Alloying effect of silver in magnesium on the development of microstructure and mechanical properties by indirect extrusion
2021-03-10BjrnWieseRegineWillumeitmerDietmrLetzigJnBohlen
Björn Wiese,Regine Willumeit-Römer,Dietmr Letzig,Jn Bohlen
aDivision Metallic Biomaterials,Institute of Materials Research,Helmholtz-Zentrum Geesthacht,Max-Planck-Str.1,Geesthacht 21502,Germany
b Magnesium Innovation Center(MagIC),Institute of Materials Research,Helmholtz-Zentrum Geesthacht,Max-Planck-Str.1,Geesthacht 21502,Germany
Received 17 April 2020;received in revised form 24 July 2020;accepted 7 August 2020
Available online 2 October 2020
Abstract The effect of Ag in solid solution on the microstructure,texture and the deformation behaviour of indirectly extruded Mg was investigated.Ag as a solid solution strengthener affects the behaviour during extrusion,resulting in enhanced deformation related heating and corresponding coarser grained microstructures.No substantial effect on the texture development is revealed.The mechanical properties simultaneously increase in stress and strain levels with increasing Ag content,especially in tension as a result of the changing impact of the slip modes which can be associated with a decrease of the lattice parameters as well as the c/a ratio of the hcp lattice structure.In compression tests with twin dominated flow,the impact is much smaller on the compressive yield stress but higher with respect to the twinning related strain hardening rate.Solid solution strength functions for Fleischer and Labusch were determined,also confirming the anisotropic behaviour of the extruded Mg alloys.
Keywords:Mg-Ag-alloys;Lattice parameters;Indirect extrusion;Microstructure;Mechanical properties.∗Corresponding author.
1.Introduction
For many years,magnesium and its alloys are in the focus as structural materials for lightweight applications[1–3].Nowadays,they also receive increasing attention for an application as biodegradable implant materials[4–6].While the comparably low corrosion resistance of magnesium alloys has been a major challenge for structural application,in the field of degradable biomaterials a controllable degradation of implants in the physiological environment is a desired property.Alloying concepts for the different application routes may also vary with respect to the impact of single elements on application related properties.
The requirements for the alloys used for biomedical applications can change compared to those for structural applications.On the one hand,a mandatory requirement is the limitation of the alloy additions used to non-toxic or even biologically active elements.On the other hand,alloying for enhancing specific functionalities can open up a new field of interest for alloying variations.In this regard,silver(Ag)has been proposed as an interesting element for alloying magnesium.It has been shown that antibacterial properties can be increased with rather dilute additions into magnesium,then leading to a decrease of survival rates of certain pathogenic bacteria[7,8].Furthermore,degradation rates remain within application related ranges if the element is kept in solid solution and cytocompatibility can be achieved.On the contrary,precipitation of the element due to low temperature processing leads to a distinct increase and inhomogeneity of the corrosion rate[7–10].The results of Estrin et al.[11]show that Ag in Mg with adjusted corrosion rate is even cytotoxic for tumor cells,e.g.for the cell line K562 in in vitro test.Liu et al.[8]show that the amount of precipitates in rolled and annealed sheet with up to 8 wt% Ag in Mg is critical for a low corrosion rate.With processing above the Solvus temperature,the corrosion rate in biological media is lower than 0.5mm/year over 7 days.Therefore,the Mg-Ag system is applicable for biological applications as it allows both low and controllably higher corrosion rates.
As an alloying element,Ag did not play a major role for structural applications besides few approaches where complex Mg-Zn-Ag alloys have been used for extraordinary precipitation hardening studies[12,13,3]or for an increase the high temperature properties QE(Mg-Ag-Rare Earth)and QH(Mg-Ag-Th)alloys[3,14].Bian et al.[15]investigated a ternary Mg–1.5Ag–0.1Ca alloy revealing a rather high Erichsen value of 8.6mm and a high average elongation to failure of 29%to 35% at room temperature in rolled and annealed extruded plates.However,they investigated also a binary Mg-1.5Ag showing a lower Erichsen value of 3.8mm with a large average elongation to failure of 22% to 25% despite a strong texture.
In an earlier work[8],sheet rolling of binary magnesium alloys has been carried out and proven feasible.An element specific tendency to grain growth has been revealed as a result of the applied heat treatment,i.e.associated with static recrystallization.While no distinct changes in the texture development were found as a result of this processing,the Ag addition resulted in increasing strength and ductility of the respective Mg alloy sheets.Potentially,this effect can also be used for the application of ductile Mg wires as suture or splinting material,as it has been suggested as early as in the beginning of the 20th century[16].The use of Mg wires was always limited due to the low ductility during any bending operation,which was noticed as low knotting strength for surgical techniques.
As an element slightly smaller than Mg(with respect to the atomic radius)a respective impact on the lattice structure is anticipated,e.g.changing the c/a ratio of the hexagonal close packed lattice[17–20].Similar effects have been revealed for Mg-Li alloys[21–25].For the special case of Mg as a pure metal an initial c/a ratio of the unit cell is 1.62354 at ambient temperature,thus slightly lower compared to the ideal ratio of 1.633[26].The two important deformation mechanisms for this type of Mg crystal have been identified as{0001}<11¯20>basal dislocation slip and{10¯12}<10¯1¯1>extension twinning.The latter mechanism is known to be active only with positive stress along the c-axis.While this consideration does not fulfill the von Mises criterion of homogeneous polycrystal deformation[27]which requires at least five independent slip systems,changes in the activity of these two mechanisms have often been associated with the mechanical behavior of Mg alloys[28,29].Especially along with distinct textures developed during extrusion of profiles(often simple shaped round bars)the dominating deformation mechanism–dislocation slip or twinning-changes if tensile stress or compressive stress is applied along the extrusion direction.
Estrin et al.[11]already showed a low corrosion rate of 1.0±0.1mm/year over 7 days of immersion in the biological environment for the same material with 2 and 4 wt%Ag,extruded at a speed of 2.2mm/s identical to this study.Therefore,the aim in this study is to reveal the effect of solid solution Ag in Mg on the microstructure development and the concurrent mechanical behavior.In particular,the explanation of the effect of Ag on the mechanical properties is the focus of the work.Three binary alloys of Mg-Ag are used in a cast and solid solution annealed condition for indirect extrusion experiments and the microstructure and texture development is associated with the alloy specific mechanical properties.
2.Material and methods
Pure Mg and three binary Mg-Ag alloys were composed from pure elements(Mg(99.98%,MAGONTEC,Sydney,Australia)and Ag(99.99%,ESG Edelmetall-Service GmbH& Co.KG,Rheinstetten,Germany))and cast into cylindrical ingots by using a modified permanent direct chill casting technique[30,31]under protective atmosphere of Ar with 3vol.%SF6.In this approach pure Mg was melted and kept at 720°C before the respective amount of Ag(0,2,4 and 6wt.%)was added into the melt.Stirring was applied for 20min for a homogenization of the element distribution.The alloys were then poured into pre-heated steel moulds(700°C)and hold for 2min before directional water quenching by lowing the moulds into a water bath.Atomic absorption spectroscopy and spark emission spectroscopy were used to reveal the resulting composition of this set of materials,see Table 1.
The ingots were machined to cylinders with a diameter of 49mm and length of 150mm for extrusion experiments.Solid solution annealing was conducted at 425–450°C for the billets prior to extrusion(above the extrusion temperature).For the extrusion experiments the billets were pre-heated for 1h to the extrusion temperature.Pure Mg was kept at 350°C whereas the Ag containing alloys were heated to 425°C in order to avoid precipitation during the heating phase.A horizontal extrusion press with maximum extrusion force of 2.5 MN from Müller Engineering GmbH(Todtenweis,Germany)and a container with 50mm diameter was used for indirect extrusion experiments.The extruded profile is a round bar with a diameter of 10mm,thus resulting in an extrusion ratio of 1:25.For each alloy a variation of the extrusion speed was applied.Ram speeds as extrusion speed(vext)of 0.6,1.1 and 2.2mm/s where scheduled,respectively.
Microstructural investigations were carried out using typical metallography procedures.The samples were embedded in an epoxy resin,DEMOTEC 30(1/3 liquid hardener and 2/3 powder)for the investigation of the longitudinal sections.The specimens were prepared by grinding with up to 2500 grit SiC paper at a speed of 150 min−1.In the first step diamond paste(3μm)were used followed by a mixture of diamond(1μm)and O.P.S(<0.05μm)(Oxide-Polishing-Suspension,Schmitz-Metallographie GmbH,Germany)hydrous suspension with at a speed of 80 min−1,for polishing the samples.For light microscopy investigation the samples were etched by picric acid solution[32].The grain sizes were determined using a linear interception method parallel to the extrusion direction,with a field of interest of 0.355 mm².
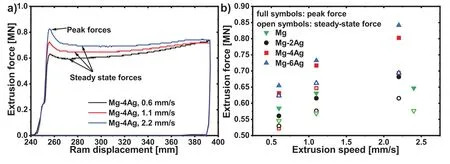
Fig.1.a)The force for the extrusion of Mg-4Ag alloy with 0.6,1.1 and 2.2mm/s extrusion speed.The peak force and steady-state force are marked.b)Peak force and steady-state force of the investigated conditions and alloys..
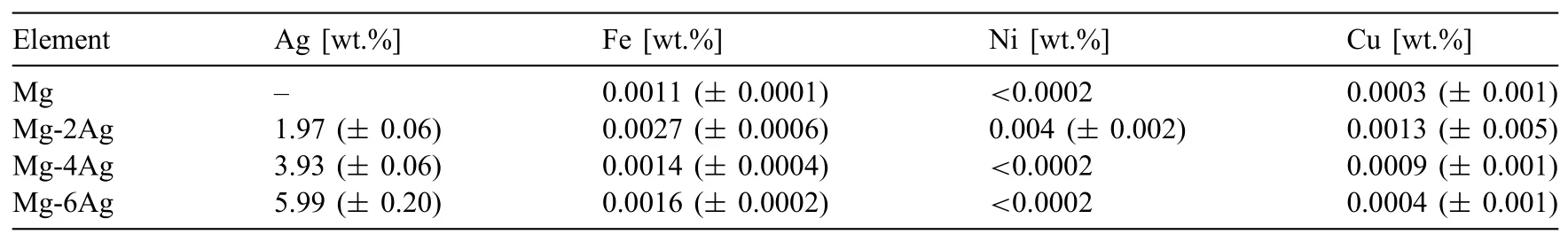
Table 1Chemical compositions of all alloys analysed with atomic absorption spectroscopy for Ag and spark emission spectroscopy for Fe,Cu and Ni,balance Mg.
Cross sections of the extruded bars were used to measure the textures of the samples by X-ray diffraction(Cu-Kα)with an X-ray diffractometer and goniometer X’Pert of Panalytical(Almelo,Netherlands).Six pole figures were measured with a maximum tilt of 70° out of the extrusion direction and used for the recalculation to inverse pole figures by using an open source code MTEX[33].
A glancing angle X-ray diffraction setup(Cu-Kα)at a D8 ADVANCE(Bruker,USA)has been used to measure the lattice parameters from diffraction peak positions with a step size of 0.01° for 2s at an angle of 3° The samples were measured on the longitudinal sections during rotation at 180 min−1.Exemplarily,samples from the lowest extrusion speed experiments were used and measured three time per sample.The diffraction angles of the respective peaks of the{10¯10}-and(0002)-planes were fitted with a gaussian distribution and the mean angles were used to calculated the lattice parameters a and c of the hcp-lattice structure.
Room temperature mechanical tests were conducted with tensile and compressive stress parallel to the extrusion direction and repeated at least three times.Tensile test samples were manufactured in the form of dog-bone samples with screws as holders according to ISO 6892 Form B with a test length of 25mm and a diameter of 5mm.Cylindrical specimens were machined to a test length of 12mm and a diameter of 8mm according to ISO 6892 for compression tests.Both,tensile and compression tests,were performed with a constant initial strain rate of 10−3s−1.
Vickers hardness measurements HV05(5kg)were carried out with a dwell time of 30s.The measurement were conducted at the cross section of the extrude profile.Every sample enabled 10 indents for the analysis using a M1C 010 testing machine(Emco-Test GmbH,Kuchl,Austria).
3.Results
During the indirect extrusion experiments,the applied extrusion force increases to an initial peak force where material flow starts.Fig.1a)illustrates an example of the force development during extrusion.A force drop to a steady-state level follows during continuous material flow.A further increase towards the end of the experiment may be correlated to a resulting billet cooling due to heat dissipation into the ram.It can be seen that the main parameters,i.e.the peak force and the steady-state force increase with the extrusion speed.
Fig.1b)collects the results of the alloys for the different extrusion speeds.While the force levels of both forces do not vary much between pure Mg and Mg-2Ag,they increase with the more Ag containing alloys for all extrusion speeds.A continuous increase with increasing extrusion speed is also found.
Fig.2 exemplarily illustrates micrographs of the alloys extruded at 1.1mm/s.The corresponding average grain sizes are shown in Table 2 and in Fig.3.In all cases fully recrystallized microstructures are found with a rather coarse-grained structure.An influence of the Ag content on the grain size is not significant for pure Mg and Mg-2Ag.A slight grain growth is observed in the alloys with more Ag.There is also an increase of the average grain size with the extrusion speed which is more pronounced for the higher Ag containing alloys.

Table 2Compilation of the mechanical properties,the grain size vs.the extrusion ram speed(vext).
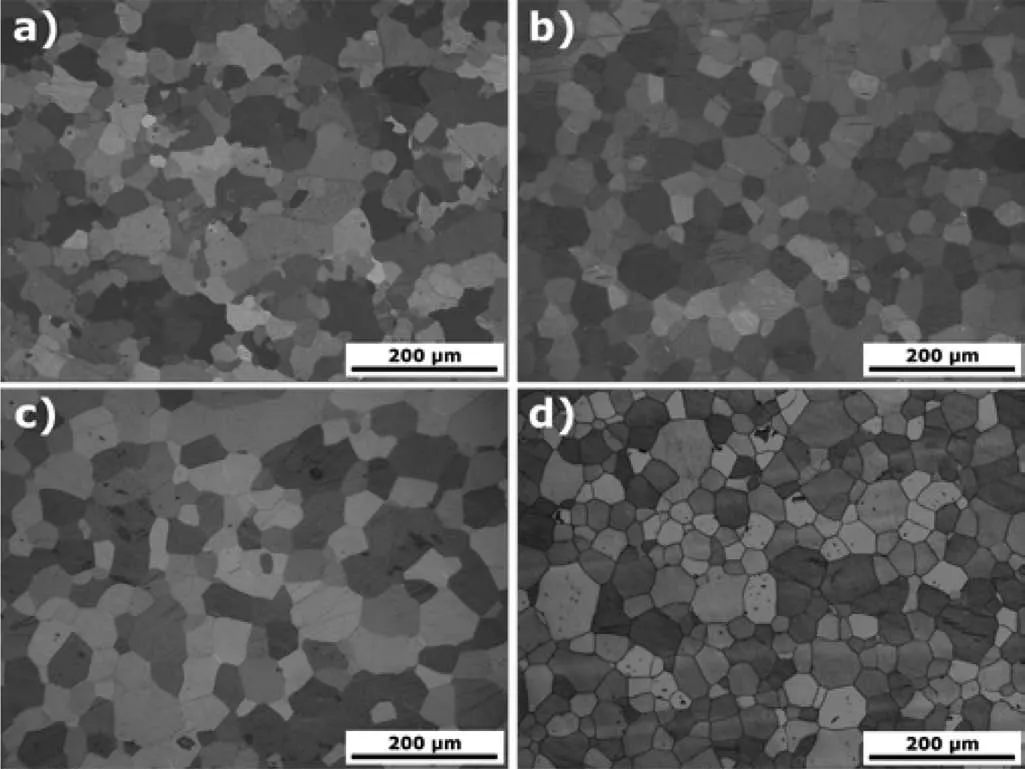
Fig.2.Exemplary light optical micrographs of a)Mg,b)Mg-2Ag,c)Mg-4Ag and d)Mg-6Ag extruded at an extrusion speed of 1.1mm/s.
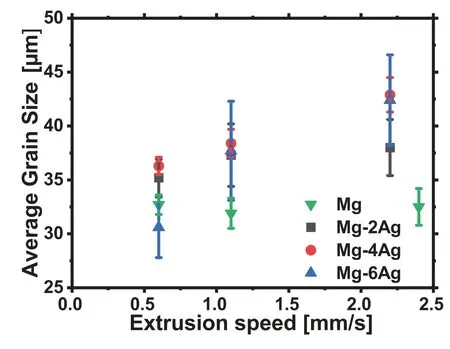
Fig.3.Grain size of the extruded alloys vs.the extrusion speed.
Fig.4 collects inverse pole figures parallel to the extrusion direction of the extruded bars.All samples favour a typical texture with highest intensities around the<11¯20>pole.The significance of this texture remains somewhat weaker for pure Mg compared to the Ag containing alloys but does not vary qualitatively.At higher Ag content a slight weakening of the texture can be observed again with increasing extrusion speed.In summary,no visible impact of the content of Ag as well as the extrusion speed on the texture development can be revealed.This typical texture leads to a preferential alignment of basal planes parallel to the extrusion direction and is suggested to be the result of a fully recrystallized microstructure[34]which is often observed in Mg alloys as a result of extrusion.
Exemplary stress-strain diagrams from tensile and compression tests of the alloys after extrusion at 0.6mm/s are presented in Fig.5.In tension,a continuous elasto-plastic transition at the yield point increases towards a pronounced elastic limit at higher content of Ag,thus indicating an interaction between the solute Ag and dislocations.This is followed in all cases by a continuous strain hardening range with decreasing slope.The uniform strain increases with the Ag content as well as the fracture strain.Unlike this,in compression lower yield points can be visualized compared to tension,which are also increasing with the Ag content.Furthermore,an Sshaped strain hardening behaviour with increasing slope up to an inflection point and followed by a decrease up to a maximum stress level is stronger in the Ag containing alloys compared to pure Mg.The result is a decrease of the fracture strain with increasing Ag content,thus the opposite of what has been found during tension testing.
The mechanical properties in tension and compression are summarised in Table 2 and visualize the above mentioned property development.The addition of Ag results in increasing tensile yield stress TYS(96 to 172MPa)as well the ultimate tensile stress UTS(189 to 251MPa)and also the uniform elongation(uniεT)(6.0% to 19.0%)and the elongation to fracture(εT)(6.8% to 24.9%).In contrast,the compression to fracture(εC)(14.6% to 9.6%)decreases with increasing Ag content,but the compressive yield stress CYS(60.0 to 81.8MPa)and the ultimate compressive stress UCS(317.0 to 372.4MPa)are also increasing with Ag.Tensile and compression values show a strong increment of TYS,UTS,CYS and UCS with increasing the Ag content from 0 to 2wt.%.
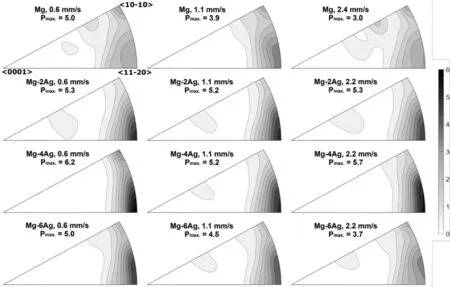
Fig.4.Inverse pole figures in extrusion direction of pure Mg,Mg-2Ag,Mg-4Ag und Mg-6Ag at extrusion speeds of 0.6,1.1 and 2.2mm/s,respectively,2.4mm/s for pure Mg.Bottom left<0001>pole,bottom right<11¯20>pole and top right<10¯10>pole.Pmax is the maximum pole density in multiples of a random distribution.
The variation of the extrusion speed has no significant effect on the shape of the compression as well as of the tensile curves.The mechanical properties for pure Mg are not affected by the increase of the extrusion speed.For the parameters of the different alloys the variation is similar with a mean value for compression to fracture of 10.7% for Mg-2Ag,10.8% for Mg-4Ag and 9.6% for Mg-6Ag.In the case of Mg-2Ag the increase in the extrusion speed leads to an increasing elongation to fracture,but to decreasing CYS and UCS.The increase in the extrusion speed leads also to decreasing TYS,UTS,CYS and UCS at higher content of Ag(Mg-4Ag and Mg-6Ag)and to increasing elongation to fracture.
The results of hardness measurements are also collected in Table 2.There is no significant change for each alloy with changing the extrusion speed.However,an increase with the Ag content is determined.
The results from X-ray diffraction experiments and determined lattice parameters a and c are potted in Fig.6a)and b)vs.the Ag content.In comparison to results from other earlier works with Mg-Ag alloys[17–20]there is a comprehensive agreement of both,decreasing a and c with increasing Ag content.The literature results are somewhat separated into two fractions,showing lower values in works until 1950(green)[17,18],but slightly higher values in works after 1950(red)[19,20].The latter show good agreement with the results of this work vs.the Ag content.Still,all works confirm a decrease of the c/a ratio with increasing content of Ag as revealed in Fig.6 for both groups with only small differences in the slope.In conclusion,the addition of Ag leads to a more pronounced decrease of c compared to a.A linear fit shows a decrease of a~0.005 °A/at.%,c~0.01 °A/at.% and c/a~0.001/at.%.
4.Discussion
The impact of Ag as an alloying element in solid solution is first visible in the forming behaviour during extrusion where there is direct correlation between the extrusion ram speed and the force.While the peak force shown in Fig.1 increases with both,increasing Ag content and increasing extrusion speed,it can be associated with the respective flow stress of the billet alloy under the applied rate dependent conditions.Then the increase of the Ag content increases both,the onset of plastic flow at the elevated temperature chosen as well as its rate dependency.Thus Ag acts as a strengthening element in solid solution in magnesium even at the higher temperature condition during extrusion.This trend is also confirmed by the mechanical values at room temperature.
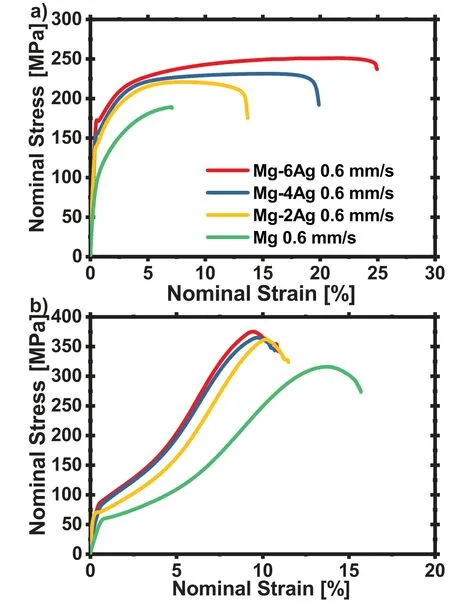
Fig.5.Stress-strain curves for a)tensile and b)compression tests on all alloys extruded at 0.6mm/s.
Principally,the same behaviour is also observed if the steady state force(Fig.1b))is reviewed instead of the peak force.However,its increase with increasing extrusion speed(i.e.forming rate)is somewhat lower.After beginning plastic flow(associated with the peak force)the decrease of the force level has been related to a deformation related temperature increase resulting in a steady state level.This decrease is more pronounced with higher extrusion speed and it is also more distinct with higher Ag content of the alloy.This reveals a higher impact on the deformation temperature with increasing speed if the Ag content is higher,e.g.as a result of the solid solution strengthening of the alloy with alloying Ag.
The peak force as well as the steady state force for pure Mg is shown after extrusion at a lower extrusion temperature(350°C instead of 425°C).Both force levels remain higher compared to those of the Mg-Ag alloys and in fact they are higher compared to the Mg-2Ag with the same extrusion speed.Still,the increase of the force levels with increasing extrusion speeds remains lowest among the alloys used,therefore confirming the above consideration about the impact of solid solution strengthening with Ag.
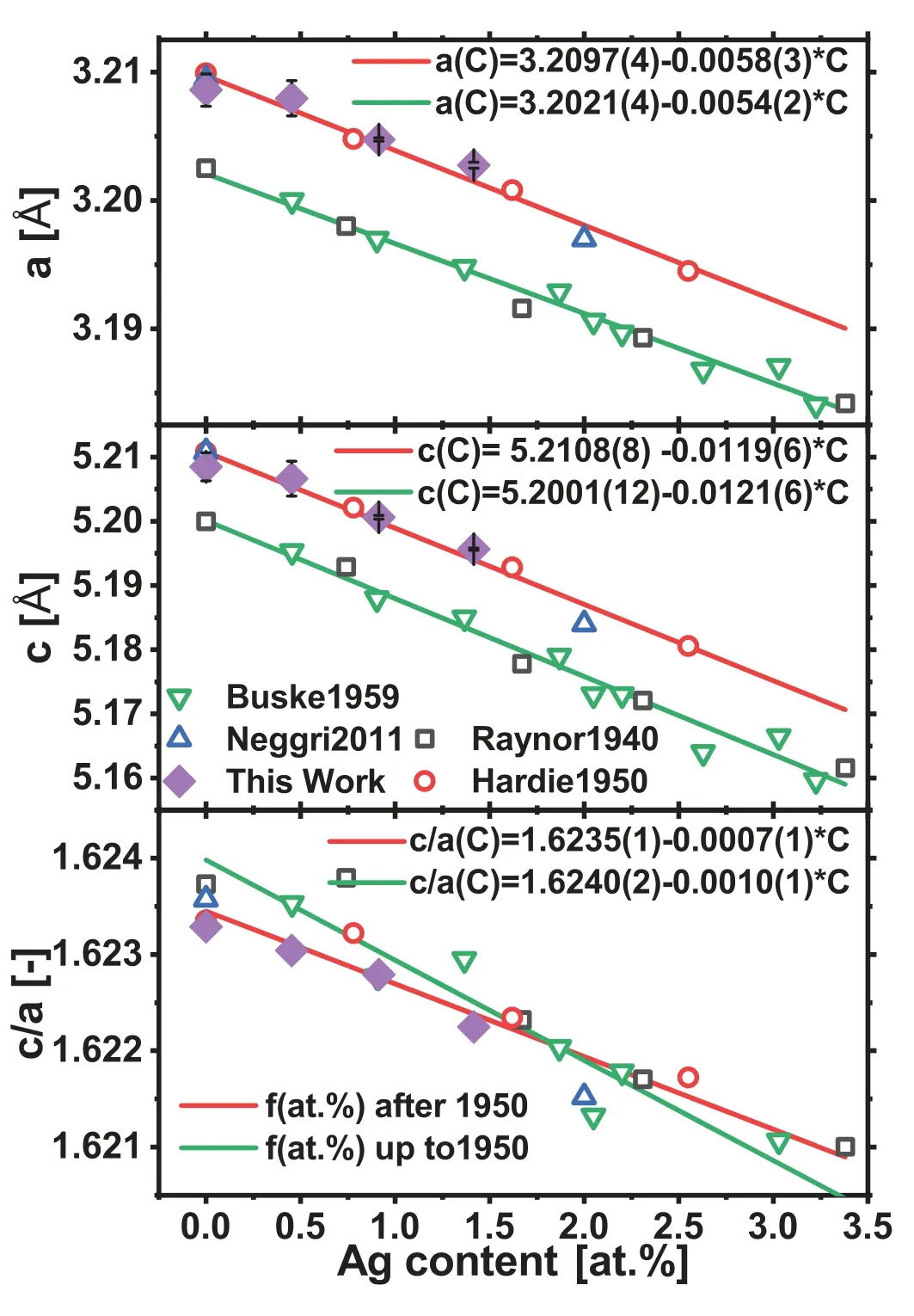
Fig.6.Collection of lattice parameters a,c and the c/a ratio;literature results are also included from Raynor1940[17],Busk1950[18],Hardie1959[19],Negri2011[20].Two separate linear fits and corresponding fit parameters are shown,standard deviations in brackets indicating the last digit.
The increase of deformation related heating and the corresponding increase of the temperature during extrusion will also have an impact on the developing grain structure as far as it is determined by the temperatures,e.g.due to higher diffusion rates[35].As a result the grain size will increase with higher temperatures if the grain structure developing mechanisms,i.e.recrystallization and grain growth,can expand further.This is in agreement with the results for the Mg-Ag alloys,especially if compared to the results for pure Mg.While in all cases fully recrystallized microstructures in Fig.2 are the result,the degree of the microstructure development is varying with the applied extrusion speed.For pure Mg there is no visible change of the grain size with increasing speed in the rather small range of varied extrusion parameters in this study.On one hand side this corresponds to the lower extrusion temperature of 350°C compared to 425°C for Mg-Ag alloys which may limit a thermal effect on the microstructure development of pure Mg.On the other hand,this is more clearly related to a general low range of extrusion speeds.Then an effect of the speed increase is too low to take effect on the microstructure.Only in the case of Ag addition to Mg a related enhancement of the recrystallization process allows visible microstructure changes to be revealed.For all Mg-Ag alloys there is an increase of the grain size with the extrusion speed which is more distinct if the Ag content is higher,therefore confirming the recrystallization based accelerating impact on the microstructure.Only at the lowest extrusion speed–where the deformation heating would be lowest following the above consideration–the Mg-Ag show a reduction of the grain size with increasing the Ag content.
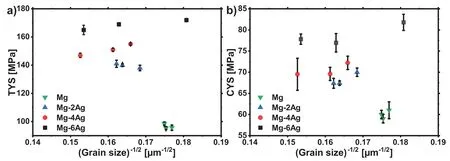
Fig.7.Yield stress vs.the inverse square root of the grain size(Hall-Petch plot)for the extruded alloys of this study;a)TYS,b)CYS.
The effect of the extrusion speed on the texture development due to the addition of Ag to Mg is less pronounced than it is on the grain size.Typical textures of extruded Mg alloys are found where basal planes are aligned preferentially parallel to the extrusion direction[36].
For extruded profiles in the form of round bars,thus with a rotational symmetry around the extrusion direction the typical texture highlights a prismatic<10¯10>fibre component parallel to the extrusion direction[37]which expresses the above mentioned alignment.A tendency to develop a tilt component towards the<11¯20>pole has been related to the recrystallization[34]which occurs during extrusion,i.e.dynamic recrystallization during forming as well as static recrystallization during the subsequent cooling of the profile[38].For the pure Mg profiles in this study both respective components are visible,which can also be addressed to the lower extrusion temperature compared to the alloys with Ag.
The textures of Mg-Ag alloys does not show a pronounced<10¯10>pole intensity any more but highest intensity around the<11¯20>pole,which indicates preferential recrystallization during the extrusion process[34].This component therefore is more pronounced and leaves the Mg-Ag alloys with higher maximum pole intensities compared to pure Mg.While the maximum texture intensity is slight reduced with increasing the extrusion speed for the Mg-6Ag materials and the angular distribution becomes broader with increasing the Ag content,there is no substantial change in the texture development.This allows the hypothesis that orientation related effects on the activity of deformation mechanisms during mechanical testing can be neglected and it allows reviewing the effect of grain size and/or solute content of Ag on the mechanical properties.
Assuming a classical impact of the average grain size on the yield stresses in tension and compression,Fig.7 collects the yield stressσyHin the form of a Hall-Petch plot following

with TYS in tension and CYS in compression,d is the average grain size of the fully recrystallized microstructure,σ0is the friction stress for dislocation motion and K is the grain boundary strengthening factor.While the data basis shown in Fig.7 is not broad enough to conclude on any of the mentioned parameters and also a positive slope of K would not always be found within the rather small variations of the underlying grain sizes,important findings can be concluded with respect to the alloying effect.
Generally,yielding exhibits a distinct asymmetry between tension and compression,always leaving the CYS significantly lower than the TYS.This asymmetry has been associated with the preferential activation of{10¯12}<10¯11>extension twinning if strain is accommodated by c-axis extension[39],as illustrated in Fig.10.As in general tendency basal planes are aligned parallel to the extrusion direction,then caxis extension by twinning is favoured along with compressive stress parallel to the extrusion direction,but not along with tensile stress.The main deformation mechanism is case without distinct twinning activity is basically basal slip[29].The main planes and directions in an hcp are illustrated in Fig.8 and in a)for basal slippage.
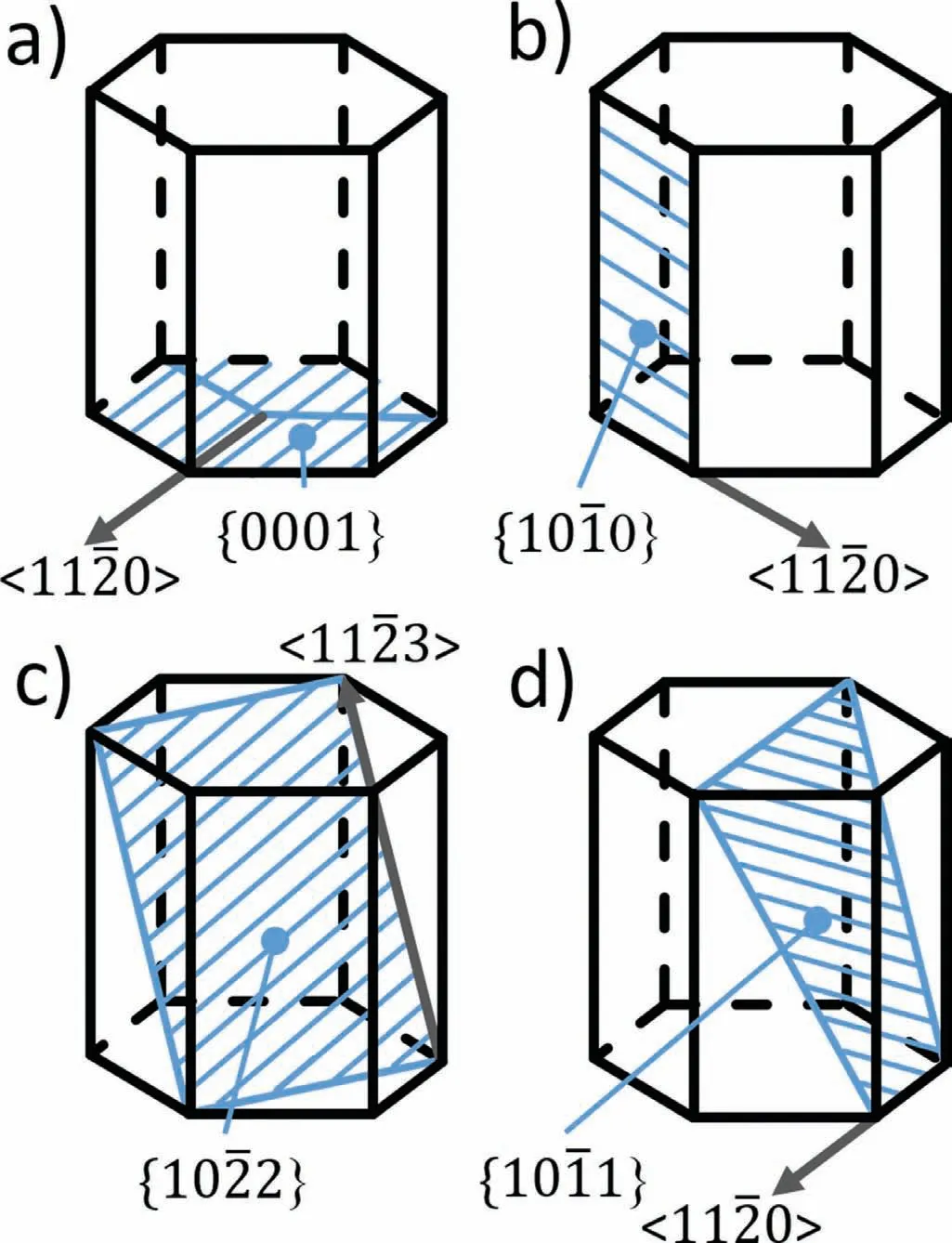
Fig.8.The major planes and directions in a hcp for a)for basal slip{0001}<11¯20>and non-basal slip as b)prismatic{10¯10}<11¯20>,pyramidal c){10¯22}<11¯23>and d){10¯11}<11¯20>.
In Fig.7 the results for pure Mg differ significantly from those of the Mg-Ag alloys although the average grain size is within the same ranges.This specific behaviour can be associated with the weaker texture of pure Mg and the concurrent less significant alignment of basal planes parallel to the extrusion direction,thus a preferential activity of basal slip and therefore lower yield stresses in tension and in compression.In the case of the Mg-Ag alloys,without distinct differences in the textures,the levels of TYS,accordingly dislocation slip dominated,increase visible with the Ag content,despite of the grain size.Contrary,the same effect is not distinct for the CYS,a condition of twin dominated yielding.Obviously,the onset of plastic flow due to the addition of Ag is influenced by dislocation glide but not so significantly by the onset of twinning.
For a consideration of solid solution strengthening by Ag,two theoretical approaches are applied to describe the effect as a function of the solute atom concentrations C(at.%)on the yield stressσy(or strength),following the works of Fleischer[40]and Labusch[41]on solid solution strengthening:
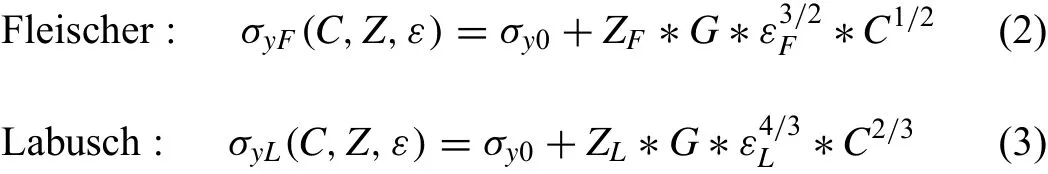
whereσy0is the yield stress of the pure metal,Z is a related constant,G is the shear modulus andεis a parameter which combines the atomic size and shear modulus misfits.The Fleischer approach is more applicable to dilute concentrations of alloying elements whereas Labusch describes better the interaction of more solute atoms with the slip planes.As there are no objective reasons to prefer one theory above the other,both are shown.
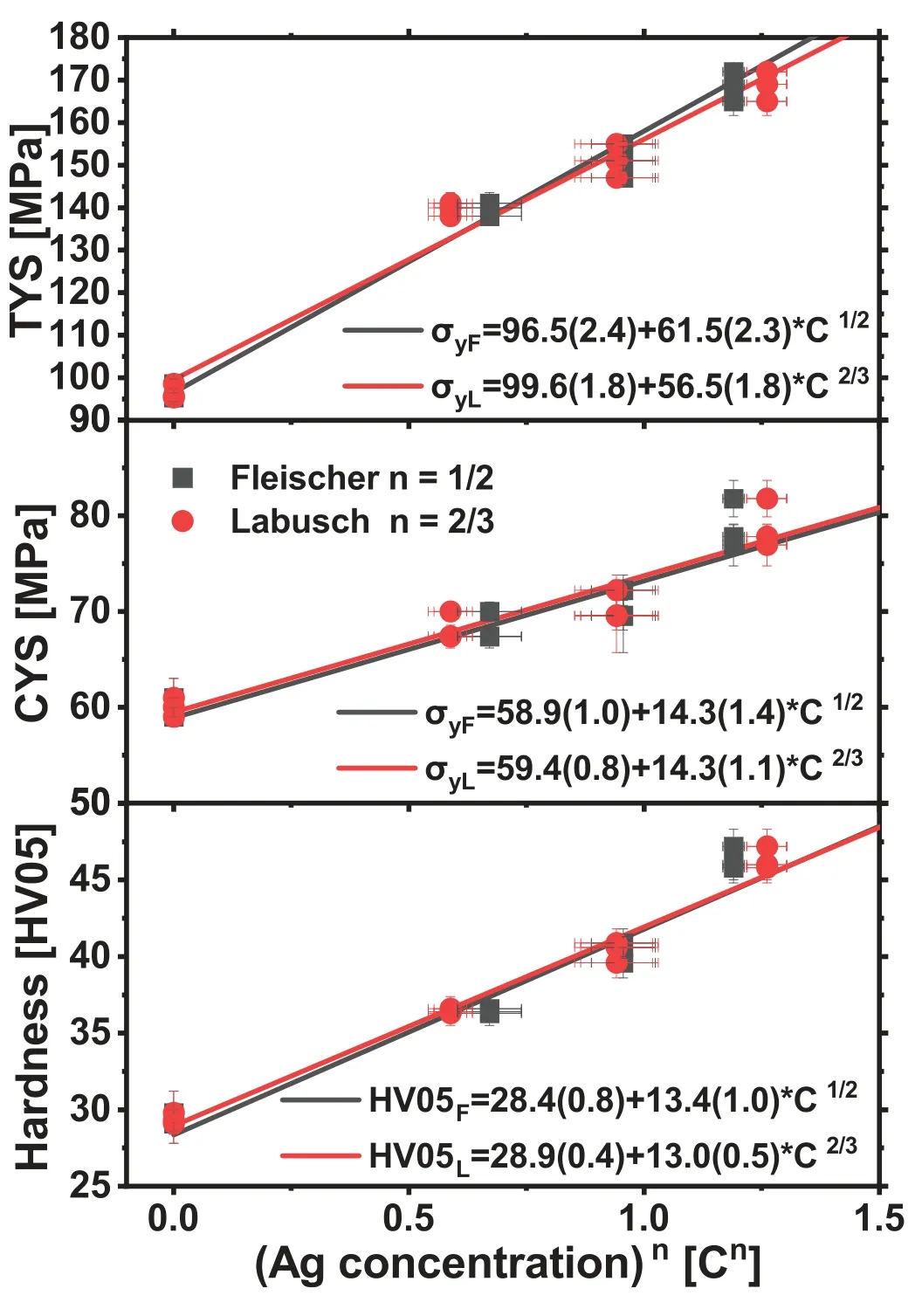
Fig.9.TYS,CYS and hardness vs.solute concentration C of Ag according to Fleischer n=1/2 and Labusch n=2/3 from all samples and corresponding linear fits.Standard deviation in brackets.
In Fig.9 the mechanical results of all samples were used by neglecting the effect of the grain size on stress properties(which turned out to be rather small in Fig.7).The TYS and CYS as well as the hardness were plotted vs.the concentration cn,where n varies for both approaches(Fleischer n=1/2 and Labusch n=2/3).The basic idea of Fleischer or Labusch should work due to similar interaction between the atoms and dislocations.Correspondingly,the variation of the linear fitted parameters is only small,i.e.the onset understood as the respective yield stress of pure Mg and the slope as a coefficient resulting from Z∗G∗εas the shear and misfit related impact of the solute element on the respective property.
In the case of the TYS the onset is 97MPa for the Fleischer approach and 100MPa for Labusch,but in the case of CYS the respective values are 59MPa for both approaches.While a difference between the models is not resolved,the significant difference of a factor of ca.1.7 between TYS and CYS corresponds to the above discussed directionality of activation of deformation mechanisms,especially twinning along with the compression tests.Furthermore,the slope of the fits,i.e.the strengthening effect with Ag is higher for the TYS(62 MPa/at.%1/2and 57 MPa/at.%2/3)than for the CYS(14 MPa/at.%1/2as well as 14 MPa/at.%2/3).This marks also an anisotropic behaviour with a factor approximately 4 between CYS and TYS for the solid solution hardening impact of Ag in this study.As both,Fleischer and Labusch,base on the effect of the atoms on the CRSS in an isotropic material,the approach assumes the same effect on all slip planes.However,the distinct textures of the materials in this study do not refer to an isotropic material at all but to preferential activation of dislocation slip in tension or twinning in compression.The different impact of Ag in these two testing directions therefore describes a much more distinct solute hardening effect due to slip rather than it affects the onset of twinning.Both fits,following Fleischer or Labusch reveal an onset of ca.29 HV05 as the fit for pure Mg and a slope of both 13 HV05/at.%1/2or 13 HV05/at.%2/3.As the hardness measurement includes an impact tensile stress,compressive stress and shear stresses,it fits well with the prediction of the effect of Ag on the hardness in this study.It reveals a substantial hardening ability due to Ag in solid solution.
In the case of the tensile curves it can be seen that the addition of Ag in Mg leads to a more pronounced yield point along with TYS(Fig.5).Similar to a Cottrell atmosphere effect,this alone indicates an interaction of Ag atoms with dislocations.This effect cannot be observed for the compression curves,only a stronger increment of the stress in the beginning is of the compression from pure Mg to the Mg-Ag alloys are visible.Again,this is in agreement with a solid solution strengthening of dislocation slip rather than twinning.A concurrent increment in the achieved uniform elongations as well as the fracture strains in tensile testing also indicates higher strain hardening ability along with the activity of dislocation slip.The increase in elongation to fracture cannot be explained by solid solution hardening and thus indicates a change in the geometric relationships in the Mg crystal as a function of the Ag content.This is not found for the compression test results.The compression to failure is somewhat reduced with a higher Ag content.
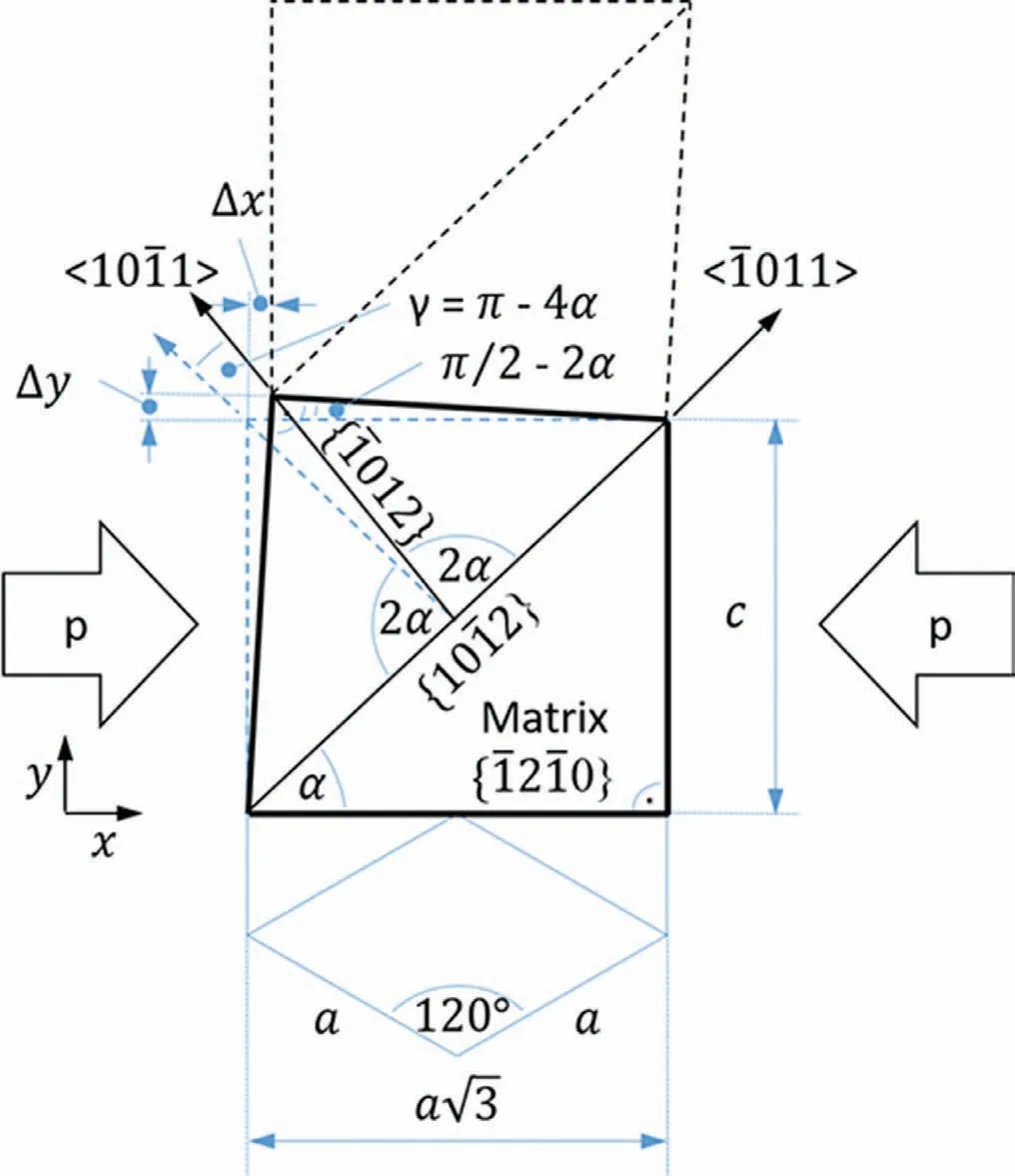
Fig.10.Geometrical relationship for the extension twin{10¯12}<10¯11>in an hcp crystal,with p as pressure or force to the crystal..
The respective measurements in Fig.6 of the lattice parameters a and c are in good agreement to other measurements in the past[17–20].The trend for c and a with the addition of Ag to Mg is similar for the results up to 1950(green)and after 1950(red)(Fig.6),but the values are slightly higher after 1950 as for the present work.This is not considered an effect of sample preparation,since all four data sets from the literature are from samples that were annealed to bring atoms into solid solutions and to dissolve the stresses from preparation.However,the trend for the c/a ratio shows a smaller deviation between these groups.In combination,this seems to be a systematic variation in the results for the c and a ratio compared to recent studies and also to the current work.Thus,Ag leads to a reduction of both parameters where c declines more than a,resulting in a reduced c/a ratio compared to pure Mg.Similar effects due to alloying elements have been investigated in the past.A reduction in the c/a ratio in the hcp were also determined for Mg-Li alloys[21,23,24].Yoshinaga and Horiuchi[24]correlated the lattice reduction in the Mg-Li system with an increase in the CRSS of basal slip.This is in an agreement with the Suzuki effect[42],the solution hardening due to the chemical interaction.It has been predicted,that this results in a reduction of the stacking fault energie due to an increase in the concentration of solid solution atoms[42].The CRSS will be increased by the square root from concentration[42].In consequence,the Li addition is promoting non-basal slip by a hardening of basal slip[24,43,44,45].Fig.8 is shows planes and directions of basal and some non-basal slip systems.In this work,Ag shows the first time a similar effect like Li did in the earlier works which allows hypothesizing that Ag also promotes non-basal slip during deformation,like prismatic[21]and pyramidal[45]slip,thus leading to higher stress levels during yielding as well as for the strength.This is the explanation for the increase in elongation to fracture with increasing Ag content or decreasing c/a ratio.Concurrently,an effect on the strain hardening ability is anticipated.
On the other hand,the compression curves–here based on twin dominated flow during and after yielding-show a completely different type of strain hardening with increasing slope of the curves up to an inflection point during the plastic deformation.Dobroˇn et al.[28]have shown exemplarily,that this inflection point corresponds to a change of the deformation process,where the dominating strain hardening behaviour due to twin growth comes to an end in the fully twinned microstructure and is then followed by dislocation slip in the fully differently oriented material.In the case ofthe present Mg-Ag alloys a stronger increase of the slope before the inflection point is associated with a higher impact of the twins after their nucleation during growth.The reduction of the c/a ratio results in an increase of the twinning shearγ as suggested in[46,47].Due to the geometrical relationship for the extension twin{10¯12}<10¯11>in an hcp crystal the twining shearγ[rad]and the displacement by a twin can be calculated,as illustrated in Fig.10.This relation can be calculated according to lattices parameter.For twinning shear γas a function of c/a ratio:

and
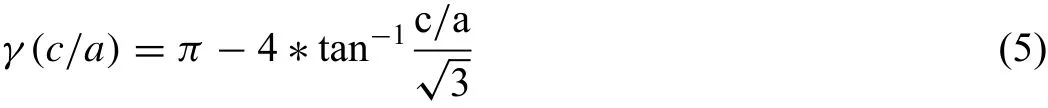
5.Conclusion
In this work,Mg-Ag alloys with the alloying element in solid solution have been indirectly extruded and the impact on processing as well as the resulting microstructure and property relationship have been investigated.
Ag is a strengthening element during extrusion as well as in mechanical.The impact on the required forces in the extrusion process increases with the alloying content and the extrusion speed.This strengthening of the material,as well as an increasing extrusion speed leads to an increase in the average grain size.The textures are all consistent with typical findings for fully recrystallized microstructures of extruded round bars with the basal planes parallel of the extrusion direction similar to pure Mg.
Ag in solid solution decreases the lattice parameters of the hcp lattice structure and leads to a reduction of the c/a ratio.A resulting impact on the activity of deformation mechanisms,hypothetically strengthening of basal slip and promoting nonbasal slip,leads to an increase of both,yield and elongation properties in tensile tests in extrusion direction.On the contrary,twinning in the compression case does not seem to be significantly influenced by the lattices changes.This result in increased twin shear reveals a more distinct strain hardening and lower compression to fracture in twin-dominated forming operations.
Solid solution strengths functions for Fleischer and Labusch on TYS,CYS and hardness were determined.The strengthening effect of Ag on the TYS is 4 time higher than for CYS and also theσy0shows factor of approximately 1.7 between the compression and tensile results.
Declaration of Competing Interest
None.
Acknowledgments
The author would like to thank Khausik Narasimhan(MSc.),Maria Nienaber(MSc.),Guadalupe Cano(MSc.)and Jochen Harmuth(MSc.)at HZG for some help with detailed microstructure and texture analyses.This research was partly funded by Helmholtz Association in the frame of Helmholtz-Russian Science Foundation Joint Research Group grant number HRSF-0025.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Recent developments and applications on high-performance cast magnesium rare-earth alloys
- Surface characterization and corrosion behavior of calcium phosphate(Ca-P)base composite layer on Mg and its alloys using plasma electrolytic oxidation(PEO):A review
- Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying
- Magnesium matrix composite reinforced by nanoparticles–A review
- The design of Co3S4@MXene heterostructure as sulfur host to promote the electrochemical kinetics for reversible magnesium-sulfur batteries
- A new die-cast magnesium alloy for applications at higher elevated temperatures of 200–300°C
