Rapid and effective treatment of traumatic spinal cord injury using stem cell derived exosomes
2021-03-01ShiqingFengJinqingGo
,Shiqing Feng Jinqing Go ,e,
a College of Pharmaceutical Sciences,Zhejiang University,Hangzhou 310058,China
b Pilot National Laboratory for Marine Science and Technology,Qingdao 266137,China
c Department of Orthopedics,Tianjin Medical University General Hospital,Tianjin 300052,China
d International Science and Technology Cooperation Base of Spinal Cord Injury,Tianjin Key Laboratory of Spine and Spinal Cord Injury,Department of Orthopedics,Tianjin Medical University General Hospital,Tianjin 300052,China
e Dr.Li Dak Sum &Yip Yio Chin Center for Stem Cell and Regenerative Medicine,Zhejiang University,Hangzhou 310058,China
Keywords:Exosome Fibrin glue Spinal cord injury Emergency treatment
ABSTRACT Traumatic spinal cord injury is a fatal acute event without effective clinical therapies.Following the trauma,immediate neural protection and microenvironment mitigation are vitally important for nerve tissue repair,where stem cell-based therapies could be eclipsed by the deficiency of cells due to the hostile microenvironment as well as the transport and preservation processes.Effective emergency strategies are required to be convenient,biocompatible,and stable.Herein,we assess an emergency cell-free treatment using mesenchymal stem cell-derived exosomes,which have proven capable of comprehensive mitigation of the inhibitory lesion microenvironment.The clinically validated fibrin glue is utilized to encapsulate the exosomes and in-situ gelates in transected rat spinal cords to provide a substrate for exosome delivery as well as nerve tissue growth.The emergency treatment alleviates the inflammatory and oxidative microenvironment,inducing effective nerve tissue repair and functional recovery.The therapy presents a promising strategy for effective emergency treatment of central nervous system trauma.
1.Introduction
As a trauma in the central nervous system (CNS),traumatic spinal cord injury (SCI) is among the most fatal diseases.Since there is a lack of clinically available therapy for effective nerve tissue repair and functional recovery,SCI has become one of the leading causes of adult death and disability [1,2].Following the acute event,progressive secondary injury develops rapidly and continuously expands the damage to adjacent nerve tissues [2] .Correspondingly,an extremely hostile microenvironment grows in the injured spinal cord,involving severe inflammation and oxidative stress,and impedes nerve recovery both from intrinsic and therapeutic approaches [3,4].Prompt administration straightly after the acute trauma is vitally important to dispute the inhibitory parameters and patch the substrate,thus preserving neurons and promoting tissue repair [5,6].The crucial role of the management in the immediate post-injury phase has been emphasized by studies showing increased risk of secondary complications posed by the failure to access emergency treatments [6,7].However,almost every trauma occurs remotely from medical facilities.In consequence,there is an urgent need for emergency treatment with feasible and biocompatible intervention to the lesion microenvironment within the initial stage of the devastating acute event.
Stem cell transplantation has proven promising for nerve tissue regeneration in extensive studies [8–11],especially for mesenchymal stem cells (MSCs),of which the therapeutic effects are mainly attributed to the microenvironment mitigation functions by cellular paracrine [12–14] .However,cell-based therapies could be largely eclipsed by the preservation and transportation procedures as well as the hostile lesion microenvironment [10] .As vehicles of paracrine secretions,exosomes hold great promise in medical treatments.MSC-derived exosomes have been demonstrated to mitigate the SCI microenvironment and promote nerve repair [15–17] .Hence,instead of stem cells,exosomes represent more qualified candidates for emergency treatment of acute SCI.And among the alternative cell sources,human umbilical cord MSCs are clinically accessible and extensively applied in experimental therapies.Therefore,exosomes are isolated from the MSCs in this study and evaluated for emergency treatment of SCI.
On the other hand,due to the damage of extracellular matrix (ECM) in the lesion site,the emergency intervention still requires a biocompatible substrate to provide an immediate supplement for ECM as well as efficient delivery of the exosomes [18] .Vector materials for exosome transplantation in nerve tissues are poorly demonstrated.Viscoelastic hydrogels mimic the ECM of soft nerve tissues,among which injectable hydrogels could meet the demand of simple and rapid operation in emergency care.Our previous study has reported effective nerve tissue regeneration and functional restoration by a transplantation strategy based on a hyaluronic acid hydrogel and human MSC-derived exosomes [17] .Herein,fibrin glue,a clinically validated injectable hydrogel,is applied to encapsulate the transplanted exosomes.The in-situ formation of fibrin glue mimics the blood clotting process,utilizing fibrinogen and thrombin from natural human blood as the two gelation constituents.Fibrin glue is extensively used in clinical practices and has been approved for its biocompatibility and wound healing properties,but rarely used in SCI medical treatment due to the absence of effective SCI therapy.In this study,the transplantation of the exosome-encapsulated fibrin glue(FG-Exo) is designed as a feasible emergency treatment to comprehensively mitigate the SCI microenvironment.Significant functional recovery and nerve tissue repair are induced by the emergency treatment,which could be possibly attributed to the attenuation of inflammatory and oxidative responses.The exosome-based strategy in the current study showed promising results in the animal study and yielded potential for translation in human SCI.
2.Materials and methods
2.1.Materials
Minimum essential medium-α
(MEM-α
) was purchased from Biological Industries (Beit Haemek,Israel).The human basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) were purchased from Pepro Tech Group (New Jersey,USA).Fetal bovine serum (FBS) and 0.25% (w:v)trypsin were purchased from Gibco BRL (Gaithersburg,MD,USA).Human umbilical cord mesenchymal stem cells were obtained from Ever Union Biotechnology (Beijing,China).Fibrinogen and thrombin were purchased from Shanghai RAAS Blood Products Co.,Ltd.(Shanghai,China).Female SD rats weighing about 230 g were purchased from SLAC Laboratory Animal Co.,Ltd.(Shanghai,China).All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee at Zhejiang University.2.2.Culture of human umbilical cord MSCs and exosome isolation
The culture medium for MSCs derived from the human umbilical cord was prepared using MEM-α
supplemented with 10% FBS and human-derived factors bFGF and EGF both at 20 ng/ml.The cells were cultured in 37 °C and 5% COatmosphere,and the medium was refreshed every two days.The culture supernatant was collected and centrifuged at 3,000 g and 10,000 g for 30 min,respectively,to remove cell debris.Then,after ultrafiltration at 2,000 g for 8 min using Amicon-ultra (100 kD,Millipore,USA),the supernatant was ultracentrifuged at 100 000 g and 4 °C for 70 min to obtain the exosome pellet.The exosomes were suspended in PBS at -80°C before use.2.3.Characterization of exosomes
The collected exosomes were detected by transmission electron microscope (TEM) (JEM-1200EX,JEOL,Japan).Formvar/carbon-coated 200-mesh copper electron microscopy grids were loaded with exosome suspension of 5 μl through incubation for 1 min at room temperature.Then,the grids were stained with standard uranyl acetate and semi-dried for TEM observation.The exosomes were examined for expression of CD9 and CD63 by Western blotting assay.Exosome suspensions were lysed and incubated for 5 min at 95 °C.The sample was centrifuged and examined in Jess Simple Western System (Bio-techne,Minneapolis,USA) using CD9 (OM198821,Omnimabs,CA,USA) and CD63 antibodies (OM245783,Omnimabs,CA,USA).The distribution of the exosome diameters was examined by nanoparticle tracking analysis (NTA) using the NanoSight NS300 system.Before using in the experiment,the exosomes were quantified according to the protein content by Micro-BCA Protein Assay(23235,Thermo Fisher Scientific,USA).
2.4.Fibrin glue fabrication and encapsulation of exosomes
Fibrinogen (1.5 mg) was dissolved in ultrapure water (30 μl)to obtain a fibrinogen solution of 50 mg/ml.Thrombin was dissolved in CaClsolution at a concentration of 500 IU/ml.The solutions were mixed at an equal volume of 30 μl to form a 60 μl fibrin glue.The hydrogel was dehydrated,fractured,and coated with gold for observation using scanning electron microscope (SEM) (SU8010,Hitachi,Japan).For exosomeencapsulated fibrin glue (FG-Exo),before gelation,the solutions of fibrinogen and thrombin were prepared at a two-fold concentration and then respectively mixed,at equal volumes,with exosome suspension containing 50 μg of exosomes in 15 μl.Finally,the density of exosomes in fibrin glue was 100 μg/60 μl.
2.5.Detection of exosomes in fibrin glue
Exosomes were fluorescently labeled through incubation with the CM-DiI (40718ES50,Yeasen,Shanghai,China) at a 2 μg/ml concentration of the dye at 37 °C for 5 min and subsequently 4°C for 15 min.After ultracentrifugation at 100 000 g for 70 min at 4 °C to remove the excess dye,the labeled exosomes were encapsulated in fibrin glue as mentioned above.The fibrin glue was observed in a confocal laser scanning microscope(CLSM),and exosomes distributed in the gel were detected by z-stack scanning.
2.6.Spinal cord transection and FG-Exo treatment
Female SD rats weighing around 230 g were randomly separated into four groups with 6 animals in each.Animals received spinal cord transection surgery under deep anesthesia as reported previously [4] .Briefly,the hair on the back near T10 was removed and the muscle was separated.Laminectomy was conducted to expose the dorsal surface of T9 to T11 segment.The spinal cord was transected at T9 to T10,leaving a lesion gap of 4–5 mm.After exact hemostasis,the fibrinogen and thrombin solutions containing exosomes each of 30 μl were injected into the lesion site simultaneously(FG-Exo group).And blank fibrin glue was formed in the lesion site through injection of exosome-free fibrinogen and thrombin (FG group).Non-treated control termed as SCI group was injected with the same volume of PBS.Animals receiving laminectomy without spinal cord transection served as the Sham group.Finally,after suture of the muscle and skin,the animals were kept warm until recovery from the anesthesia.The animals received penicillin within 7 d post-surgery.Manual bladder massage was performed for postsurgical care twice daily until the reflexive bladder activity returned.
2.7.Evaluation of motor function restoration
Locomotor function evaluation was conducted by a Basso,Beattie,Bresnahan (BBB) locomotion rating scale and a horizontal ladder rung walking test.For BBB assessment,the rats were tested weekly after the surgery in an open field by observers blinded to the treatment groups.The behavioral outcomes were evaluated by the 21-pointed rating scale.A ladder rung walking test was conducted by allowing the rats to walk from one to the other end of a horizontal ladder with irregular distance arrangements between the rungs.The walking ability was evaluated according to the placement of the limbs during stepping.The tests were recorded by a camera.Effective steps in the ladder were defined as pedal activities of the hind limb against the metal rungs.The outcome was analyzed by GraphPad prism 8.0.
2.8.Tissue processing and immunohistochemistry
At 4-week post-surgery,animals were perfused under deep anesthesia by isotonic physiological saline,and tissues of the spinal cord,heart,liver,spleen,lung,kidney,and bladder were harvested.For immunofluorescence staining of the spinal cord,after fixation in 4% paraformaldehyde in PBS for 24 h,the tissue was dissected to collect a 1.5 cm segment encompassing the lesion site and dehydrated by 30% sucrose.The sample was then embedded in an optimal cutting temperature compound (OCT) (Thermo Fisher,USA)and cryosectioned into 20 μm thick slices.The sections were stained for interested markers through incubation with primary antibodies of glial fibrillary acidic protein (GFAP)(BA0056,Boster,Wuhan,China),neurofilament (NF) (#2836,Cell Signaling Technology,USA),4-hydroxynonenal (4-HNE)(AB46545,Abcam,USA),8–hydroxy-2 ′ -deoxyguanosine (8-OHdG) (OM176273,Omnimabs,USA),CD68 (MCA341GA,Bio-Rad,USA),Arginase-1 (Arg-1) (A1847,ABclonal,China),inducible nitric oxide synthase (iNOS) (18985–1-AP,Proteintech Group,USA),choline acetyltransferase (ChAT)(OM247037,Omnimabs,USA),and myelin basic protein(MBP) (OM264373,Omnimabs,USA) overnight at 4 °C,then after washing,with fluorescence conjugated secondary antibodies (34206ES60,34106ES60,34212ES60,34112ES60,Jackson ImmunoResearch,USA) at 37 °C for 1 h.The sections were finally stained with DAPI (62248,Invitrogen,USA).For other tissues,after fixation in 10% neutral formalin,the tissues were trimmed,dehydrated,and paraffin-embedded.Paraffin slices of 4 μm were prepared for hematoxylin and eosin (HE) (G1120,Beijing Solarbio Science &Technology,China) and Masson staining (G1346,Beijing Solarbio Science&Technology,China) by using the assay kits.
2.9.Statistical analysis
Quantitative analysis of immunohistochemistry results was processed using ImageJ 1.52n.The data were shown as mean± standard deviation (SD) values.Statistical analysis was performed by GraphPad prism 8.0 using the one-way ANOVA test.
3.Results and discussion
3.1.Exosome encapsulation in fibrin glue
MSCs derived from the human umbilical cord were culturedin vitro
,and the exosomes were isolated from the cell culture supernatant.The exosomes were observed in TEM and showed cup-shaped morphologies around 100–200 nm(Fig.1 A).The exosome particles were further detected by NTA,revealing most particles were about 150 nm (Fig.1 B).CD63 and CD9 are two surface markers on the membrane of exosomes.Expression of the proteins analyzed by Western blot assay(Fig.1 C) suggested successful isolation of the exosomes.To make efficient delivery of the exosomes during transplantation and provide biocompatible ECM to the lesion site,the strategy applied fibrin glue,a clinically validated in situ hydrogel.Solutions of fibrinogen and thrombin were mixed to form the fibrin glue.The gel was dehydrated and detected by SEM,which showed a highly porous three-dimensional (3D) structure with nanofibers(Fig.1 D).MSC-derived exosomes were suspended in the gel solutions to construct exosome-encapsulated fibrin glue.Encapsulation of exosomes showed no obvious difference in gelation of fibrin glue.Exosomes labeled by CM-DiI were observed in the fibrin glue and exhibited a high-density 3D spatial distribution (Fig.1 E).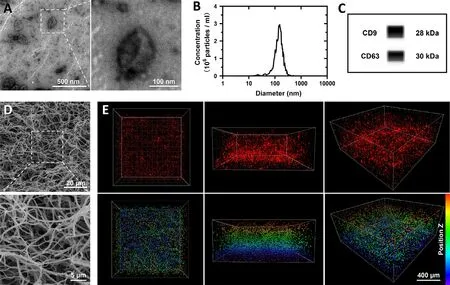
Fig.1-Characterization and encapsulation of exosomes.(A) Morphology of exosomes tested by TEM.(B) Particle size analysis of exosomes by NTA.(C) Western blot results of tetraspanins CD9 and CD63 expression in exosomes.(D)Three-dimensional structure of fibrin glue observed in SEM.(E) Three-dimensional distribution of CM-DiI-labeled exosomes(red) in the fibrin glue scanned by CLSM.The images showed views of different angles,and the spatial distribution of exosomes was presented by images in lower lines with pseudo colors according to position Z.
3.2.Nerve tissue repair by the emergency treatment
The treatment strategy was subsequently evaluated in a rat spinal cord transection model (Fig.2 A).After transection injury of the spinal cord,the exosome-suspended fibrinogen and thrombin solutions were synchronously injected into the lesion site and allowed for gelation (FG-Exo group).PBS (SCI group) and blank fibrin glue (FG group) treatments served as controls.According to BBB score assessment,rats without effective treatment after the acute injury failed to obtain functional recovery and exhibited almost total paralysis during the whole post-injury period (Fig.2 B).The emergency therapy using fibrin glue-encapsulating exosomes effectively promoted recovery of hindlimb motor functions,indicating a significant advantage of the emergency treatment strategy over both the SCI and FG groups.The rats presented similar outcomes in the ladder rung walking task,which has proven sensitive to movement disorders following an injury of the motor system in rat models of stroke and SCI [19] .The FGExo group exhibited more effective steps on the ladders of about 30% compared to those lower than 10% in the other two groups (Fig.2 C).The performance of rats in BBB (Videos S1–S4) and ladder tests (Videos S5) were recorded by videos.The motor function outcomes suggested a promising role of the emergency treatment strategy using FG-Exo and predicted the effects of the therapy on nerve tissue regeneration.Nerve tissue regenerative effects of the FG-Exo emergency treatment were further evaluated (Fig.2 D–Fig.2 F,Fig.S1A).As for indicators of mature neurons and astrocytes in glial scars respectively,NF and GFAP were considered correlatedly indicating the nerve tissue regeneration extent [20,21].The FG-Exo treated tissue exhibited reduced lesion volume and increased integrity,with a more abundant distribution of NF in both the lesion and adjacent areas.Investigation of ChAT and MBP showed similar results among the groups (Fig.S2).FGExo treated tissue obtained significantly increased extensive distribution of ChAT,an indicator for cholinergic neurons.And the recovery of MBP in the lesion site also indicated improved remyelination in the FG-Exo group compared to both SCI and FG groups.
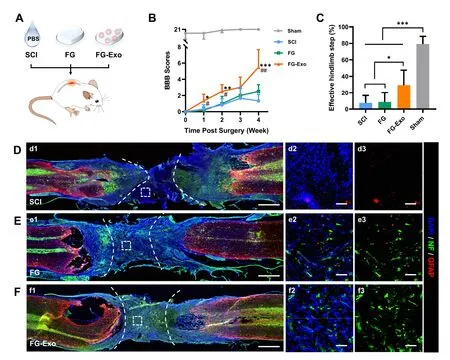
Fig.2-Evaluation on functional recovery and nerve tissue regeneration effect of FG-Exo treatment in rat spinal cord transection injury.(A) Schematic illustration of the therapeutic experiment.(B) BBB scores (mean ±SD,n=6) of animals in different groups within 4 weeks post-injury.∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001 compared to SCI group;# P < 0.05,## P < 0.01 compared to FG group.(C) Effective hindlimb steps (mean ±SD,n=6) tested by ladder rung walking task on Day 28.∗P < 0.05 and ∗∗∗P < 0.001.(D-F) Representative images showing immunostaining of NF (green) and GFAP (red) staining in SCI (D),FG (E) and FG-Exo (F) groups.Micrographs in (d2,e2 and f2) show the magnified views of the boxed lesion areas in(d1,e1 and f1).Images of NF and GFAP channels are presented in (d3,e3 and f3),respectively.The dashed lines indicate the lesion edges.Scale bar,1 mm (d1,e1 and f1),50 μm (d2,d3,e2,e3,f2,f3).
By using the clinical materials,human umbilical cord MSCs and fibrin glue,the strategy elicited significant nerve repair effects.In the experiment,the therapy was performed following the injury of spinal cord transection.The insitu formed 3D system serves as a biocompatible ECM containing comprehensive microenvironment regulators.The functional restoration,indicated by BBB scores and ladder rung walking test,showed an obvious advantage of the composite system over the blank fibrin glue.On the other hand,however,this effect is inferior to several previous studies of the authors using other functional materials and stem cells [4,17,22].More studies,especially clinical translation,of superior functional biomaterials are crucial to develop effective emergency treatments.And the dosage of transplanted exosomes remains to be optimized in further investigations.Besides,the introduction of antiinflammatory and antioxidant drugs in the system could also contribute to protecting the nerve tissue in the acute phase.

Fig.3-Immunofluorescence staining of oxidative damage products 4-HNE and 8-OHdG in spinal cord tissues.(A,B)Representative micrographs of 4-HNE (A) and 8-OHdG (B) staining in spinal cord tissues of different groups.(C,D) Quantified analysis of the fluorescence signals in 4-HNE (C) and 8-OHdG (D) staining.Data represent mean ±SD,n=6.∗P < 0.05 and∗∗P < 0.01.
3.3.Mitigation of microenvironment
After the acute trauma,extensive secondary damage occurs in the spinal cord tissue,involving ischemia,severe oxidative stress,and inflammatory responses [23] .Exosomes derived from MSCs have been reported to alleviate inhibitory environment and secondary injury.Especially,our previous research has reported the promotive effects of exosomes derived from the human placental amniotic membrane MSCs in SCI therapy [17] .In the current study,stem cellderived exosomes are delivered by fibrin glue in order to comprehensively regulate the microenvironmentvia
mitigating inhibitory factors and supplementing substrates,which are vitally important for the protection of neurons during the acute phase [24,25].Herein,the emergency treatment using FG-Exo was also further evaluated for the effects on the lesion microenvironment.Excessive production of ROS lastingly causes damage to the nerve tissues [26,27],leaving 4-HNE and 8-OHdG as markers of tissue peroxidative damage.Detection of the two markers showed obviously more extensive distribution of the peroxidative damage products in the lesion of SCI group,while administration with FG and FG-Exo effectively prevented the oxidative procedures(Fig.3,Fig.S1B-S1C).This result was especially significant for the lipid peroxidation indicated by 4-HNE analysis (Fig.3 C).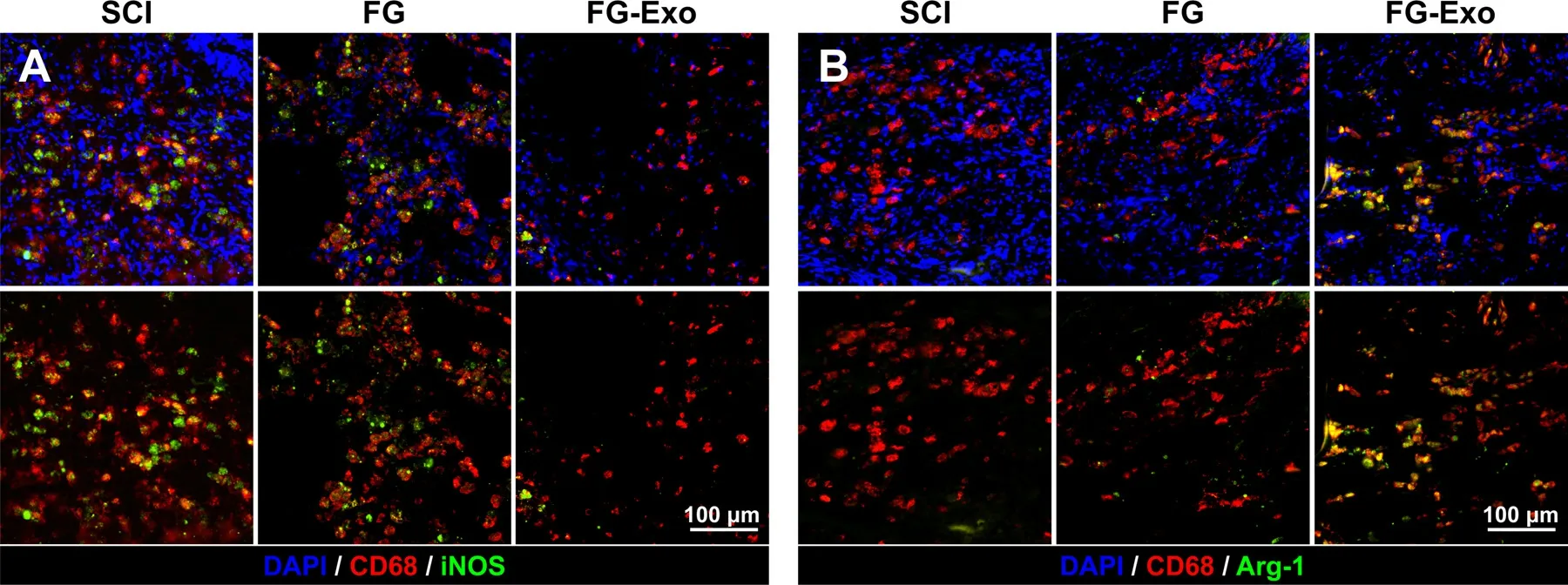
Fig.4-Detection of M1 and M2 macrophages in lesion site of spinal cords.(A) Staining of CD68 (red) and iNOS (green) for M1 macrophages in spinal cord tissues of SCI,FG and FG-Exo groups.(B) Staining of CD68 (red) and Arg-1 (green) for M2 macrophages in the spinal cord tissues.Immunofluorescent images of M1 and M2 macrophages with or without DAPI channel are displayed at the top and bottom lines,respectively.
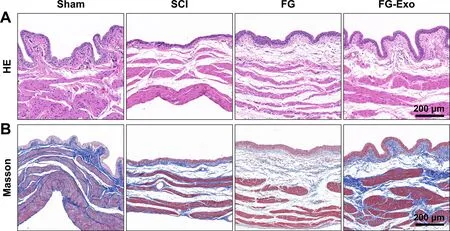
Fig.5-HE staining (A) and Masson staining (B) of the bladder tissues in different groups after a 4-week treatment.
On the other hand,inflammation is a leading cause of neural damage.Macrophages were detected for both M1 and M2 phenotypes,which proved opposite impacts in inflammation.Examination and colocalization of CD68 and iNOS suggested more distribution of pro-inflammatory M1 macrophages in the SCI and blank FG group than the FGExo group (Fig.4 A,Fig.S3A-S3B).The overexpression of iNOS also intensified the inflammatory progression and neuron degeneration after injury [24] .A more obvious difference was exhibited in the result of M2 macrophage detection(Fig.4 B,Fig.S3C and S3D),showing a significantly extensive distribution of Arg-1 positive M2 macrophages in FG-Exo treated spinal cord tissue.These results indicate the antiinflammatory and neuronal protective effects of the FGExo emergency treatment.The exosomes elicited significant anti-inflammatory effects through regulation on macrophage phenotypes,which mechanism is inconsistent with previous reports about systemically administrated exosomes [15] .
3.4.Urinary protection effect and safety of the FG-Exo treatment
Injury of spinal cord tissue always results in dysfunction of the urinary system.Urinary disorders cause irreversible damage in kidney and bladder tissues and are severe complications among SCI patients,even threatening their survival [28–30] .HE staining (Fig.5 A and S4A),as well as Masson staining (Fig.5 B and S4A) of the bladder tissues after a 4-week treatment,showed attenuation effect of the FG-Exo therapy on disorders in the bladder wall and the muscle bundle.Repair of spinal cord nerve tissues could restore urinary functions and alleviate neurogenic bladder.Therefore,urinary damage is a critical indicator of the nerve regeneration effect.The examinations of the bladder tissue damage revealed the urinary protective effects of the emergency treatment by FG-Exo,suggesting the impact on spinal cord repair.More than that,there rises new aspirations in the current study.As mentioned above,the authors have evaluated the therapeutic functions of exosome transplantation in the previous study.Although the strategies and materials are quite different in the current study,significant effects on bladder tissues were exhibited by both two experiments,in which exosome encapsulation effectively relieved neurogenic bladders compared to the blank material groups.The exosome-based therapies also induced better bladder conditions than stem cell treatments in the same SCI model (data not shown).These results may prompt further investigation on the possible related mechanisms.
Damage of the ECM is an inhibitory parameter blocking nerve tissue regeneration,and supplement for ECM by biomaterials has been considered an important approach in SCI treatment.Herein,the FG group was able to obtain slight extents of functional recovery and oxidative stress mitigation.This could be attributed to the tissue bridging effect of fibrin glue,which is also a basis for the application of the material to deliver exosomes.But the overall microenvironment mitigation effects in the FG group were significantly insufficient compared to those in the FG-Exo group (Figs.3 and 4),especially for the anti-inflammatory effects (Fig.4).Also,the therapeutic outcomes including the performance in open field walking and horizontal ladder rung walking (Fig.2),and the urinary bladder conditions (Fig.5),further proved the advantages of FG-Exo over FG.
The safety of this emergency therapy was evaluated through the investigation of rat organs in addition to blood indicators for liver and kidney conditions.Treatment by FGExo poses no apparent effect on general tissues (Fig.6).Detection of tissues including the heart,liver,spleen,lung,and kidney showed consistent morphologies among all the experiment groups (Fig.6 A,Fig.S4B).The indexes in the serum for liver and kidney damages,including alanine transaminase(ALT),aspartate aminotransferase (AST),blood urea nitrogen(BUN),and creatinine (CR),exhibited similar levels (Fig.6 B).
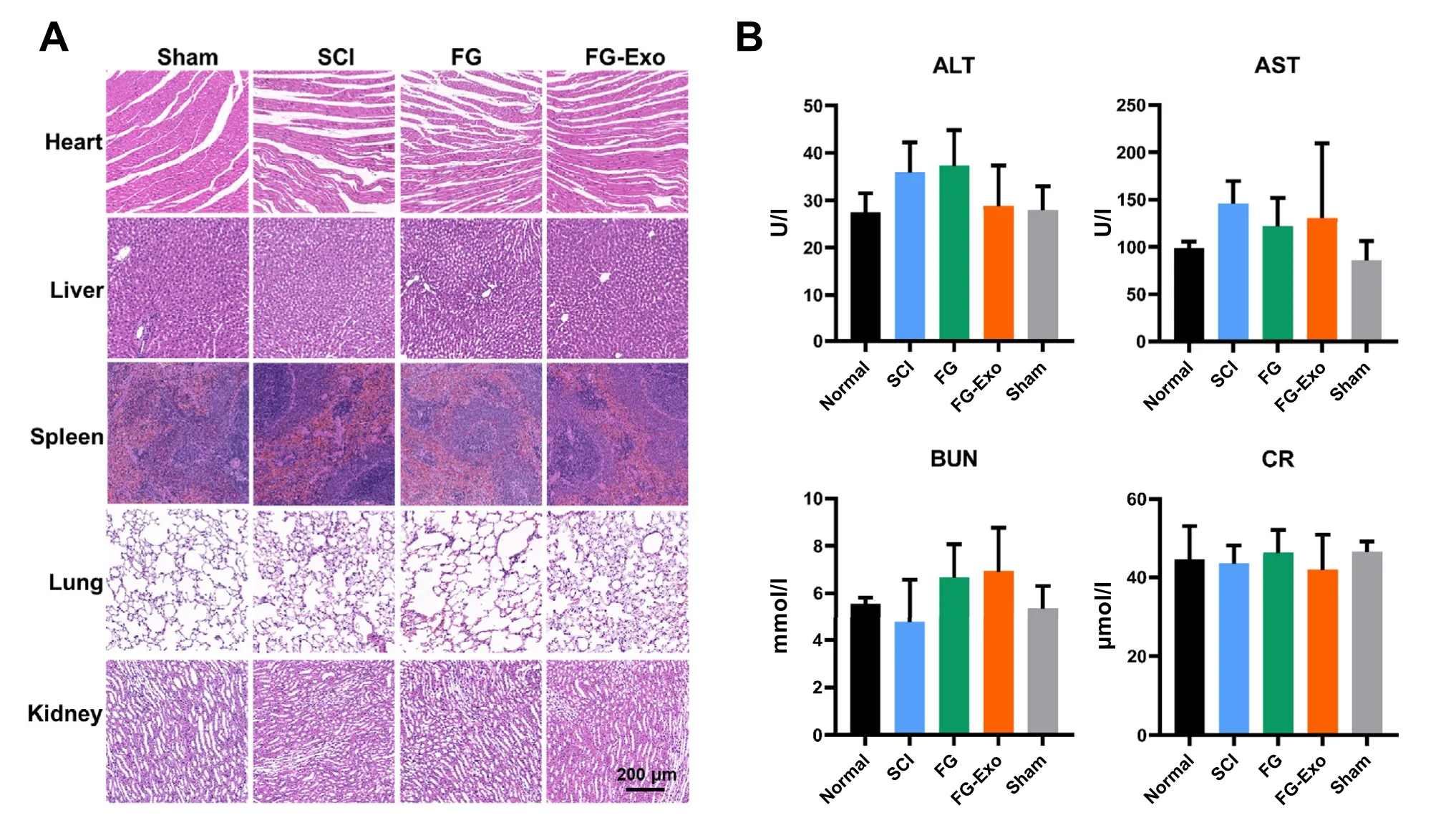
Fig.6-Safety evaluation of the FG-Exo treatment.(A) HE staining of heart,liver,spleen,lung,and kidney after the 4-week treatment.(B) Levels of liver and kidney condition indexes in the serum,including ALT,AST,BUN,and CR (mean ±SD,n=6).
4.Conclusion
Timely access to skilled management in the acute phase of traumatic SCI is essential to restrain the secondary injury and is largely decisive for the final nerve recovery outcomes.SCI patients who failed to obtain acute management are accompanied with an increased risk of secondary complications,showing higher mortality and severity of complications [6,31].Despite the awareness of the importance of emergency treatment in clinical SCI management,the development of effective emergency treatments remains in its infancy.In experimental studies,transplantation of stem cells with biomaterials has been considered promising in SCI therapy.But further efforts are needed to answer the strict requirements in cell viability and material compatibility when stem cell-based strategies are applied in urgent management after storage and transportation processes.Also,the transplanted cells usually exhibit significant death and deficiency in the acute lesion microenvironment.Recent evidence on the therapeutic effects of stem cell-derived exosomes lightens the development of emergency treatment for SCI.From the aspect of clinical practice,the current study is aimed to develop a feasible emergency treatment that involves clinical accessible cell sources,validated materials,facile operation,and effective functional nerve recovery.The proposed FG-Exo treatment is a relatively simple therapy with the injection of the exosome-encapsulated in-situ fibrin glue.The therapeutic system could be preserved and transported conveniently for pre-hospital management with the rapid operation.
In conclusion,the study demonstrates a facile and efficient therapeutic strategy based on MSC-derived exosomes and fibrin glue for effective emergency treatment of acute SCI.The FG-Exo treatment proved functional in mitigation of the oxidative and inflammatory microenvironment,thus eliciting superior impact on nerve tissue repair and urinary tissue protection in the rat severe transection SCI model.Exosome transplantation strategies and the vectors used were both poorly reported in SCI treatments.In this study,although relatively simple and rapid,the proposed strategy exhibited highly promising for effective SCI treatment in clinical application.Besides,clinically approved,human umbilical cord MSCs and fibrin glue represent accessible and biocompatible materials.Therefore,we report that the FGExo transplantation provides a promising and safe exosomebased strategy for emergency treatment of acute SCI.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was financially supported by the National Key Research and Development Project of Stem Cell and Transformation Research (2019YFA0112100,2019YFA0112102),National Natural Science Foundation of China (81973252,81803451 and 81620108028).We thank the Center of Cryo-Electron Microscopy (CCEM),Zhejiang University for the technical assistance on Confocal Laser Scanning Microscopy.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2021.10.002 .
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Advances in photosensitizer-related design for photodynamic therapy
- Applications and developments of gene therapy drug delivery systems for genetic diseases
- Aspects of high-performance and bio-acceptable magnetic nanoparticles for biomedical application
- Nanomedicine potentiates mild photothermal therapy for tumor ablation
- Carrier-free prodrug nanoparticles based on dasatinib and cisplatin for efficient antitumor in vivo
- Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19
