Enhanced hydrogen generation from hydrolysis of MgLi doped with expanded graphite
2021-02-24KngChenJunJingLiuzhngOuyngHuiWngJingwenLiuHuiyuShoMinZhu
Kng Chen ,Jun Jing ,Liuzhng Ouyng,b,∗ ,Hui Wng ,Jingwen Liu ,Huiyu Sho ,Min Zhu,b
a School of Materials Science and Engineering,Guangdong Provincial Key Laboratory of Advanced Energy Storage Materials,South China University of Technology,Guangzhou,510641,China
b China-Australia Joint Laboratory for Energy &Environmental Materials,Key Laboratory of Fuel Cell Technology of Guangdong Province,Guangzhou,510641,China
cJoint Key Laboratory of the Ministry of Education,Institute of Applied Physics and Materials Engineering(IAPME),University of Macau,Macao SAR,China
Abstract Hydrolysis of Mg-based materials is considered as a potential means of safe and convenient real-time control of H2 release,enabling efficient loading,discharge and utilization of hydrogen in portable electronic devices.At present work,the hydrogen generation properties of MgLi-graphite composites were evaluated for the first time.The MgLi-graphite composites with different doping amounts of expanded graphite(abbreviated as EG hereinafter)were synthesized through ball milling and the hydrogen behaviors of the composites were investigated in chloride solutions.Among the above doping systems,the 10 wt.% EG-doped MgLi exhibited the best hydrogen performance in MgCl2 solutions.In particular,the 22h-milled MgLi-10 wt.% EG composites possessed both desirable hydrogen conversion and rapid reaction kinetics,delivering a hydrogen yield of 966mL H2 g−1 within merely 2min and a maximum hydrogen generation rate of 1147mL H2 min−1 g−1,as opposed to the sluggish kinetics in the EG-free composites.Moreover,the EG-doped MgLi showed superior air-stable ability even under a 75 RH% ambient atmosphere.For example,the 22h-milled MgLi-10 wt.% EG composites held a fuel conversion of 89% after air exposure for 72h,rendering it an advantage for Mg-based materials to safely store and transfer in practical applications.The similar favorable hydrogen performance of MgLi-EG composites in(simulate)seawater may shed light on future development of hydrogen generation technologies.
Keywords: Hydrogen generation;MgLi-graphite composites;Hydrolysis;Air-stable ability.
1.Introduction
The ever-increasing concerns over dwindling resources and the environmental issues(such as global warming and consequent climate change,etc.)caused by burning fossil fuels have triggered extensive interests in exploration of green and renewable energy sources [1,2].Hydrogen,possessing particular advantages of extremely abundant content,environmentally benign products of oxidation(water),high-energy density(142MJ kg−1),and so forth,has been considered to be a leading candidate as an energy vector[3,4].However,its production and storage are the most demanding and challenging parts of achieving the so-called“hydrogen economy”[5,6]as far as mobile applications are concerned.Over the past decades,considerable attention has been paid to solid hydrogen storage materials,including metal hydrides(e.g.MgH2)[7],chemical hydrides(e.g.LiBH4[8,9],NaBH4[10,11],and NH3BH3[12]),because of their numerous favorable attributes with ultrahigh hydrogen capacity and energy efficiency,high safety,and the environmentally benign by-products.Although some encouraging progress has been made in potentially reversible hydrogen storage systems [13],hydrogen-rich hydrides remain confronted with challenges of harsh reversibility/cyclability and intrinsic thermodynamic and kinetic barriers,falling short of the targets set by the US Department of Energy(DOE)[14].Compared to pyrolysis,hydrogen generation from hydrolysis of hydrides [15,16](NaBH4,for example)can proceed at room temperature without external heat input,exhibiting significant advantages of rapid and well-controlled hydrogen release kinetics,high-purity hydrogen and nontoxic by-products [17].Moreover,the slightly humid hydrogen produced from hydrolysis can be directly conveyed to portable polymer electrolyte membrane fuel cells(PEMFCs)without dehumidification treatment [18].
However,the above hydrides are in particular expensive irreversible materials for one-time utilization.The prohibitively high hydrogen-production cost is caused by the lack of energy-efficient and mature regeneration technologies for spent fuel,thereby greatly hampering the commercial viability of hydrides as an off-or on-board hydrogen carrier.Motivated by this situation,extensive interests have been directed towards the exploration of cost-effective hydrogen generation systems based on abundant and readily available materials that enable high-efficiency energy conversion [19].Here,an important candidate is magnesium-based materials(Mg or MgH2,etc.)[20–22]:
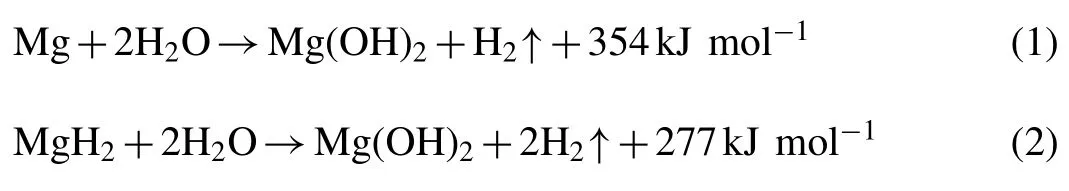
Mg/MgH2is a well-known water-splitting agent,which can release hydrogen by reacting with water following Eqs.(1)and(2).The Mg/MgH2–H2O system possesses significant advantages that merit its hydrogen applications,including abundant content in the earth’s crust,low cost,desirable operation temperatures,and mature Mg/MgH2recycling technology [23,24].Nevertheless,in the hydrolytic process for hydrogen supply,the Mg-based systems are plagued by a serious self-restraining problem,i.e.the hydrolysis reaction can be rapidly interrupted by the formation of a coherent passive Mg(OH)2layer deposited on the surface of Mg/MgH2,resulting in poor kinetics and low hydrogen yield[25,26].So far,several approaches have been developed to address the issues,e.g.ball milling [27],alloying [28],tailoring the solution components [29,30],dopants and catalysts[20,21].Doping of catalysts is regarded as one of the simplest and most effective strategies to enhance the hydrogen generation performance of Mg-based materials.However,the doping of metals,chlorides,oxides,and hydrides towards Mg-based materials [21,31,32]would not only increase the dead weight of hydrolysis systems,but also complicate the regeneration of spent fuels.More recently,the light-weight EG has been proven to be a favorable ball-milling agent and the Mg-graphite composites obtained by P-milling exhibited greatly enhanced hydrolysis properties as well as high hydrogen density [33],whereas the hydrogen generation kinetics was relatively low.Alternatively,some aqueous solutions such as organic/inorganic acids [34,35]or salt solutions(MgCl2[32],NH4Cl [36],and NaCl [37],etc.)are also found to be effective in obtaining rapid hydrogen release kinetics.In this regard,it may be possible to achieve favorable system performance of Mg-based materials by incorporating the addition of light-weight EG and modified solutions.
Herein,the ultralight MgLi alloy was chosen as the host material due to its superior corrosion resistance among Mgbased alloys [38]and high theoretical hydrogen generation capacity(9.7 wt.% H2).The hydrolysis reaction of MgLi is described as Eq.(3)and the calculation equation for the hydrogen generation capacity(HGC)is given as Eq.(4):
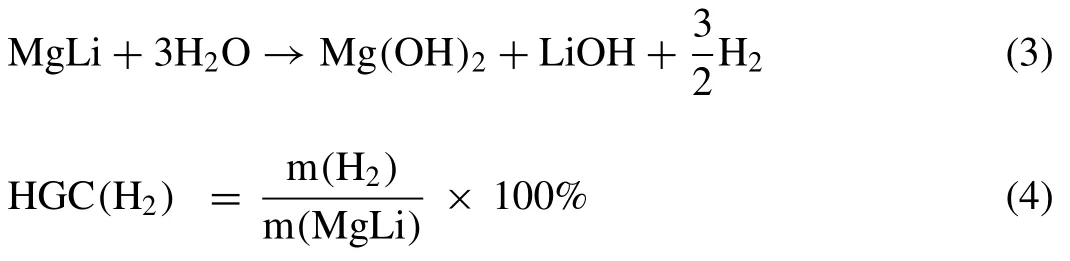
MgLi alloy,is well-known as a relatively soft material,making the ball milling process less efficient.Thus,the lightweight and readily available EG was applied as a grinding agent to inhibit the agglomeration of MgLi during ball milling course.In particular,the hydrogen behaviors of MgLi-EG composites affected by salt solutions,ball milling time,EG-doping amount,and operation temperature were investigated here.The results indicated that the MgLi alloy doped with a trace of EG exhibited desirable hydrogen yield and fast kinetics in MgCl2solutions or(simulate)seawater,which may provide a promising hydrogen supply technology for future hydrogen society.
2.Experimental section
2.1.Sample preparation
The MgLi alloy was synthesized through fused-salt electrolysis of MgCl2and LiCl under Ar atmosphere.The obtained alloy ingots were initially polished to remove the surface oxide layer,then the pre-treated ingots were pulverized into small particles using saws and finally filtered with a 100-mesh sieve.Fig.S1presents the XRD pattern of the as-received alloy,where the peaks coincide well with those in the standard PDF card of MgLi.As shown inFig.S2,the sieved MgLi powder is composed of coarse particles ranging from 50 to 300μm.Sodium chloride(NaCl,99.8% purity)and magnesium chloride hexahydrate(MgCl2,99.99% purity)were purchased from Aladdin and used as received.On the other hand,EG was obtained by calcining expandable graphite(99.5% purity,Nanjing Pioneer Nano Material Technology Co.,Ltd.)at 950°C for 5min under ambient atmosphere.The MgLi-EG composites were prepared through mechanochemical process in a QM-3SP-4 planetary ball miller(Nanjing,China)using stainless steel vial which was loaded with two types of balls of 6 and 10mm in diameter under Ar atmosphere.For a typical ball milling procedure,the ball-to-powder ratio was determined to be 30:1 with a rotation speed of 400rpm.The milling process was performed by alternating 30min of milling and 30min of rest.Moreover,the pristine MgLi powder without further pre-treatments was used as a reference sample.All samples were stored and handled in an Ar atmosphere glove box(Mikrouna,China)to prevent samples from oxidation.
2.2.Characterization
The phase structures and morphology of the solid samples collected at different conditions were investigated by powder X-ray diffraction(XRD,PANalytical EMPYREAN,Cu-Kαradiation)with a range of diffraction angles(2θ)from 10°to 90° and scanning electron microscopy(SEM,TESCAN GAIA3).The density of the EG-doped composites was determined by a tap density meter(Hengrui,China).
2.3.Hydrogen evolution measurements
The hydrogen evolution measurements were performed in a home-made device described in a previous paper [39].The reaction was carried out in a 50mL three-neck flask,wherein the solid powder samples were preloaded and then deionized water or MgCl2/NaCl solution was fed to react with the hydrolyzable materials.The evolved hydrogen gas was passed through a Monteggia washing bottle filled with water,where the drained water was collected with a beaker placed on an electronic scale that was connected to a computer used for recording the hydrogen generation volume over time.The reaction temperature was set as 278,285,298,and 308K to calculate the activation energy.In particular,each experiment was repeated at least twice to ensure reliability.Here,the calculation equation of hydrogen yield per unit weight of composites is described as follows:
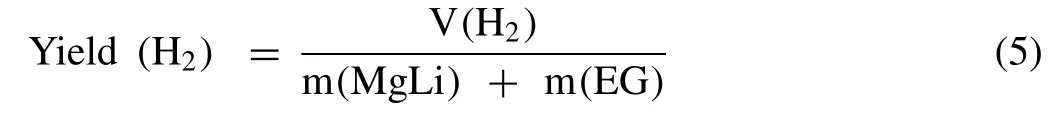
3.Results and discussion
3.1.Synthesis of MgLi-EG composites and characterization
Owing to the good ductility and plasticity of pristine MgLi,it tends to agglomerate into large particles during ball milling process without additives(Fig.S3).Herein,the lightweight and cheap EG was doped towards MgLi as a grinding agent,leading to the promotion of hydrogen production performance of MgLi.As shown inFig.S4,the morphology of the raw expandable graphite is flake-like,whereas the macroscopic worm-like EG is composed of numerous porous flocculent structures.In the present study,the MgLi-x wt.% EG(x=5,10,20,50)composites were synthesized through ball milling the counterparts.Fig.1 shows the XRD curves of hybrids with various loading amounts of EG,where the diffraction peaks are mainly referred to MgLi as the doping content is less than 50%.However,the major components of ball-milled products consist of MgLi with a body-centered cubic(bcc)structure and EG as well as a minor Mg-rich hexagonally close-packed(HCP)phase of Li3Mg17as the loading of EG reaches 50%.The newly emerged Li3Mg17could be attributed to the lack of Li caused by the formation of Li-C compounds [40,41],where Li atoms were embedded into the porous flocculent structures of EG during the high-energy ball milling process.Notably,the appearance of negligible Li2O was the result of oxidation,wherein the oxygen in Li2O may be absorbed upon EG over the aforementioned calcination.

Fig.1.XRD patterns of MgLi-x wt.% EG(x=5,10,20,50)composites milled for 22h.
In order to figure out the morphologies of the ball milled products,especially the distribution state of MgLi and EG,the microstructure of the MgLi-10 wt.% EG composites milled for different durations was characterized by SEM.As shown in Fig.2,the dispersed EG particles are distributed homogeneously on the MgLi substrate,leading to the formation of massive micro-galvanic cells in the subsequent hydrolysis process.The corresponding elemental mapping images of the MgLi-10 wt.% EG are shown inFig.S5.Noticeably,the average particles of EG for the 15h-milled samples are bigger in comparison with others.As the ball milling time prolonged from 18h to 26h,the distribution of EG became more and more homogeneous and the numbers of the microgalvanic cells increased markedly,which could account for the hydrolysis behaviors mentioned in following sections.
3.2.Effect of MgCl2 solutions on hydrogen behaviors of MgLi-EG composites
The hydrolysis kinetics of the pristine MgLi alloy was extremely sluggish,though it exhibited desirable hydrogen yields in pure water or MgCl2solution.Fig.3a presents that the 100-mesh MgLi powder released 656mL H2g−1in 60min with a H2conversion of 61%.The hydrolysis reaction of MgLi was subject to the formation of the coherent passive Mg(OH)2layer,exerting relatively slow kinetics.There was nearly 1050mL H2g−1liberated after 5h in deionized water,with a fuel conversion of 97%.Considering the sluggish kinetics of MgLi in pure water,chloride solutions were adopted to accelerate the hydrogen generation kinetics.Unfortunately,even in 2M MgCl2solution,the pristine MgLi alloy could only produce 739mL H2g−1within 60min with a H2conversion of 97% over 200min,which showed a slight promotion in hydrolysis kinetics.
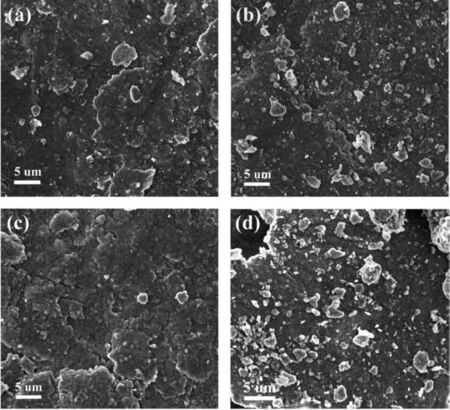
Fig.2.SEM images of MgLi-10 wt.% EG composites milled for various durations:a)15h;b)18h;c)22h and d)26h.
To further enhance the hydrolysis kinetics of MgLi alloy and realize a high-rate hydrogen generation system,the MgLi-graphite hybrids with EG loading of 5 wt.%,10 wt.%,20 wt.%,and 50 wt.% were synthesized through ball milling.Based on the optimal hydrolysis performance in 10 wt.%EG-doped sample,thus it was chosen for the candidate in the subsequent study.As shown inFig.S6,the 15h-milled composites can liberate 755mL H2g−1within 30min with a conversion rate of 77% in 0.5M MgCl2solution,surpassing that(658mL H2g−1)of the reference sample(i.e.handgrinding 10 wt.% EG-doped sample).As the milling time was prolonged over 18h,the composites showed significantly enhanced hydrolysis performance than that of 15h-milled samples,delivering a nearly theoretical hydrogen content(8.6 wt.% H2)within only 2min.And the volumetric hydrogen storage capacity of the composites was calculated to be 0.027g H2cm−3.In this regard,the hydrogen behaviors of the 22h-milled samples were further investigated by tailoring the components of salt solutions.As shown in Fig.3b,the composites show excellent reaction kinetics while reacting with MgCl2solutions,which can release 959,966,and 965mL H2g−1in less than 2min in 0.1,0.5,and 1M MgCl2solutions,corresponding to a H2conversion over 98% in the three cases.Interestingly,the hydrolysis kinetics of the MgLi-EG composites was not positively correlated with the MgCl2concentration.The composites exhibited fast hydrolysis kinetics even in low-concentration MgCl2solution,as opposed to sluggish kinetics in pure water.Therefore,the corrosion of Cl−towards hydrogen production devices may be well-controlled.To further increase the universality of hydrogen supply,(simulate)seawater was applied to react with the MgLi-EG composites.Fig.3c shows the hydrogen generation curves for hydrolysis of MgLi-EG composites in 3.5%NaCl solution and seawater.The 22h-milled composites delivered a hydrogen yield of 836mL H2g−1in 2min and 891mL H2g−1in 5min as well as a final H2conversion of 99% in the NaCl solution,exhibiting rapid hydrogen generation performance,which was comparable to that in MgCl2solution.Unfortunately,the hydrogen generation performance of MgLi-EG in real seawater was slightly inferior to that in 3.5% NaCl solution(Fig.3c)under same conditions.
Compared to the hydrolysis of the pristine MgLi,the doping of EG and ball milling treatment facilitated the formation of numerous micro-galvanic cells,thus remarkably enhancing the reaction kinetics with lots of hydrogen evolution sites.Actually,the hydrolysis process of MgLi-EG is composed by two galvanic reactions:

Fig.3.a)The hydrolysis curves of pristine MgLi in different concentration MgCl2 solutions;b)Effects of MgCl2 concentration on the hydrogen yield of the 22h-milled MgLi-10 wt.% EG;c)The hydrogen evolution curves of the 22h-milled MgLi-10 wt.% EG in 3.5% NaCl solution and seawater.
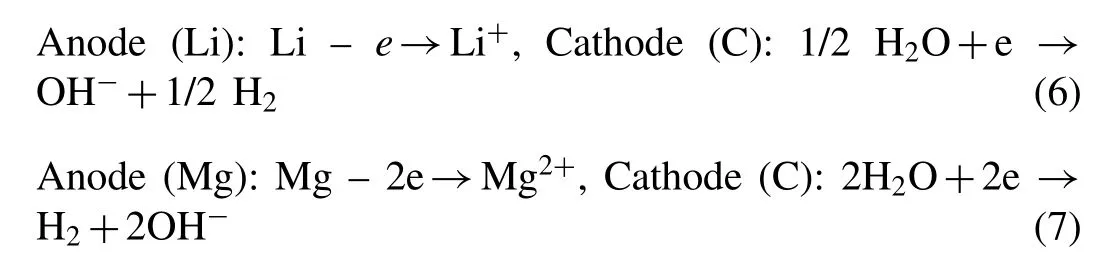
However,there was a limited promotion(188mL/g H2in 10min)in hydrogen kinetics of the ball milled MgLi-EG composites in pure water.Though the addition of EG and ball milling delivered numerous hydrogen evolution sites,the hydrolytic process of MgLi-EG was subject to the rapidly formed passive Mg(OH)2layer deposited on the surface of MgLi,exerting relatively slow kinetics.In this regard,Cl−was introduced to break the passivation layer and increased the micro-galvanic corrosion rate [38].Fundamentally,the formation of the galvanic cells should not contribute much to the hydrogen kinetics,because the MgLi/H2O reaction is not controlled by the cathodic water reduction,but by the chemical dissolution steps that disrupt the in situ formed hydroxide layer [42].Therefore,the favorable hydrogen generation performance of the MgLi-EG composites in chloride solutions can be ascribed to the synergistic effect of the Cl−corrosion towards the hydroxide layer and the formation of numerous galvanic cells by doping with EG.
3.3.Effect of EG-doping amount on hydrolysis behaviors of MgLi
Next,we evaluated the effect of the EG-doping amount on the hydrogen generation performance of MgLi.Since the MgLi-EG composites milled over 18h exhibited the fairly favorable hydrogen behaviors,the 22h-milled samples were selected as candidates in the present study.Fig.4a shows the hydrogen generation plots for the 22h-milled MgLi-EG composites with an EG-doping content ranging from 5 wt.%to 50 wt.% in 0.5M MgCl2solution.Although the 5 wt.%EG-doped MgLi composites exhibited relatively effective promotion in the hydrolysis kinetics with a hydrogen yield of 825mL H2g−1within 20min in comparison with the EG-free samples,the H2release rate still falls short of the high-rate hydrogen generation requirements.Then the doping amount of EG was further increased to accelerate the hydrogen generation rate of the EG-doped composites.The 10 wt.%EG-doped system delivered a hydrogen yield of 966mL H2g−1in 2min,as illustrated by Fig.4a.More specifically,the MgLi-EG composites with EG loading of 10 wt.% presented remarkably faster hydrogen kinetics than that of the expandable graphite doped system under same conditions(Fig.S8).Among the above-mentioned MgLi-EG composites,the 10 wt.% EG-doped system held the optimal hydrolysis performance with a fuel conversion of ∼100% and a maximum hydrogen generation rate of 1147mL H2min−1g−1(Fig.4b).Unfortunately,the increment of the loading amount of EG posed a compromising hydrogen release capacity per unit weight of MgLi-EG composites though the fuel conversion of the 20 wt.%and 50 wt.%EG-doped systems was over 96%.In the case of the 50 wt.% EG-doped samples,the major component of the products transferred into Li3Mg17with a hcp structure after 30h of ball milling,accompanying with the disappearance of MgLi remained in the 20h-milled samples as well as the visible reduction on the diffraction peaks of EG(Fig.S7a).Increasing the EG-loading content to 70 wt.%caused the formation of the Mg-rich phase of Li3Mg17in advance,which was driven by the embedding of Li atoms into the graphite structure [40,41].The deviation of the diffraction peak of C towards a lower angle re-confirmed the formation of the aforementioned Li-C compounds,as illustrated byFig.S7b.Thus,both the Li loss and the deadweight in the doped systems with EG loading over 20% would incur lower hydrogen yield,albeit with a good air-stable ability.Overall,the addition of 10 wt.% EG towards MgLi possessed both fast reaction kinetics and desirable hydrogen yield,delivering clear significance for the development of practical Mg-based hydrogen generation systems for off-or on-board applications.
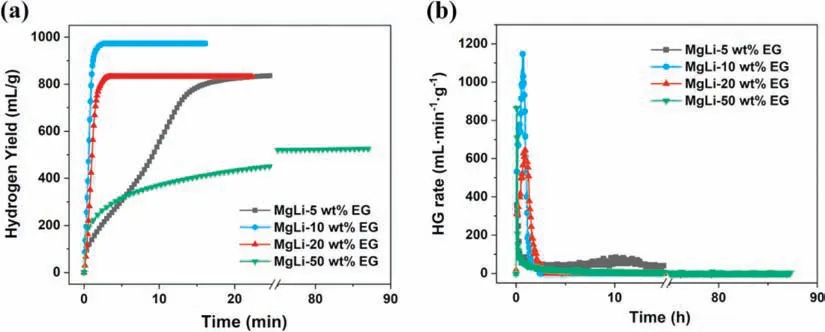
Fig.4.a)Effects of the EG-doping amount in the MgLi-EG composites on a)the hydrogen yield and b)the hydrogen generation rate.
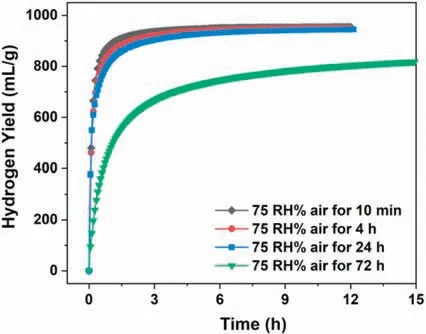
Fig.5.The comparison for hydrogen generation curves of the 22h-milled MgLi-10 wt.% EG composites after exposure to 75 RH% air for various durations.
3.4.Air-stability of MgLi-graphite composites
A safe and effective means of storing and transporting hydrogen-based materials for practical use remains a challenge.In this regard,developing air-stable highly reactive hydrogen supply materials with excellent hydrogen performance is of great significance.In the present study,the 22h-milled MgLi-10 wt.% EG composites,possessing both rapid reaction kinetics and optimal hydrogen yield,were selected as candidates to examine the air stability.In particular,each powder sample was exposed to an ambient atmosphere with relative humidity(RH)of 75% at 25 °C,and the hydrogen evolution curves of the MgLi-EG composites exposed to the air for various durations are present as Fig.5.After air exposure for 10min,no attenuation was observed in the hydrogen yield,where the doped system liberated 923mL H2g−1within 2min and achieved ∼98% fuel conversion in the end.It indicated that the hydrogen yields were almost the same at 948 and 945mL H2g−1after air exposure for 4h and 24h,corresponding to a H2conversion over 97%.Even exposed to the air for 72h,there was still 819mL H2g−1liberated within 15min for the composites.Finally,the fuel conversion of the exposed system reached 89%,showing a yield loss less than 10% compared to the unexposed one.The good air-stability property of MgLi-10 wt.% EG was mainly ascribed to the gas impermeability of EG coated on the surface of MgLi alloy and superior corrosion resistance of MgLi itself.Table 1 presents the hydrogen yields of the MgLi-EG composites exposed to 75 RH% air for different time.The superior air-stability exhibited in the MgLi-EG composites makes it much safer to be store and transfer samples in practical applications,greatly increasing the convenience and practicability of Mg-based materials in hydrogen fields.
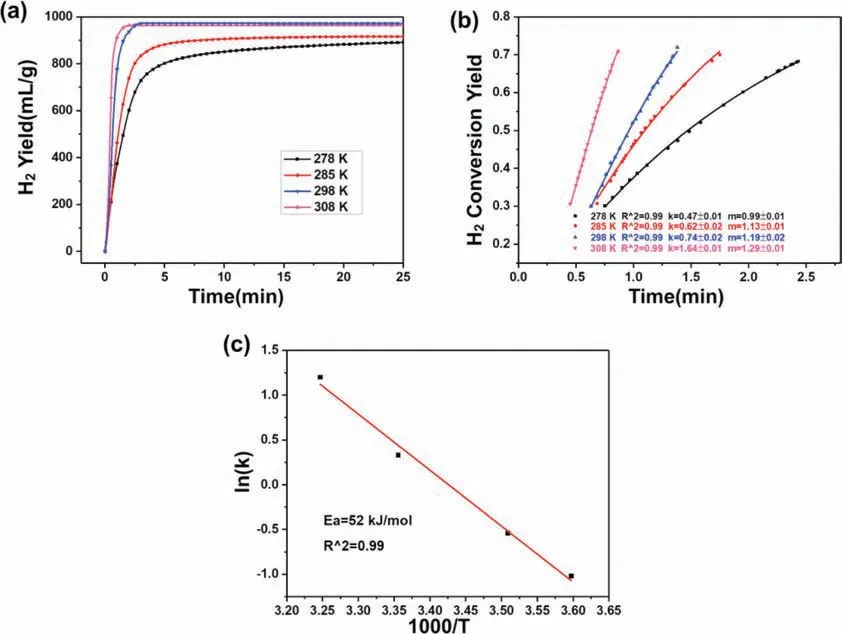
Fig.6.a)Hydrogen generation plots of the 22h-milled MgLi-10 wt.% EG composites versus time at different temperatures;b)Hydrolysis kinetic curves of the composites conducted under different temperatures;c)Arrhenius plots of ln k vs the reciprocal absolute temperature 1000/T for the composites.
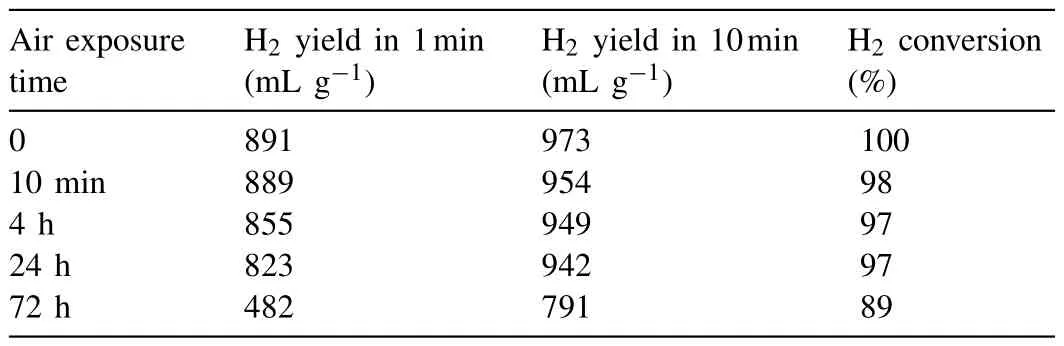
Table 1 Hydrogen yield of the MgLi-EG composites exposed to 75 RH% air for different time.
3.5.Effect of reaction temperature on hydrogen generation of MgLi-EG composites
To determine the effect of temperature on hydrogen behaviors of the MgLi-EG composites,the temperature range in the case of the MgLi-10 wt.% EG composites was from 5 to 35 °C.The impact of temperature is clearly shown in Fig.6a,by both the increasing slope values of hydrogen generation plots and the decreasing reaction completing time which is reduced from 30min at 5 °C to 2min at 35 °C.Encouragingly,even at 5 °C,there were ∼800mL H2g−1released within 5min as well as an ultimate fuel conversion of 92% for the composites.As expected,the hydrogengeneration yield could reach 882mL H2g−1within 5min at a temperature of 12 °C,and the final yield was 916mL H2g−1,corresponding to a H2conversion of 94%.The hydrogen yield of the MgLi-EG composites would surpass 964mL H2g−1at an operating temperature of over 25 °C,nearly achieving a full H2conversion within only 3min.To evaluate the exact kinetics of the above composites,the hydrogen release rate was treated through the Arrhenius equation[15,43].The H2conversion rate vs time curves at different temperatures are shown in Fig.6b,where the correlation factor(R2)values are over 0.99,indicating good fitting accuracy.The relationship between temperature and reaction rate k could be described as the following equation:

where k is the reaction rate constant,A is the pre-exponential factor,Ea is the apparent reaction activation energy(J mol−1),R is the gas constant(8.314J mol−1K−1),and T is the absolute temperature(K).Fig.6c presents the Arrhenius plot of ln k vs the reciprocal absolute temperature 1000/T for the MgLi-EG composites in 0.5M MgCl2solution.The apparent reaction activation energy(Ea)was determined to be 52kJ mol−1,which is much lower than that of the Mg-EG composites(67.6kJ mol−1H2)[33].
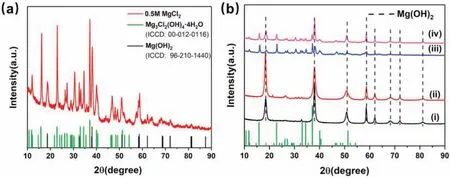
Fig.7.XRD patterns of a)the solid products from the MgLi-10 wt.% EG composites in 0.5M MgCl2 solution and b)the hydrolyzates of the MgLi-10 wt.%EG composites in 0,0.1,0.5 and 1M MgCl2 solutions.
3.6.Hydrolysis mechanism and discussion
To elucidate the hydrolysis process of the MgLi-C composites,the hydrolysis solid products were characterized by XRD,as presented in Fig.7.The XRD patterns of the solid products from the MgLi-10 wt.% EG composites in 0.5M MgCl2solution are shown in Fig.7a,wherein the major diffraction peaks are indexed to Mg3Cl2(OH)4·4H2O and Mg(OH)2.Fig.7b presents the comparison of XRD patterns for the hydrolyzates of the MgLi-10 wt.% EG composites in different concentration MgCl2solutions.As the concentration increases,so does the peak intensity of Mg3Cl2(OH)4·4H2O.The observation of Mg3Cl2(OH)4·4H2O could be ascribed to the corrosion of Cl−towards Mg(OH)2during the hydrolysis process [38,44].In this regard,the MgLi alloy milled with various amounts of EG exhibited rapid kinetics and desirable hydrogen yield in chloride solutions,whereas the hydrolysis for either pristine MgLi in MgCl2solutions or MgLi-EG composites in pure water showed sluggish kinetics though they obtain good hydrogen conversion(Fig.3).The enhanced hydrogen behaviors could be attributed to the synergistic effect of the formation of numerous galvanic cells by doping with EG after long-time ball milling and the Cl−corrosion towards the hydroxide layer.Overall,the hydrogen supply of MgLi can be described as the following pathway:
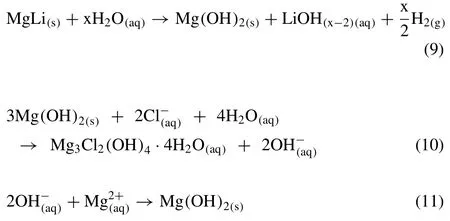
Interestingly,the light-weight EG was suspended on water and Li was dissolved into solution in the form of LiOH(As shown in Fig.S9),whereas Mg was converted to white solid precipitation.In this regard,the bottom-up self-separation of the hydrolysis products is favorable for recycling and re-utilization of spent fuels.
4.Conclusion
In this work,the expanded graphite was milled with MgLi to enhance the hydrogen behaviors.The hydrolysis performance of the MgLi-EG composites in MgCl2solutions was more superior to that for either pristine MgLi in MgCl2solutions or MgLi-EG composites in pure water.Among the various amounts of EG-doping systems,the 10 wt.%EG-doped MgLi held optimal hydrolysis performance in 0.5M MgCl2solution,delivering a hydrogen yield of 966mL H2g−1within 2min and a maximum hydrogen generation rate of 1147mL H2min−1g−1.Moreover,the composites exhibited similar superior hydrogen behaviors in 3.5 wt.%NaCl solution(simulate seawater),releasing 891mL H2g−1of hydrogen in 5min with a final H2conversion of 99%.The results indicated that the enhanced hydrogen properties of the EG-doped MgLi could be ascribed to the synergistic effect of the formation of numerous galvanic cells by doping with EG after long-time ball milling and the Cl−corrosion towards the hydroxide layer.The MgLi-EG composites exhibited excellent air-stability even exposed to the 75 RH%air for 72h,there was still 819mL H2g−1liberated within 15min with a fuel conversion of 89%.These findings are of importance for the development of practical Mg-based hydrogen supply system for mobile or portable applications.
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China Projects(Nos.51771075),the National Key R&D Program of China(No.2018YFB1502101),the Foundation for Innovative Research Groups of the National Natural Science Foundation of China(No.NSFC51621001)and by the Project Supported by Natural Science Foundation of Guangdong Province of China(2016A030312011).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.02.008.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Rapid-developing Mg-based biodegradable materials:The editorial of the special issue on Mg-based functional materials -biomaterials section
- Magnesium-Based Materials for Energy Conversion and Storage
- A special editor’s issue on Mg-based functional materials:Design and development
- Assessing the microstructure and in vitro degradation behavior of Mg-xGd screw implants using μCT
- A combination strategy of functionalized polymer coating with Ta ion implantation for multifunctional and biodegradable vascular stents
- In vitro corrosion-fatigue behavior of biodegradable Mg/HA composite in simulated body fluid
