Effects of oxytetracycline dihydrate and sulfamethoxazole on Microcystis aeruginosa and Chlamydomonas microsphaera*
2021-02-22XudongZHOUXinchengJIANGShanGAOZhenjiaWANPengchengGAO
Xudong ZHOU , Xincheng JIANG, Shan GAO, Zhenjia WAN, Pengcheng GAO ,
1 College of Natural Resources and Environment, Northwest A&F University, Yangling 712100, China
2 PowerChina Huadong Engineering Corporation Limited, Hangzhou 311122, China
Abstract The increasing use of pharmaceuticals has become a major environmental issue in China. The presence of antibiotics in water may have deleterious effects on non-target aquatic organisms such as microalgae. In this study, a cyanobacterium and an alga species in surface waters, Microcystis aeruginosa and Chlamydomonas microsphaera, were exposed to 0, 0.1, 0.5, 1.0, 2.0, 5.0, 10.0, and 20.0 mg/L of oxytetracycline dihydrate (OXY) and sulfamethoxazole (SMZ) for 96 h to determine the effects of these antibiotics on the growth and surface morphology. Moreover, the photosynthetic activity and the contents of superoxide dismutase (SOD), malondialdehyde (MDA), and protein were measured to examine the biochemical characteristics of M. aeruginosa and C. microsphaera under OXY and SMZ stress. The effects of both antibiotics on the growth of both species were concentration-dependent and characterized by low-dose stimulation and high-dose inhibition. C. microsphaera was more sensitive to both antibiotics than M. aeruginosa was. The algal cell membranes of both species disintegrated after exposure to a high concentration of OXY. All of the physiological parameters measured in this study were relatively stable at low concentrations of OXY and SMZ. After exposure to high concentrations of OXY and SMZ, photosynthetic activity decreased significantly, whereas lipid peroxidation and the abundance of SOD, MDA, and protein increased significantly. Thus, low-dose antibiotics may increase algal blooms in eutrophic waters.
Keyword: antibiotics; cyanobacterium; green algae; physiology; growth
1 INTRODUCTION
Antibiotics have been employed extensively in the areas of human healthcare, veterinary pharmaceuticals, and aquaculture for several decades (Kemper, 2008). The increasing use of pharmaceuticals has become a major environmental issue, especially in China, where they are severely overused (He et al., 2016). Chinese people readily turn to antibiotics when they become sick, and antibiotics are used widely in farms and in livestock to meet the growing demand for food. In 2013, human consumption of antibiotics in China reached 77 760 tons, and the consumption of veterinary antibiotics was 84 240 tons. In veterinary antibiotics, tetracyclines and sulfonamides accounted for 6 950 and 7 890 tons, respectively (Zhang et al., 2015). After metabolism by humans and animals, antibiotics that cannot be absorbed completely pass into sewage systems or are discharged directly into the environments (Xu et al., 2007a). Animals can contribute a significant amount of antibiotic waste; in 2015, it was estimated that 84% of the 54 000 tons of antibiotics discharged into the river basins in China was excreted from animals. (Zhang et al., 2015).
Various antibiotics have been detected in surface waters and groundwater in China in recent years, including tetracyclines, quinolones, chloramphenicol, macrolides, fluoroquinolones, sulfonamides, and trimethoprim (Jiang et al., 2011; Li et al., 2014). The recorded levels of antibiotics in water are usually low (from ng/L to μ g/L) (Xu et al., 2007b; Jiang et al., 2011; Li et al., 2014), but they are considered “pseudopersistent” contaminants due to their continued release and permanent presence in water (Hernando et al., 2006). The presence of antibiotics in water may have deleterious effects on non-target aquatic organisms such as microalgae.
Algae are primary producers in aquatic ecosystems that provide energy and oxygen for other aquatic organisms. Some green algae are more sensitive to contaminants in comparison with rotifers, crustaceans, and fish (Isidori et al., 2005; Ferreira et al., 2007). Therefore, various algal bioassays are used to estimate the potential toxicity of chemicals and to evaluate the water quality (Kobraei and White, 1996; Wen et al., 2010). In a previous study, T etraselmis suecica was selected as a non-target aquatic organism for experiments testing the toxicity of chloramphenicol, florphenicol, and oxytetracycline in 96-h bioassays that calculated the 50% inhibitory concentration (IC50) of these compounds and chlorophyll a content (Seoane et al., 2014). Tetracycline significantly inhibited the growth and metabolism of Microcystis
aeruginosa (Kützing) and Selenastrum capricornutum (Printz) according to assays of algal biomass, chlorophyll fluorescence index ( Fv/ Fm) and indicators of cell injury, including superoxide dismutase (SOD) and malondialdehyde (MDA) content (Yang et al., 2013). Plants produce antioxidative enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxide dismutase (POD), glutathione reductase (GR) and glutathione S-transferase (GST), to eliminate reactive oxygen species (ROS) and avoid oxidative damage (Wu et al., 2015). SOD is considered an essential component of the plant antioxidative defense system, because it has the ability to convert ·O2-into H2O2and O2and thus prevent cell damage (Shi et al., 2009; Wu et al., 2015). MDA is the final decomposition product of the lipid peroxidation of polyunsaturated fatty acids in biomembranes (Shalata et al., 2001; Qian et al., 2009), from which they are released to react with proteins and nucleic acids. Chlamydomonas microsphaera (Pascher & Jahoda) is a dominant species in surface waters in China (Zhang et al., 2013). M. aeruginosa is known for its dominant role in algal bloom outbreaks in freshwater worldwide, especially in eutrophic non-circulatory aquatic systems, and is a producer of hepatotoxic microcystins (Gumbo and Cloete, 2011; Han et al., 2015). M. aeruginosa is resistant to some pollutants, including cadmium and lead (Rzymski et al., 2014), and it is relatively adaptable to the presence of tetracyclines (Yang et al., 2013). Increasing pharmaceutical pollution creates an urgent need to test whether and how such pollution can affect algae, and it is important to understand the interactions between various pollutants.
In the present study, we tested the effects of antibiotics oxytetracycline dihydrate (OXY) and sulfamethoxazole (SMZ) on M. aeruginosa and C. microsphaera, because these types of antibiotics are among the most frequently used drugs by the Chinese population and are detected often in Chinese surface water (Jiang et al., 2011; Li et al., 2014). The effects and mechanisms of action of OXY and SMZ were investigated by assessing algal growth, chlorophyll fluorescence, SOD, lipid peroxidation, and protein content, as well as observation of cell surface characteristics. The results of this work can be used to evaluate whether there is a potential for increased blooms under the influence of different concentrations of antibiotics.
2 MATERIAL AND METHOD
2.1 Organisms and reagents
Microcystis aeruginosa FACHB-1343 (class Cyanophyceae) and C. microsphaera FACHB-52 (class Chlorophyceae) were supplied by the Freshwater Algae Culture Collection at the Institute of Hydrobiology of the Chinese Academy of Sciences (Wuhan, China). M. aeruginosa and C. microsphaera were maintained under axenic growth conditions in BG-11 medium and SE medium, respectively (Bold, 1949; Stanier et al., 1971). The culture medium and the tools used in the inoculation were all autoclaved. All inoculation procedures were operated in a laminar flow cabinet. The light incubator used for the culture was sterilized by ultraviolet light for 1 hour before use. Both species were cultured in 250-mL Erlenmeyer flasks containing 150 mL of medium at 25± 1 ° C under illumination by a cool-white fluorescent light (50 μmol photons/(m2·s)) in light:dark cycle of 12 h:12 h.
OXY and SMZ were purchased from Aladdin (Shanghai, China). The antibiotics were dissolved in sterile water at concentrations of 1 000 mg/L as stock solutions. The stock solution of each antibiotic was added to the organism suspensions to obtain the following final concentrations: 0, 0.1, 0.5, 1.0, 2.0, 5.0, 10.0, and 20.0 mg/L. The concentration range of the antibiotics was based primarily on the IC50of antibiotics for both species.
Before the test, both species were cultured in an antibiotic-free medium, after which the antibiotics were added to the medium during the logarithmic growth phase. The initial biomass of M. aeruginosa was 6.0×106cells/mL, while that of C. microsphaera was 4.9×104cells/mL. Both species were exposed to the antibiotics for 96 h. Three replicates were performed for each treatment and the flasks were shaken twice every day.
2.2 Analysis of biological indicators
2.2.1 Biomass and photosynthetic yield
The cell counts and optical density at 675 nm were highly correlated for M. aeruginosa and C. microsphaera ( R2>0.99), in accordance with a previous report (González-Pleiter et al., 2013; Pivokonsky et al., 2014). The optical density of M. aeruginosa and C. microsphaera in each flask was measured using a spectrophotometer (UV-1600PC, Mapala, China) at 675 nm every 24 h during the experimental period. Algal cell counts were calculated using the regression equations between cell concentration and optical density shown in the supplementary material.
The Fv/ Fmratio represents the maximum variable fluorescence relative to the total fluorescence, which is an important indicator of photosynthetic eき ciency (Krause, 1988). Thus, the Fv/ Fmratio is used as a stress indicator that describes the potential yield of the photochemical reaction (Björkman and Demmig, 1987). After the experiment, the photosynthetic yield parameters for both species were determined using a fluorometer (SN-AP-332, Photon Systems Instruments, Czech Republic). Samples were adapted to darkness for at least 15 min before fluorescence parameters ( Fv/ Fm) were measured (Roháček and Barták, 1999).
2.2.2 SOD, lipid peroxidation, and total soluble protein concentration
SOD, lipid peroxidation, and total soluble protein parameters were determined at the end of the culture period. First, 25 mL of the culture fluid was collected and centrifuged at 12 000× g for 10 min (4 ° C) to collect algal cells, which were then re-suspended in 0.05 mol/L phosphate buffer. The freeze-thaw process was repeated rapidly three times. Finally, the samples were centrifuged at 12 000× g for 8 min (4 ° C) and the supernatant was used to determine SOD, MDA and soluble protein content.
SOD activity was measured based on its capacity to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm (Beauchamp and Irwin, 1971). One unit of SOD activity was defined as the amount of enzyme required to inhibit NBT reduction by 50% in comparison with that of tubes lacking the algal extract. Lipid peroxidation was analyzed by monitoring MDA content, which was determined using the thiobarbituric acid method (Ohkawa et al., 1979). The total soluble protein concentration was determined using Coomassie Brilliant Blue G-250 according to the Bradford assay (Bradford, 1976). Bovine serum albumin was used to prepare the protein standard curve.
2.2.3 Morphological observations of organisms
For subsequent observation, 30 mL of each algal solution was concentrated to 2 mL by centrifugation at 1 935× g for 15 min (25 ° C). A glass slide was soaked in the concentrated solution and air-dried. After fixing in 2.5% glutaraldehyde and 0.1 mol/L phosphate buffer for 2 h (Millonig, 1961), the cellcoated glass slide was washed three times with 0.1 mol/L phosphate buffer for 10 min, dehydrated with a graded ethanol series (30% ethanol, 50% ethanol, 70% ethanol, 80% ethanol, 90% ethanol, and 100% ethanol), and soaked in isoamyl acetate (Villagrasa et al., 2019). Dehydration in each solution was performed for 10 min. The glass slide was dried in a critical point dryer (K850, Emitech, UK) and coated with gold (E-1045, Hitachi, Japan). Finally, the samples were examined by scanning electron microscopy (S-4800, Hitachi, Japan).
2.3 Statistical analysis
All of the results were expressed as the mean ± standard deviation based on triplicate analyses. The relationships between the antibiotic concentrations and the biological parameters determined for the organisms were analyzed by one-way ANOVA using SPSS 23.0 (IBM Inc., Armonk, NY, USA). IC50values were obtained by Probit analysis with SPSS 23.0. The figures were produced using Origin 9.0.
3 RESULT
3.1 Algal growth inhibition rates
The two antibiotics inhibited the growth of both species (Fig.1) in a concentration-dependent manner. However, lower antibiotic concentrations (0.1, 0.5, and 1.0 mg/L) inhibited the growth of both species by less than 10%. The growth of M. aeruginosa was slightly enhanced by both antibiotics at the lowest concentration (0.1 mg/L). After 96 h without any antibiotics, the density of the control group of M. aeruginosa increased by 3.0×107cells/mL, whereas that of the control group of C. microsphaera increased by 2.7×105cells/mL, as shown in Supplementary Fig.S1.

Fig.1 Mean and standard deviation of the percent growth inhibition of M . aeruginosa and C. microsphaera in different concentration of OXY and SMZ over 96 h
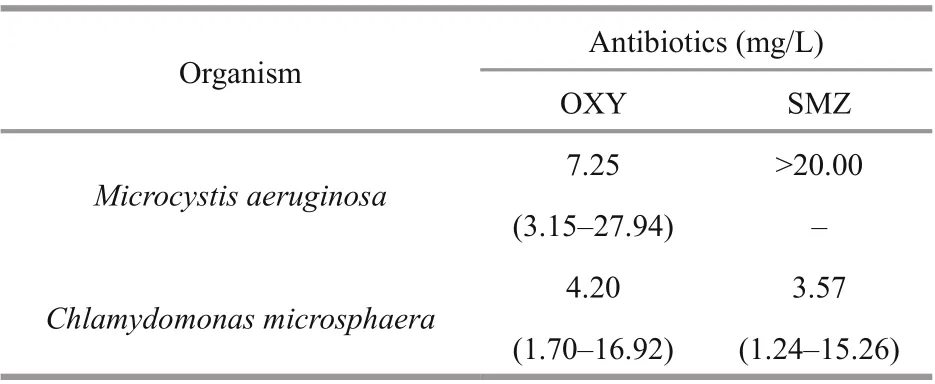
Table 1 The 96-h IC 50 values of OXY and SMZ for M. aeruginosa and C. microsphaera
The 96-h IC50can visually reflect the acute toxicological effects of harmful contaminants on organisms. As shown in Table 1, the toxicity endpoints for the antibiotics in C. microsphaera were less than 5.0 mg/L. The growth of M. aeruginosa was inhibited by less than 30% at the highest SMZ concentration (20.0 mg/L), so the IC50exceeded 20.0 mg/L. C. microsphaera was more sensitive to both antibiotics in comparison with M. aeruginosa according to IC50values. In comparison with SMZ, OXY was less toxic to C. microsphaera and much more toxic to M. aeruginosa.
3.2 Photosynthetic activity
Antibiotics can significantly affect the physiological and biochemical characteristics of both species. As shown in Fig.2, after exposure to low concentrations of OXY and SMZ (0.1 and 0.5 mg/L) for 96 h, the Fv/ Fmratio of both test species remained unchanged. However, in comparison with the control group, there was a significant difference ( P <0.01) in the photosynthetic activity of M. aeruginosa following exposure to high SMZ concentrations (2.0, 10.0, and 20.0 mg/L), and the Fv/ Fmratio of C. microsphaera responded in a similar manner. However, the effect of OXY on the photosynthetic activity of C. microsphaera was different from its effect on that of M. aeruginosa. The Fv/ Fmratio of C. microsphaera decreased significantly following exposure to OXY concentrations greater than 1.0 mg/L, whereas Fv/ Fmratio of M. aeruginosa decreased sharply only at the highest OXY concentration (20.0 mg/L). In general, these tests showed that both species were insensitive to low concentrations (0.1 and 0.5 mg/L) of OXY and SMZ in this experiment.
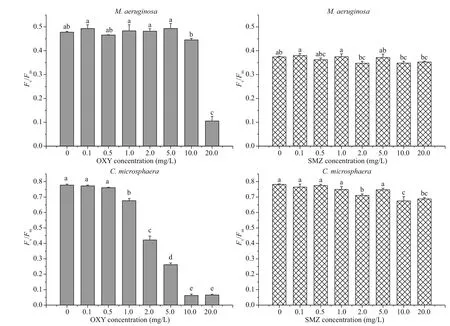
Fig.2 Mean and standard deviation of the F v/ F m ratio of M. aeruginosa and C. microsphaera in different concentration of OXY and SMZ after 96 h
3.3 SOD
In comparison with the appropriate control groups, both species generally showed relatively stable SOD activity at low antibiotic concentrations (0.1-5.0 mg/L), but SOD activity increased significantly ( P <0.01) following exposure to 10.0 or 20.0 mg/L of OXY or SMZ (Fig.3). After exposure to 10.0 mg/L of OXY or SMZ, the relative magnitude of the change in M. aeruginosa SOD was smaller than that of C. microsphaera.
Thus, exposure to OXY and SMZ affected the release of SOD by both species starting at the same concentration (i.e., 10.0 mg/L). However, the SOD activity of the groups treated with OXY and SMZ differed. The SOD activity of both species under the effect of 20.0 mg/L OXY was significantly greater than that observed under treatment with 20.0 mg/L SMZ. After exposure to OXY, SOD activity was 9.17±0.71 U/109cells in M. aeruginosa and 9.88±0.98 U/107cells in C. microsphaera. However, after exposure to SMZ, SOD activity was 6.53±0.38 U/109cells in M. aeruginosa and 4.84±0.87 U/107cells in C. microsphaera.
3.4 Lipid peroxidation
As shown in Fig.4, after 96 h of exposure to OXY, the MDA content of M. aeruginosa and C. microsphaera generally increased with the OXY concentration, but there were no significant differences between the control and low-concentration treatments (0.1 and 2.0 mg/L for M. aeruginosa, 0.1 and 0.5 mg/L for C. microsphaera). In contrast, the effects of SMZ on the MDA content of M. aeruginosa and C. microsphaera were very different; the MDA content remained almost constant at 2 μmol/108cellsin M. aeruginosa, whereas the MDA content of C. microsphaera increased sharply when the antibiotic concentration was increased from 1.0 mg/L to 2.0 mg/L. Treatments with 2.0-20.0 mg/L of SMZ significantly increased the MDA content of C. microsphaera to a level that was almost twice that of the control group.
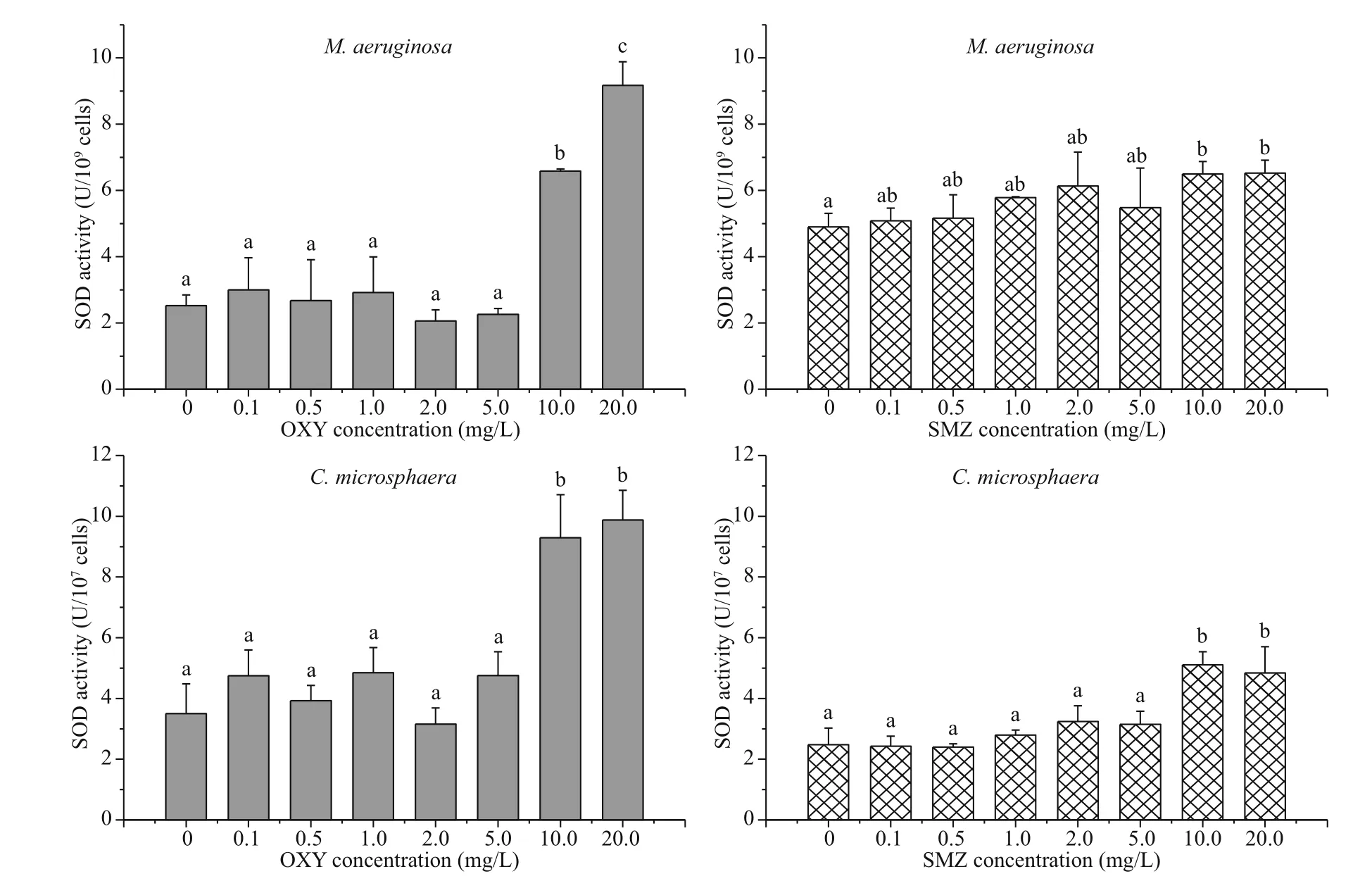
Fig.3 Mean and standard deviation of the SOD activity of M. aeruginosa and C. microsphaera in different concentration of OXY and SMZ after 96 h
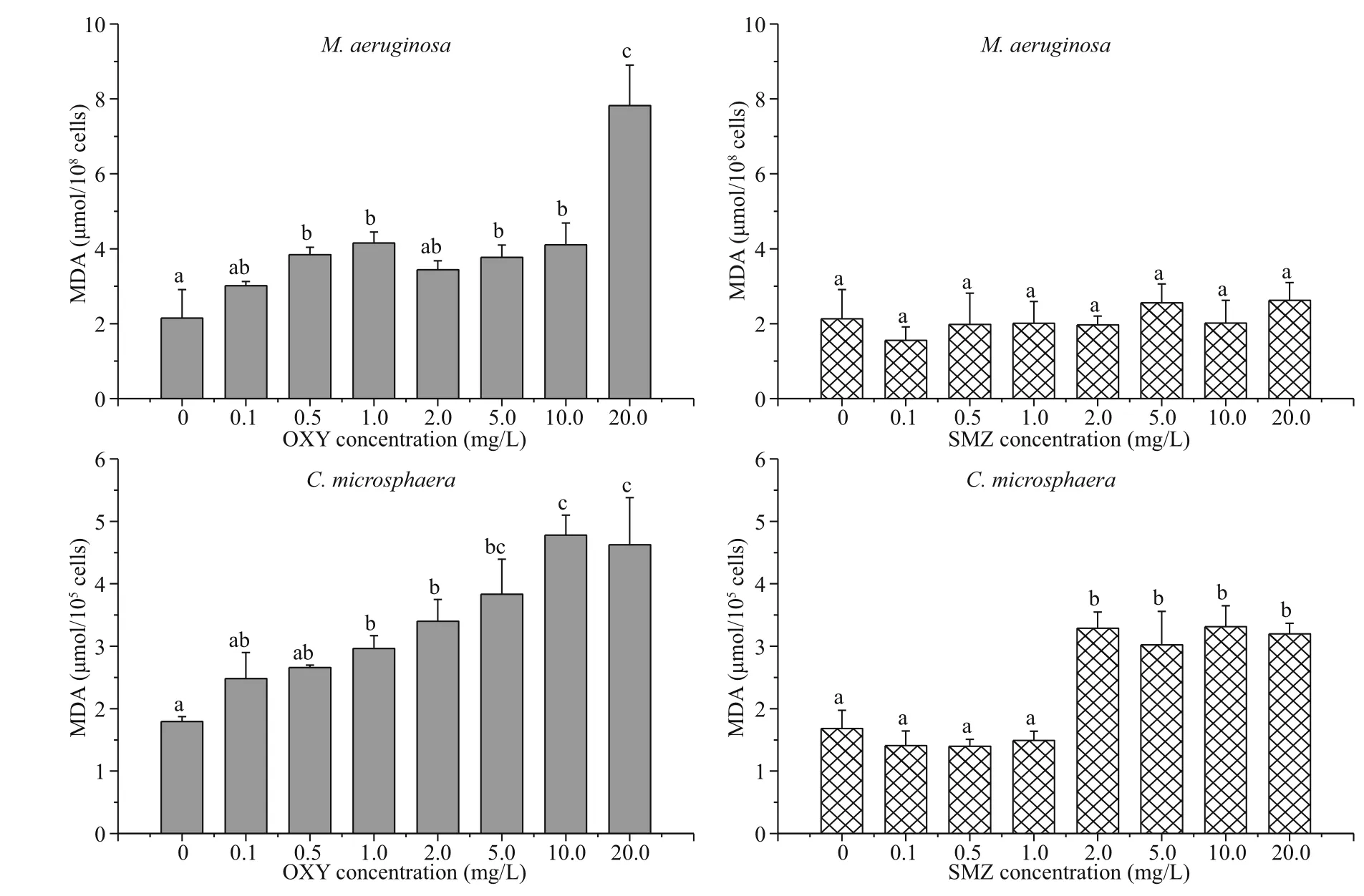
Fig.4 Mean and standard deviation of the MDA content of M. aeruginosa and C. microsphaera in different concentration of OXY and SMZ after 96 h
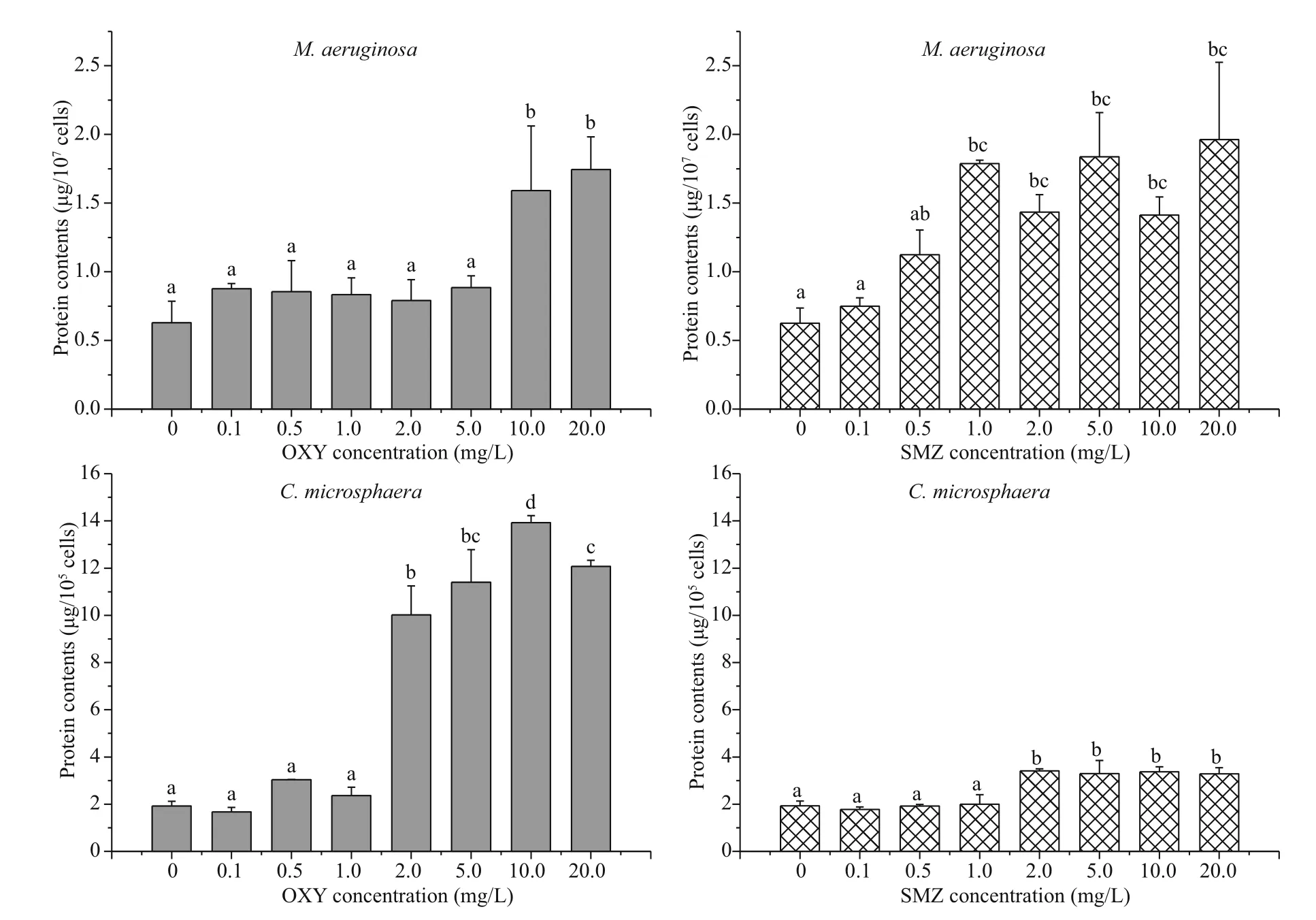
Fig.5 Mean and standard deviation of the total soluble protein content of M. aeruginosa and C. microsphaera in different concentration of OXY and SMZ after 96 h
3.5 Protein content
The total soluble protein content of each species did not change with exposure to antibiotic concentrations of 0.1 or 0.5 mg/L, but it increased when higher concentrations were utilized (Fig.5). In M. aeruginosa, the no observable adverse effect level (NOAEL) for OXY and SMZ were 5.0 mg/L and 0.5 mg/L, whereas the lowest observable adverse effect level (LOAEL) for OXY and SMZ were 10.0 mg/L and 1.0 mg/L, respectively, while both antibiotics had a LOAEL of 2.0 mg/L in C. microsphaera. Similar to the effects of the tested antibiotics on MDA content, the effect of OXY on the protein content of C. microsphaera was greater than the effect of SMZ, and there were significant differences in the protein content of the control group and those of the groups exposed to high OXY concentrations (2.0-20.0 mg/L).
3.6 Morphological observations
In the absence of antibiotics, M. aeruginosa (Fig.6a) and C. microsphaera (Fig.7a) maintained their integrity, while the cells of both species gradually disintegrated as the concentration of OXY increased (Figs.6 & 7). Some M. aeruginosa cells began to show cellular damage when they were exposed to 1.0 mg/L OXY (Fig.6b), while cells showed obvious damage under exposure to 10.0 mg/L (Fig.6c) and 20.0 mg/L OXY (Fig.6d). Some C. microsphaera cells were also damaged at high concentrations (5.0 mg/L and 20.0 mg/L) of OXY (Fig.7c & d).
4 DISCUSSION

Fig.6 Scanning electron microscopy images of M. aeruginosa cells exposed to OXY at a concentration of 0 (a), 1.0 (b), 10.0 (c), or 20.0 (d) mg/L
Photosynthetic activity is affected by contaminants, and species respond differently to different contaminants. When photosynthetic eき ciency was used as an acute endpoint after 24 h of antibiotic exposure ranging from 0.1 μg/L to 10.0 mg/L, OXY was more toxic than SMZ in tests using green algae ( Pseudokirchneriella subcapitata), in which the Fv/ Fmratio decreased following exposure to OXY at concentrations greater than 0.57 mg/L, but not following exposure to SMZ at any tested concentration. However, for cyanobacteria ( M. aeruginosa), OXY was less toxic than SMZ (van der Grinten et al., 2010); their Fv/ Fmratio decreased following exposure to OXY concentrations greater than 5.4 mg/L or SMZ concentrations greater than 0.57 mg/L. In comparison with SMZ, OXY had a greater effect on photosynthesis by M. aeruginosa following exposure to concentrations greater than 10.0 mg/L in our study. The difference in the results of our work and the previously mentioned study may be due to differences in the exposure times of the organisms to the tested pollutants or the difference in the inoculum cell density utilized in each study; the inoculum cell density in our study was three times that used in the Grinten study. In addition, photosynthetic activity can respond differently to the same contaminant in different organisms. For example, cyanobacteria were found to be less sensitive to OXY in comparison with green algae (van der Grinten et al., 2010). Similar to previous studies, measurement of the Fv/ Fmratio demonstrated that the sensitivity of C. microsphaera to antibiotics was greater than that of M. aeruginosa, and the IC50values showed a similar trend.
The activity levels of antioxidative enzymes and lipid peroxidation enzymes in plants generally increase as concentrations of pollutants increase. For example, in Scenedesmus vacuolatus (Shihira & Krauss), MDA and SOD content increased as the concentration of copper increased (Sabatini et al., 2009). A correlation between antioxidative enzyme activity and stress tolerance capacity in transgenic plants has also been demonstrated (Allen et al., 1997). Exposure to concentrations of tetracycline and anhydrotetracycline greater than 5.0 mg/L significantly increased the SOD activity (Xu et al., 2019). In the present study, SOD activity increased in a concentration-dependent manner in both species, especially at high concentrations (10.0 and 20.0 mg/L). The increase in SOD activity may be explained by increased production of reactive oxygen species, as well as by increased expression of multiple genes encoding SOD (Bowler et al., 1992; Mishra et al., 2006).
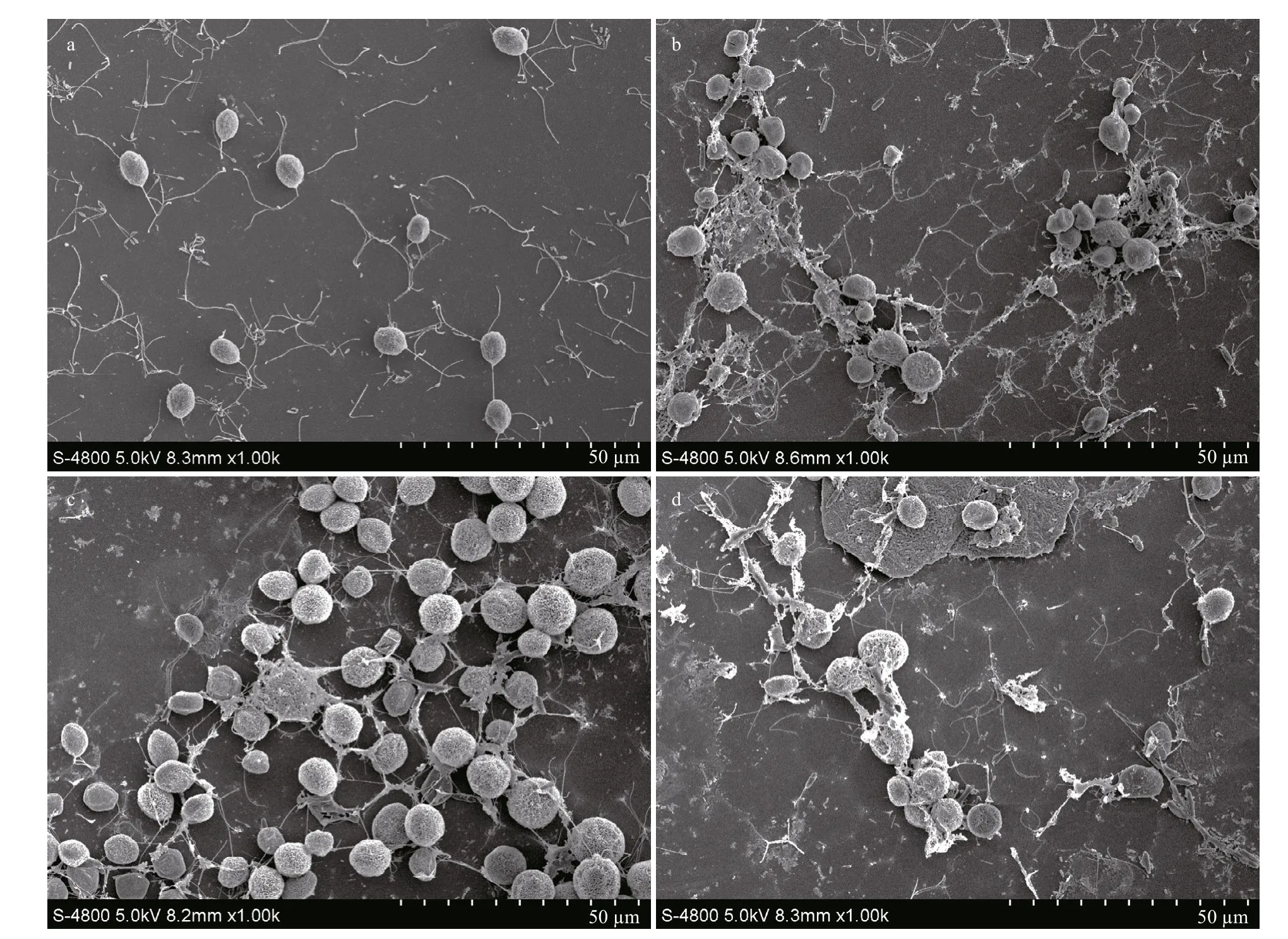
Fig.7 Scanning electron microscopy images of C. microsphaera cells exposed to OXY at a concentrations of 0 (a), 1.0 (b), 5.0 (c), and 20.0 (d) mg/L, respectively
Accumulation of MDA can indicate lipid membrane and cell damage (Dixit et al., 2002; Tang et al., 2007). In the present study, MDA content increased rapidly with the OXY concentration, thereby indicating that the integrity of the cell membranes was damaged by OXY, in agreement with the morphological observations. This effect suggests that cell membranes were damaged in this study as a result OXY inhibiting bacterial protein synthesis by combining with the 30S ribosomal subunit to prevent the association between aminoacyl-tRNA and the bacterial ribosome (van der Grinten et al., 2010; Hoerr et al., 2016).
Proteins are important components of biomolecules and catalysts in biochemical reactions, and they are used as indicators of changes in plant metabolism (Lu et al., 2015). The soluble protein content of algae generally increases after exposure to pollutants because algae can eliminate the toxic effects of pollutants by accumulation of proteins (Osman et al., 2004; Sabatini et al., 2009). The total soluble protein content of both tested species increased as the concentrations of OXY and SMZ were increased, thereby indicating that stress due to antibiotics stimulated protein production. Moreover, the release of soluble proteins may be caused by cell rupture under the effect of high concentrations of OXY. According to our observations of both species by scanning electron microscopy, there were no evident changes in the cell surface after exposure to different concentrations of SMZ, as shown in the Supplementary Figs.S2 & S3. Under exposure to SMZ, cell surface morphology was unchanged and the abundance of soluble proteins significantly increased, suggesting that SMZ may change the permeability of alga cell membranes, leading to the release of intracellular proteins.
According to our results, the growth of both species was inhibited at high concentrations of OXY, the inhibitory effect on M. aeruginosa was smaller than that on C. microsphaera, while the inhibitory effects of all concentrations of SMZ on the growth of C. microsphaera were markedly greater than their effects on M. aeruginosa. These findings indicate that M. aeruginosa is more adaptable under high concentrations of both tested antibiotics. It was reported that M. aeruginosa showed survivability greater than that of chlorophyceae Selenastrum capricornutum after twice exposure to tetracycline (Yang et al., 2013).
On the other hand, OXY and SMZ did not inhibit growth or physiological metabolism until the concentration of the antibiotic exceeded 0.5 mg/L. This finding appears to be positive, because the antibiotic concentrations found in actual water bodies are usually far less than 0.5 mg/L (Jiang et al., 2011; Li et al., 2014; Du et al., 2017; Fu et al., 2017; Liu et al., 2018), and the highest concentration of antibiotics reported in any surface water is less than 0.02 mg/L (Deng et al., 2016), which a lack of a toxicological threat to the growth of microalgae in natural water bodies by antibiotics. Song et al. (2016) confirmed that low concentrations of antibiotics did not influence the growth of algae in fishponds. However, the potential for hormesis effects on microalgae by lowdose antibiotics was ignored (Liu et al., 2012; Yang et al., 2013; Lu et al., 2015; Wan et al., 2015). In China, algal blooms in water bodies occur frequently and are a serious issue because they lead to diminished water quality because of excessive algal growth. Excessive nitrogen and phosphorus in water have been focused on as the most likely causes of algal blooms (Wang et al., 2016; Tang et al., 2018), but low-dose antibiotics are likely to be an unnoticed factor that aggravates algal blooms due to hormesis effects.
Moreover, because different algal species have different levels of sensitivity to antibiotics, a species with greater antibiotic tolerance could replace a less tolerant species as the dominant species in an aquatic ecosystem following antibiotic exposure (Lürling and Roessink, 2006). Both M. aeruginosa and C. microsphaera are common algae with different effects on surface water; the former produces toxins and is a primary species of bloom-forming cyanobacteria, which are a major threat to fresh waters worldwide because of their impact on drinking water supplies and human health, whereas the latter species is much less harmful (Tang et al., 2018). Unfortunately, we found that low-dose antibiotics stimulate the hazardous cyanobacteria M. aeruginosa, rather than the non-hazardous green algae C. microsphaera. Therefore, the presence of low-dose antibiotics in water could enhance the risk of algal blooms by changing the community structure and increasing the dominant position of M. aeruginosa in blooms, which could lead to the degradation of aquatic environments. Additional studies should aim to further illuminate the hormesis effects of low-dose antibiotics on microalgae and the risk of such effects on aquatic ecosystems.
5 CONCLUSION
It is shown in this study that OXY and SMZ affect the growth of M. aeruginosa and C. microsphaera in a dose-response relationship characterized by lowdose stimulation and high-dose inhibition. The 96-h IC50values of OXY and SMZ for M. aeruginosa were 7.25 mg/L and >20.0 mg/L, respectively, whereas they were 4.20 mg/L and 3.57 mg/L, respectively, for C. microsphaera. Thus, C. microsphaera was more sensitive than M. aeruginosa to both antibiotics. The concentrations of OXY and SMZ required to inhibit the growth of both species were greater than 0.5 mg/L. The physiological characteristics of both species were affected significantly by high concentrations of OXY and SMZ. With low concentrations of OXY and SMZ, all of the physiological indicators determined in this study, including photosynthetic activity, SOD activity, lipid peroxidation, and protein content, were relatively stable. However, exposure to high concentrations of OXY and SMZ significantly decreased photosynthetic activity and increased SOD activity, lipid peroxidation, and protein content. Scanning electron microscopy and measurement of MDA and protein content indicate that OXY can destroy the structure of cell membranes.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Influence of sequential tropical cyclones on phytoplankton blooms in the northwestern South China Sea*
- Simulated perturbation in the sea-to-air flux of dimethylsulfide and the impact on polar climate
- Performance of ecological restoration in an impaired coral reef in the Wuzhizhou Island, Sanya, China*
- Investigating factors driving phytoplankton growth and grazing loss rates in waters around Peninsular Malaysia
- Reproductive cycle of Ophiopholis mirabilis (Echinodermata: Ophiuroidea) in Zhangzi Island area, northern Yellow Sea*
- Partial function prediction of sulfate-reducing bacterial community from the rhizospheres of two typical coastal wetland plants in China*
