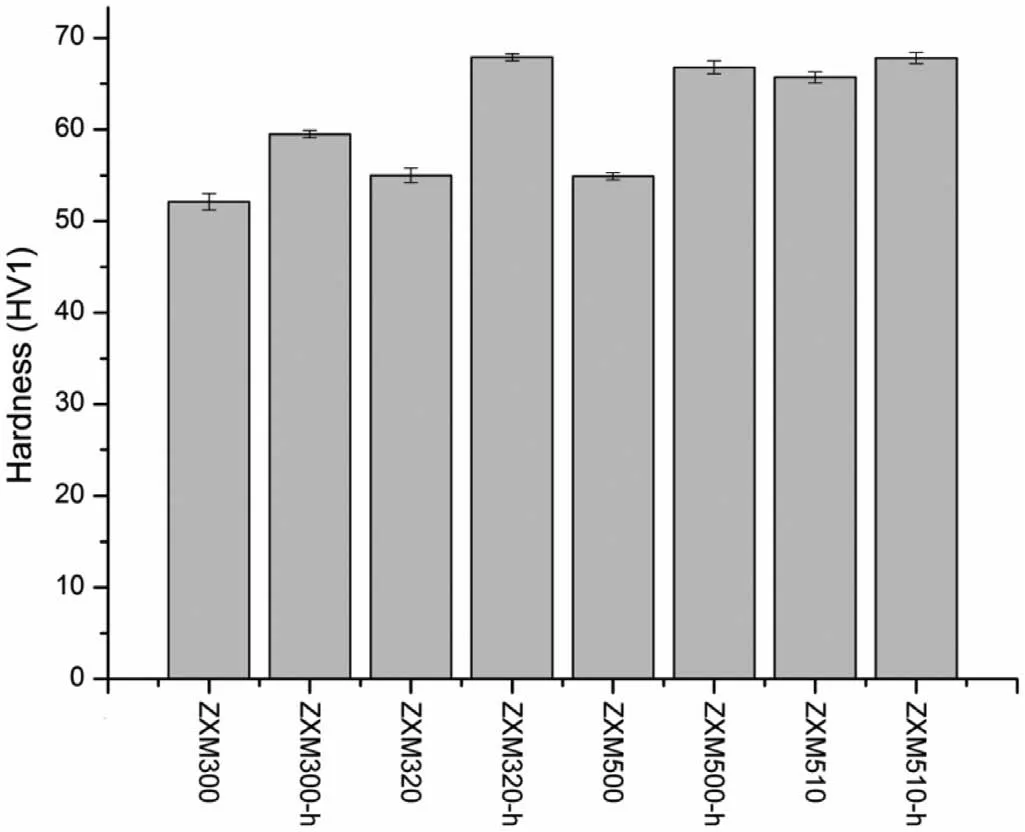
Fig.14.Vickers hardness of the homogenized and hot rolled alloys.
4.Conclusion
In this study,four different magnesium alloys with a constant ratio of Mn and two different ratios of Zn and Ca were produced,homogenization heat treatment and hot rolling were applied.The following results were obtained:
•It is determined that the microstructures of the alloys are composed ofα-Mg as the matrix phase and Ca2Mg6Zn3as the secondary phase.
•Zn promotes Ca2Mg6Zn3phase formation and the amount of Ca2Mg6Zn3phase increases with increasing Zn and Ca% of the alloy.
•In Mg-Zn-Ca-Mn quaternary system,Zn is a more effective grain refiner than Ca.
•The electrochemical corrosion resistance of the alloys decreases with increasing Zn/Ca atomic ratio.
•According to the results obtained from the corrosion tests,it is seen that the immersion corrosion rates of all alloys(before and after the rolling)are below the bio-degradation rate(<0.5mm/yr.)that is required for the broken bone support plate.
•When the alloys and cortical bone are compared in terms of mechanical properties,it is seen that ZXM300-h and ZXM500-h alloys have better mechanical properties than cortical bone.
•Although immersion corrosion rates of these alloys after 48hrs.(0.029mm/yr.for ZXM300-h and 0.219mm/yr.for ZXM500-h)satisfy acceptable corrosion rates,ZXM300-h alloy has the best mechanical properties and corrosion rate to be used as a biodegradable fracture bone plate alloy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was supported by the Scientific Research Projects Coordination Unit of Karabuk University.Project Number:KBU-BAP-16/2-DR-100.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
