Improved methodological concepts for processing liquid Mg at high temperature
2021-03-10ArturKudyNtliSozkWojiehPolkowskiGrzegorzBruzdAdeljdPolkowskDontellGiurnno
Artur Kudy,Ntli Sozk,Wojieh Polkowski,Grzegorz Bruzd,Adeljd Polkowsk,Dontell Giurnno
aŁukasiewicz Research Network-Krakow Institute of Technology,Zakopia´nska 73 Str.,30-418 Kraków,Poland
b Institute of Metallurgy and Materials Science,Polish Academy of Science,25 Reymonta St.,30-059 Kraków,Poland
c National Research Council of Italy,Institute of Condensed Matter Chemistry and Technologies for Energy,Via De Marini 6,16149,Genova,Italy
Received 20 April 2020;received in revised form 29 May 2020;accepted 11 June 2020
Available online 30 June 2020
Abstract In this paper,new improvements of methodological concepts upon examining wettability of high vapor pressure liquid metal systems(e.g.Mg-based alloys)in contact with refractory materials,are presented and discussed.In this regard,high-temperature experiments on molten magnesium(Mg)in contact with graphite as a refractory substrate,were performed by utilizing a newly developed testing device and by applying a suitable experimental procedure.The wetting experiments were carried out by the sessile drop method and under identical testing conditions(700°C/10min under a protective gas atmosphere).Two different procedures were applied:the classical contact heating(CH)or a newly introduced capillary purification(CP)one.The contact angle behaviors observed under the same conditions were strongly influenced by the applied procedure.Specifically,in the case of using the CH procedure,a presence of native surface oxide layer on the metal surface hinders the observations of melting process,making not possible to experimentally determine the wetting kinetics curveθ=f(t).Contrarily,during the wetting test performed on the Mg/graphite couple by applying the CP procedure,the native surface oxide layer was mechanically removed during the squeezing of the molten Mg through the hole of a capillary.Indeed,an oxide-free squeezed Mg-drop with regular and spherical shape was successfully obtained and dispensed on the graphite substrate.Consequently,the reliable contact angle value around θ=150° for the Mg/graphite system,was measured within the wetting test.
Keywords:Mg-alloys;Magnesium matrix composites;Sessile drop;Capillary purification procedure;Wettability;Contact angle.
Introduction
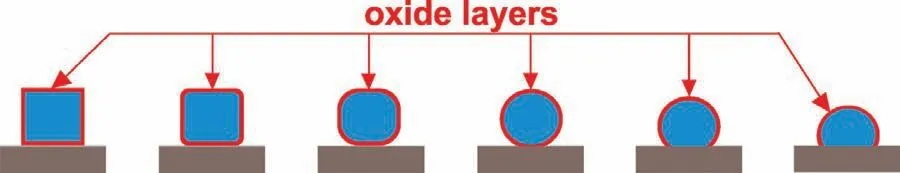
Fig.1.A scheme of high-temperature wettability test by classical sessile drop method(SD)combined with a contact heating procedure(CH).
In recent years,a growing interest in experimental measurements of thermophysical,physicochemical and technological properties of molten Mg and Mg-based alloys is observed in both basic and applied research areas[1–10].Mgbased alloys,and especially Mg-based metal matrix composites(MMCs),are attractive candidates for many structural applications,mainly due to their high specific strength and stiffness or a very good wear resistance[1].The light-weight and high strength-to-weight Mg-based MMCs offer incredible advantages in the aerospace use.Beside of space applications,light-weight materials are extremely desired for various structural and functional applications in automotive,military,medicine and others industrial branches[2–4].However,in order to ensure a high quality of products made by Mg-based MMCs,it is necessary to deeply understand and determine the interfacial phenomena taking place between molten Mg(a matrix)and various reinforcements.On the other hand,the measure of a set of reliable thermophysical,physicochemical and technological properties of liquid Mg and Mg-based alloys is a very challenging task,due to the high chemical affinity of Mg to oxygen leading to an almost instant formation of a continuous and thick surface oxide layer.The presence of such oxide impedes the intimate contact between the molten metal phase and the substrate.Consequently,it makes really difficult to conduct molten Mg drop-assisted experiments and the reliability of the results obtained is highly questionable.Furthermore,a well-recognized intensive evaporation of molten Mg upon high-temperature measurements leads to a potential contamination,or in the worst scenario,to a fatal damage of expensive components included or assembled into the experimental devices.For this reason,only few research laboratories are capable to carried out properly such experiments,and a limited number of works on wetting characteristics measurements of Mg-based alloys are reported in literature.The literature survey[5–10]revealed that available papers are mostly concerned on experiments involving a classical sessile drop method combined with a contact heating(CH)procedure,as shown in Fig.1.Since in this attempt a native oxide film cannot be removed from the Mg drop surface,the results obtained by the CH procedure should be discussed by taking into account the presence of the mentioned oxide layer.
The affecting phenomena of oxidation and evaporation of Mg upon wettability tests,have been carefully described by Contreras et al.[5],and the effect of the testing temperature on the wetting and spreading behavior of pure Mg on TiC substrate,were analyzed.In this regard,the CH procedure at T=800,850 and 950°C,in a static Ar atmosphere,was applied.The authors report that TiC is not wettable by liquid Mg in the temperature range of T=800–850°C.Namely,at T=800°C a decrease of contact angle was observed from 120° to 100° and a further decreasing was observed by increasing the temperature up to T=850°C and after an holding time of 2700 s.The authors concluded that the wetting kinetics observed at the Mg/TiC triple line was strongly affected by the presence of an oxide layer at the liquid metal surface.Aiming to provide“true”contact angle values(i.e.not affected by any“external”factor like surface oxide or surface roughness of the substrate,etc.),the testing temperature was increased up to 950°C(i.e.300°C above the melting point of Mg).By taking into account thermodynamic considerations,at such temperatures the oxide layer at the surface of the liquid Mg drop is thermally removed,and as a consequence,a reliable contact angle value at the Mg/TiC triple line(θ~15°after t=2700 s)is exhibited.However,at temperatures much higher than the melting point of Mg,the substantial evaporation of the material should be carefully considered,in order to limit the contamination of the experimental environment and to protect the apparatus against a severe damage.In addition,a strong material evaporation increases the ratio of the solid base diameter to solid height,which in case of reactive phenomena at the interface,makes the chemical-physical meaning of the measured contact angle values as“apparent”[11].
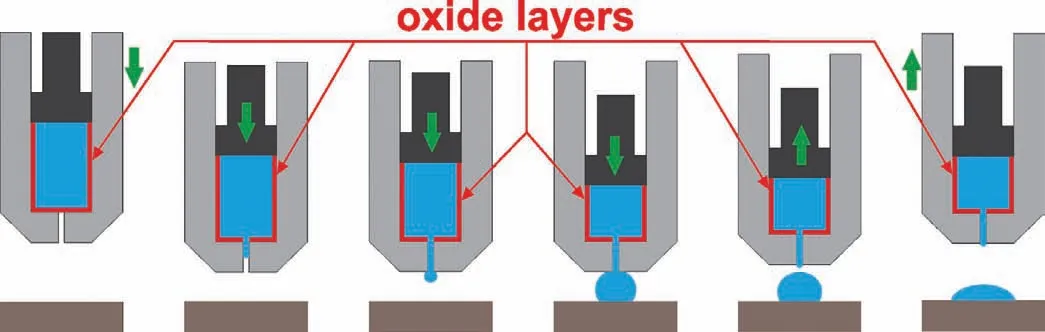
Fig.2.A scheme of high-temperature wettability test by classical sessile drop method(SD)combined with no-contact capillary purification procedure(CP).
For the mentioned reasons,experimental procedures involving a sessile drop obtained by the capillary purification method(CP)(Fig.2)could be pointed out as the more efficient way in successfully avoiding the negative effect of native surface oxide layer.In the CP procedure both the metal and the substrate are heated up separately up to the testing temperature.Specifically,at the beginning of the experiment,the metal is placed in a capillary located above the substrate and after reaching the selected testing temperature,as mentioned previously,it is squeezed through a hole of the capillary.Nevertheless,a more detailed description of the CP procedure is described elsewhere[11,12],while here the main benefits provided by applying the mentioned procedure are highlighted:
(I)non-contact heating of the couple-i.e.undesirable effect of the heating history are negligible,which is relevant in the case of highly reactive systems such Mg/metals or Mg/oxides;
(II)purification of the drop from surface oxide layer–as shown in Fig.2,the native oxide films is mechanically removed during the squeezing of the liquid Mg-drop form the capillary,which allows the measurement of true contact angle values.
As already introduced,the number of reported works on wetting properties of Mg-based alloys using the sessile drop method combined with the CP procedure is still very limited.To our best knowledge,as documented in[13,14],only Fujii et al.have reported results about wetting behavior of molten pure Mg and Mg–Al alloy in contact with a pure Ti substrate.Namely,the CP procedure was applied to evaluate the effect of both the Mg evaporation during the wetting test;and the presence of surface oxide film of the Ti substrate on the contact angles value at T=800°C[13].It is worth to be highlighted that the CP procedure was applied,but the liquid metal was squeezed out by imposing an external pressure.In particular,pressurized Ar was used as the squeezing medium(instead of a piston,as shown in Fig.2)and consequently the molten Mg drop was formed at the hole of the MgO-capillary and dispensed onto the Ti substrate.Fujii et al.concluded that the Mg-evaporation during the wetting test significantly affected the obtainedθvalues.Accordingly,they proposed a post-processing re-calculation taking into the consideration a change of height and diameter of the molten drop.The initial contact angle of the molten Mg with the oxidized substrate was in the range ofθ=95–110° However,the authors correctly considered the presence of an oxide layer at the liquid Mg/Ti interface as affecting factor of the wetting kinetics observed within the first instants of the experiment.In addition,by taking into account the oxide removal process at the Ti surface(allowing the“intimate”contact between Mg and Ti),the contact angle decreased toθ=68° and this value was kept constant until the end of the wetting experiment.
In order to overcome the existing technical difficulties in the characterization of high vapor pressure liquid metals prompt to be oxidized(including Mg)the authors ad-hoc developed a new experimental device,as documented in[15,16].A similar complex device already exists in theŁukasiewicz-Krakow Institute of Technology laboratories,as detailed elsewhere[11,12,17–19],which allows an unique set of experimental capabilities.On the other hand,its complex construction makes it very expensive and time-consuming for maintenance and for cleaning up the components of the experimental chamber(i.e.removing the products formed via evaporation/condensation mechanism)after each single experiment.Consequently,a pre-contamination of the chamber components may either(I)significantly affect a phase equilibria and various chemical reactions taking place during next experiments or(II)lead to unexpected lowered efficiency or even failure of the crucial components inserted into the experimental device.In order to overcome the technological difficulties encountered mainly in investigating Mg-based materials,the newly designed and assembled apparatus includes:(I)a simplified assembling of the high-temperature chamber allowing easy and fast cleaning;(II)the possibility for examining different samples in a unique experiment and under the same experimental conditions,which allows the user to make shorter the time for investigation;(III)the capability to perform sessile drop experiments by applying the CP procedure that increases the reliability of the results obtained even when high-vapor pressure molten phases are processed,such as liquid Mg and Mg-based alloys.
In the following sections of the paper,all the related issues concerning the design,a detailed description of the device and some testing possibilities will be provided.In this regard,relevant examples of high-temperature experiments safely carried out for molten Mg in contact with graphite,will be given
Experimental design
A view of the device for safely processing liquid Mg and its alloys is shown in Fig.3a,as well as the available testing methods and procedures are schematically presented in Fig.3b.
A detailed scheme and description of the device is provided in the Appendix A.Here,the following main advantages respect to the already existing apparatuses,are drawn:
1)A simplified construction of the high-temperature chamber allowing easy and fast cleaning of its interior parts and walls from deposited metal materials as residual of the carried out experiments.
2)An unique capability to simultaneously test up to 10 samples during one single experiment under the same test conditions(atmosphere,temperature etc.).
3)A possibility of using different testing methods and procedures(Fig.3b)for investigating molten Mg alloys with a particular emphasis on the CP procedure(with up-and-down movement of a capillary containing liquid metal).Additionally,other testing procedures including the drop sucking and drop pushing,especially useful for in situ“opening”of the drop/substrate interface at the testing temperature for the characterization of interfacial reaction products,are also available.
Beside of aforementioned measurements capabilities,the following attractive features of the new apparatus are listed:
1)A wide range of testing temperatures(up to 1000°C).
2)A possibility to carry out experiments under high vacuum conditions(up to 10−7 hPa),under the inert gases atmosphere or both(a flowing gas with controlled rate at required level of pressure inside the vacuum chamber).
3)An automatic real-time checking of the temperature by 4 thermocouples located in different positions of the chamber.
4)A fully automatic and remotely controlling/recording computerized system of the overall testing parameters.
Examples of experiments on molten Mg
In order to testify and present a wide range of possible options offered from the new/improved ad-hoc designed experimental device,the wettability of dense graphite substrates(Svenska Tanso AB,Sweden)by liquid Mg(99.98 wt%-Stanchem,Poland)was investigated at high temperature by using the SD method.Before the experiments each piece of Mg used in described experiments was subjected to a mechanical grinding with SiC papers followed by ultrasonic cleaning in C3H8O alcohol(isopropanol)for 5 min.After that,cleaned and degreased Mg pieces were put either on the graphite substrate(for the CH procedure)or in the graphite capillary(for the CP procedure).Subsequently,the samples were immediately transferred into the experimental chamber.After positioning the samples,a high vacuum of 5×10−6was produced,and then a flowing Argon(99.999% was introduced).Finally,the CH and CP experiments were performed in a one single trial,under exactly the same conditions carried out under a flowing Ar(99.999%)atmosphere at T=700°C/10min.During the experiments the Mg/graphite couple images were recorded by the high-speed digital CCD camera at 50 frames per second,and then the images were processed to measure contact angle values over time and to analyze the related wetting kinetics,by using ASTRA 2 software.
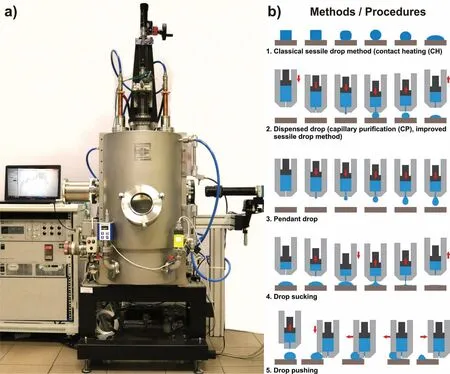
Fig.3.High-temperature device for investigation of properties of high vapor pressure liquid metals and their interaction with refractory materials:a–front view;b–possible testing methods and procedures[15,16].
Selected images of the liquid Mg/graphite couple recorded during the high-temperature wetting test performed by the CH procedure,are shown in Fig.4.The entire video recorded upon the test is available as a SUPPLEMENTARY MATERIAL #1.
As it can be seen,during the wetting experiment performed by using the CH procedure,the melting of the Mg piece placed on the graphite substrate(Fig.4a)was detected at T=650°C close to the contact area(Fig.4b).After a further heating up to 700°C,the Mg/graphite couple was held for 600s.The shape of the Mg piece did not significantly change during the whole test.It was caused by the presence of a native surface oxide layer hindering the achieving of the complete melting of Mg sample,as shown in Figs.4c-h.Consequently,it was not possible to measure a“true”value of the contact angle and to analyze the Mg/graphite wetting kinetics(compare with Fig.1).
The existence of a stable and thick oxide scale at the surface was confirmed by the results of SEM/EDS analyses performed at the top of the solidified Mg/graphite sample after the CH test,as shown in Fig.5.As it comes from the collected EDS spectra,the appearance of the two singular spectra peaks revealing the presence of Mg and O confirms that the native oxide layer was not removed during the test.
In Fig.6,selected images recorded during the hightemperature wetting test performed on the Mg/graphite couple by the CP procedure are collected and shown(the corresponding video is available as the SUPPLEMENTARY MATERIAL#2).
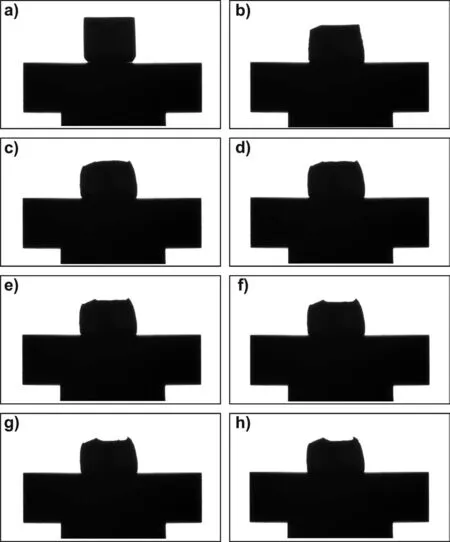
Fig.4.Images recorded by the CCD camera during the test of Mg/graphite using contact heating(CH)procedure at T=700°C for t=600s,under flowing Ar(99.999%)atmosphere:a)the couple before test at T=25°C;b)the beginning of melting at T=650°C;c)the beginning of the test at T=700°C,t=0s;d)T=700°C,t=120s;e)T=700°C,t=240s;f)T=700°C,t=360s;g)T=700°C,t=480s;h)the end of the test at T=700°C,t=600s.

Fig.5.The representative results of SEM/EDS analyses taken on the surface of re-melted solidified Mg sample during the CH test of Mg/graphite couple(T=700°C,t=600s).
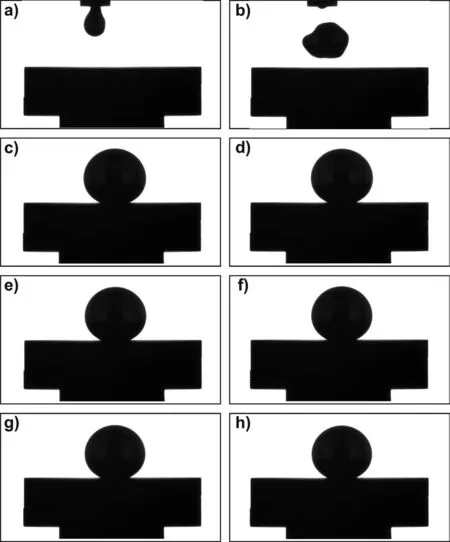
Fig.6.Images recorded by the CCD camera during the test of Mg/graphite by using graphite capillary(the CP procedure at T=700°C for t=600s,under flowing Ar(99.999%)atmosphere):a)the start of dropping liquid Mg from graphite capillary at T=700°C;b)falling drop on graphite substrate;c)the beginning of the test at T=700°C,t=0s;d)T=700°C,t=120s;e)T=700°C,t=240s;f)T=700°C,t=360s;g)T=700°C,t=480s;h)the end of the test at T=700°C,t=600s.
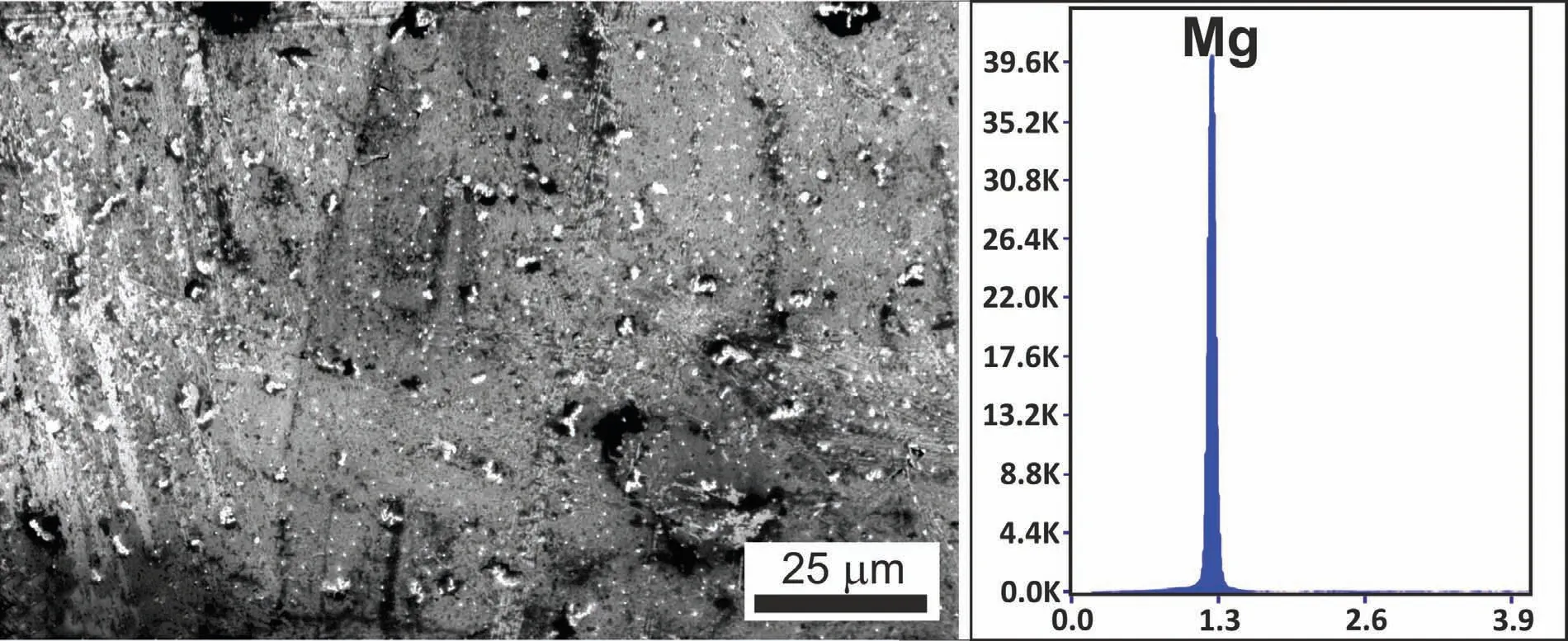
Fig.7.The representative results of SEM/EDS analyses taken on the surface of solidified Mg drop formed during the CP test of Mg/graphite couple(T=700°C,t=600s).
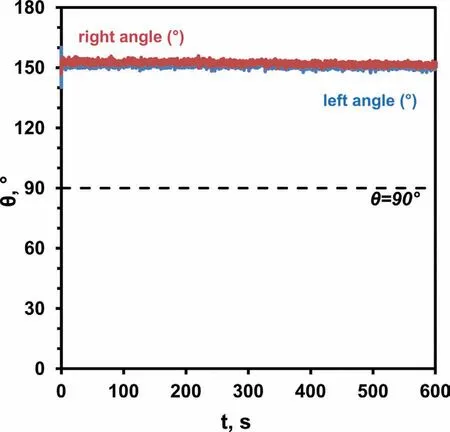
Fig.8.Wetting kinetics of molten Mg(99.98 wt%)on the graphite substrate recorded at T=700°C for 300s under flowing Ar(99.999%)atmosphere using the CP procedure.
In the case of high-temperature wetting experiment performed by the CP procedure,as compared to that obtained by applying the CH procedure,a different behavior of the liquid Mg sample deposited on the graphite substrate,was observed.The different behavior results from the mechanical removal of the native oxide layer by squeezing the molten Mg drop through a graphite capillary(Figs.6a,b).In Fig.6c the Mg/graphite couple corresponding to the start(t=0s)of the test performed at T=700°C,is shown.In particular,the squeezed Mg drop exhibited regular and spherical shape which allows concluding that the complete melting of Mg in contact with the graphite substrate was reached and maintained(Fig.6c–h)until the end of the experiment(t=600s at T=700°C).In this case,a“true”triple line was achieved and reliable contact angle values,as well as wetting kinetics were obtained.Within the wetting experiment,the absence of the oxide layer at the liquid Mg/graphite interface was confirmed by the SEM/EDS analyses,as shown in Fig.7.
The wetting behavior of the Mg drop/graphite couple observed by applying the CP procedure at 700°C for 600s under a flowing Ar 99.999% atmosphere,is shown in Fig.8.The contact angle behaviors as a function of time(i.e.right and left contact angle values)indicate that under the mentioned testing conditions,graphite is not wettable by liquid Mg(θ>90°).In addition,by focusing on the average contact angle,the value ofθ=150° was measured and kept constant at the liquid Mg/graphite triple line from the beginning to the end of the experiment(t=600s).
Some selected images showing the solidified Mg/graphite couples after the wetting tests performed at 700°C/600s by using the two different testing procedures(CH and CP),are compared in Fig.9.
In the case of Mg processed by the CH procedure,the developed native oxide scale preserved after the test carried out at T=700°C,is clearly distinguished at the surface of the Mg sample,as shown in Fig.9a.As discussed before,it acts as a barrier for the complete melting of the metal sample which impedes the correct measurement of the wetting characteristics and proper interpretation of the wetting kinetics.In contrast,the CP procedure(Fig.9b)allows to mechanically remove the native oxide and to avoid any further oxidation phenomena upon the imposed testing conditions.In this case,a mirror-like surface of the Mg drop was obtained,as expected.
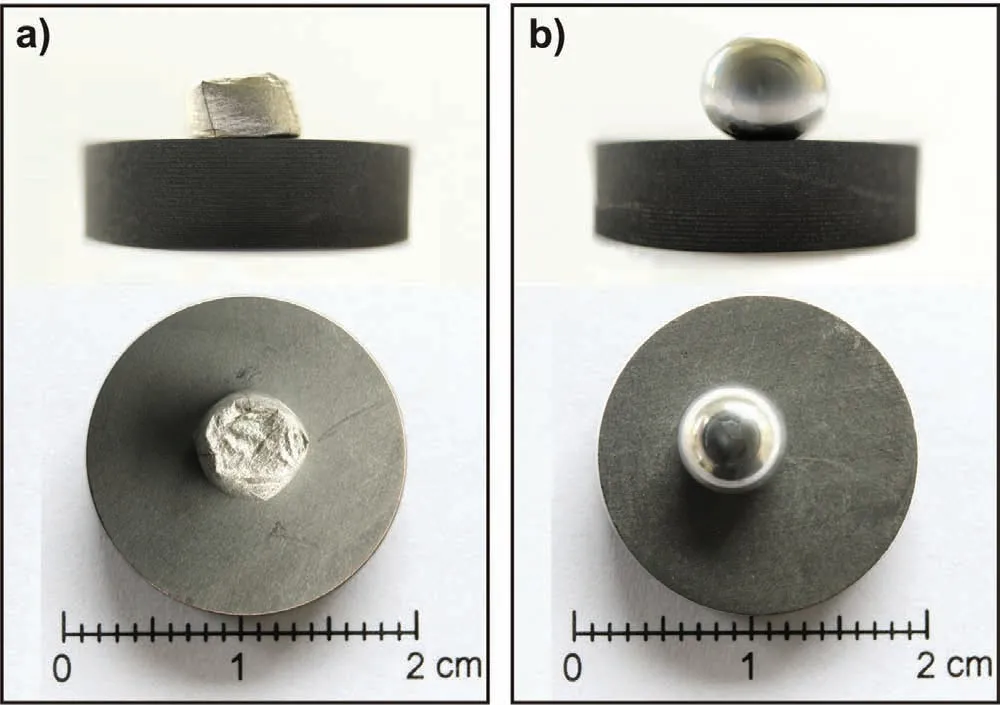
Fig.9.The images of Mg/graphite couples after the wettability test at 700°C for 600s,by using two different testing procedures:a)the CH;and b)the CP.
Conclusions
By using the newly developed experimental set-up,the wetting behavior of Mg-based materials might be safely examined at temperatures up to 1000°C both under static and flowing inert gases atmosphere.A pre-heating stage of high vacuum up to 10−7hPa may be applied.In addition,the classical sessile drop method combined with capillary purification procedure can be applied.In this way,the native oxide can be successfully removed.Improved methodological and design concepts implemented make the new ad-hoc developed experimental set-up unique at the world scale,in particular for investigating liquid metals having high vapor pressures like Mg and Mg-based alloys.The documented update in methodological issues will provide considerable inputs to scientific studies on high-temperature wettability of liquid Mg and its alloys.It also will allow the better understanding of the role of test-sensitive wettability-related parameters in liquid-assisted materials synthesis processing as well as in joining dissimilar materials.Furthermore,reliable data concerning thermophysical and technological properties of Mg,measured by using the new device,should allow in optimizing the selection of refractory mould materials used for foundry processes(e.g.crucibles,molds,casting spoons etc.).
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The studies were performed within the financial support given by the National Science Centre(NCN)in Poland,under the project MINIATURA 2,No.2018/02/X/ST8/03044 in 2019–2020.
Appendix A
A scheme of the high-temperature device is shown in Fig.A.1.The new testing device contains the following constituents marked on the scheme(Figs.A.1a,b):
1)An experimental vacuum chamber.
2)A window for observations of rotatable loading table.
3)A window for observation and recording.
4)Microtron 1310 high-speed digital CCD camera(rate of 10–100 fps depending on the stage of the test)with a system for real-time recording of sample images during high-temperature studies.
5)A light source illuminating of sample during hightemperature studies.
6)A table rotation controller with stepper motor.
7)Universal ports for connecting vacuum sensors(measuring range from atmospheric pressure to ultra-high vacuum).
8)A capillary manipulator mechanism with up-and-down movement.
9)A vacuum diffusion pump.
The interior of experimental chamber and a test setup are schematically presented in Fig.A.1d.The following components of the chamber can be listed:
•A replaceable heater(Mo,Ta or steel)(a).
•Screens system shielding the heater(b-d).
•A replaceable capillary with up-and-down movement(e).
•Thermocouples(f-i).
•A drop of liquid metal(j).
•A ratable loading table(l)with the possibility to load 10 substrates(for CP tests)or metal/substrate couples(for CH tests)(k).
•A change and raise system of samples(m).
•A protective gas supply tube with distribution hole for keeping the static or flowing gas at a controlled rate and required level of pressure(n).
•A plate separating high and low temperature areas(o).
•A sample transfer stick with up-and-down movement(p).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
