A new species of Micryletta (Amphibia: Anura:Microhylidae) from the Langbian Plateau in southern Vietnam
2021-02-10NikolayPoyarkovTanVanNguyenJianHuanYangVladislavGorin
Nikolay A.Poyarkov, Tan Van Nguyen,Jian-Huan Yang, Vladislav A.Gorin
1 Faculty of Biology, Department of Vertebrate Zoology, Moscow State University, Moscow, 119234, Russia
2 Laboratory of Tropical Ecology, Joint Russian-Vietnamese Tropical Research and Technological Center, Hanoi, Vietnam
3 Department of Species Conservation, Save Vietnam’s Wildlife,Ninh Binh, Vietnam
4 Kadoorie Conservation China, Kadoorie Farm and Botanic Garden, Tai Po, Hong Kong SAR, China
We report a new species of the genusMicrylettafrom the montane evergreen forest in the Bidoup-Nui Ba National Park,Lam Dong Province, Langbian Plateau, southern Vietnam,based on molecular and morphological evidence.The new species is diagnosed by a combination of the following morphological characters: body size small (snout-vent length(SVL) 22.4 mm, single female); iris uniform black; snout nearly truncate in dorsal view, slightly rounded in profile; tibiotarsal articulation of adpressed limb reaching level of eye; dorsal surface smooth; supratympanic fold present, prominent; outer metatarsal tubercle absent; tips of toes very weakly dilated into small discs; finger webbing absent, toe webbing rudimentary; dorsal surfaces of head and body orange-red with small irregularly shaped dark-brown patches; dorsal surface of limbs pale dark brown with small reddish speckles;body flanks dark brown anteriorly fading to grayish brown posteriorly with brown spots in groin area; lateral sides of head immaculate blackish brown without white patches; coloration of ventral surfaces immaculate dark gray.The new species is divergent from all other congeners in 16S rRNA gene sequences (2.6%–5.8%).Following the IUCN Red List Categories and Criteria, we propose the new species be listed as Data Deficient (DD).
Paddy frogs of the genusMicrylettaDubois, 1987 are a little-known group of microhylids, with 10 nominal species currently recognized: i.e.,M.aishaniDas, Garg, Hamidy,Smith & Biju, 2019;M.dissimulansSuwannapoom, Nguyen,Pawangkhanant, Gorin, Chomdej, Che & Poyarkov, 2020;M.erythropoda(Tarkhnishvili, 1994);M.hekouensisLiu, Hou, Mo& Rao, 2021;M.lineata(Taylor, 1962);M.nigromaculataPoyarkov, Nguyen, Duong, Gorin & Yang, 2018;M.immaculataYang & Poyarkov, 2021;M.inornata(Boulenger,1890);M.steinegeri(Boulenger, 1909); andM.sumatranaMunir, Hamidy, Matsui, Kusrini & Nishikawa, 2020 (Liu et al.,2021; Miller et al., 2021; Munir et al., 2020; Suwannapoom et al., 2020; Yang & Poyarkov, 2021).Members ofMicrylettahave a wide distribution from southern China including Taiwan and Hainan islands, the northeast portion of India and Myanmar, southwards through Indochina and the Malayan Peninsula to Sumatra, Nicobar, and the Andaman Islands(Frost, 2021) (Figure 1A).Several recent phylogenetic studies have revealed that this genus contains several species groups, including theM.steinegeriandM.inornatacomplexes, as well as several undescribed lineages, hence the taxonomy ofMicrylettais still far from complete (Alhadi et al., 2019; Das et al., 2019; Miller et al., 2021; Poyarkov et al.,2018; Suwannapoom et al., 2020; Yang & Poyarkov, 2021).For example, the status ofM.inornatalineata(Taylor, 1962)remains controversial.Several recent studies have suggested that the striped specimens ofMicrylettafrom the Tanintharyi Region of Myanmar areM.lineata, that the taxon is a full species, or that it may represent a senior synonym ofM.erythropodaoriginally described from southern Vietnam (Miller et al., 2021; Zug & Mulcahy, 2020).Miller et al.(2021)reported the occurrence ofM.inornatain southern Myanmar,which also has a striped dorsal pattern resembling that ofM.lineata.However, Miller et al.(2021) did not examine the type specimens ofM.lineata, nor did they include in their analysis materials from the type locality of this taxon in the Nakhon Si Thammarat Province of Thailand.Thus, their identification of the Tanintharyi population asM.lineatashould be considered with caution pending further research and sampling effort(Poyarkov et al., 2021); we tentatively indicate this lineage asM.cf.lineatabelow.
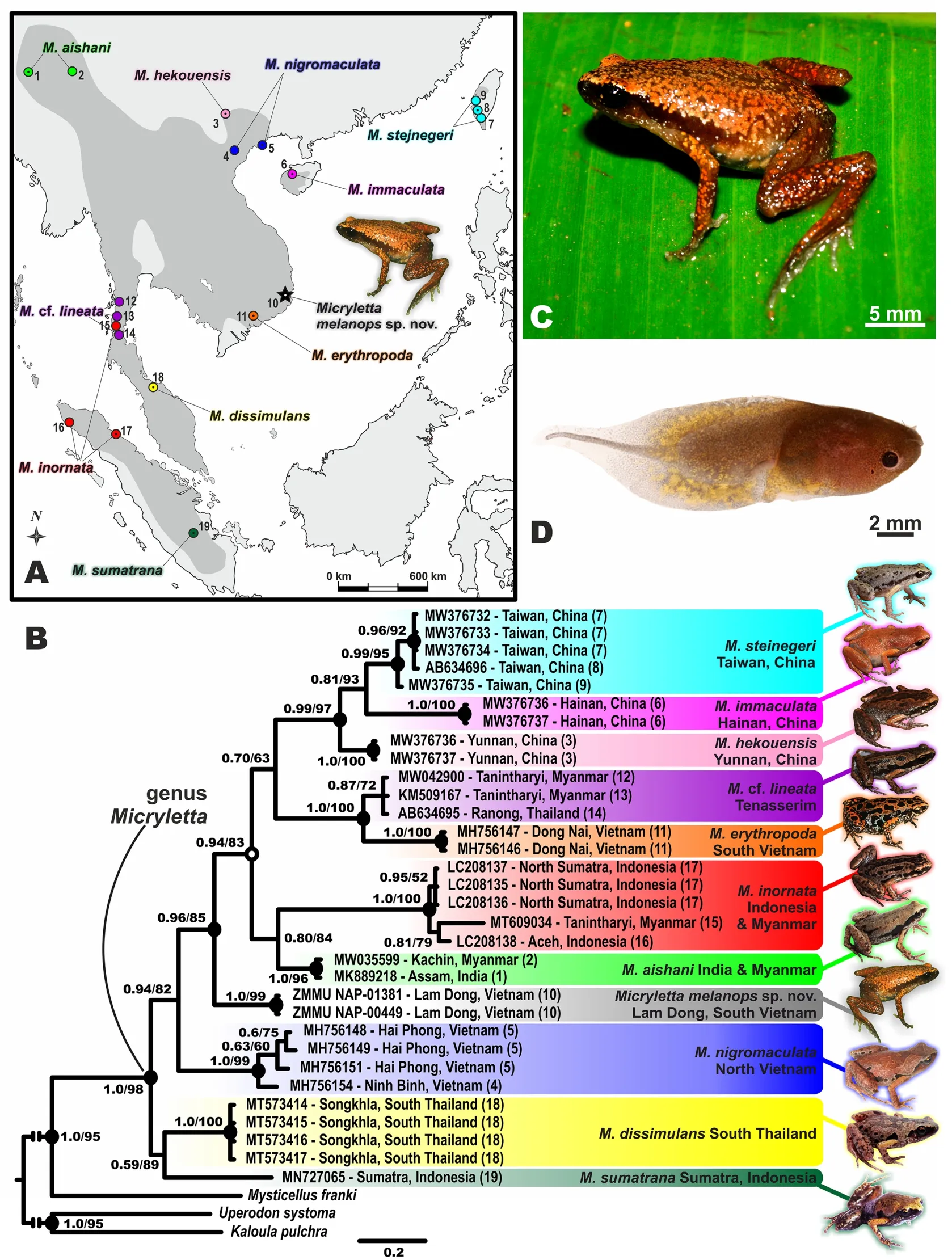
Figure 1 Phylogenetic relationships and distribution of Micryletta genus, holotype of Micryletta melanops sp.nov.(ZMMU A7583) in life,and tadpole of Micryletta melanops sp.nov.in life (ZMMU NAP-01381-5)
The Bidoup-Nui Ba National Park (hereinafter NP), located in Lam Dong Province on the eastern edge of the Langbian Plateau in southern Vietnam (Figure 1A), covers an area of 69 663 ha and includes the two highest peaks of the plateau:i.e., Mt.Bidoup 2 287 m above sea leave (a.s.l.) and Mt.Nui Ba 2 167 m a.s.l.(Nguyen & Kuznetsov, 2011).Although this protected area was established in 2004, the herpetofauna of the Bidoup-Nui Ba NP is still insufficiently studied, with 10 new amphibian species described from the NP within the last decade alone (Hoang et al., 2020; Poyarkov et al., 2014,2017, 2020; Rowley et al., 2010, 2011a, 2011b, 2016; Stuart et al., 2011).Overall, the Langbian Plateau is recognized as a local amphibian hotspot with high endemism in Indochina(Poyarkov et al., 2021).
In 2009, during fieldwork in the montane evergreen forests of Lam Dong Province in southern Vietnam, Nikolay A.Poyarkov encountered one unusual adult microhylid specimen and a series of tadpoles, which were tentatively identified asM.cf.inornata(Poyarkov & Vassilieva, 2011).Subsequent phylogenetic analysis of the 16S rRNA mtDNA gene confirmed the placement of this population withinMicrylettaand its formation of a lineage deeply divergent from all other recognized species of the genus.Morphological analysis of the adult specimen further revealed that this individual is distinct from all other congeners by a unique combination of morphological and chromatic characters and represents an undescribed species.Geographic coordinates and elevation were obtained using a Garmin GPSMAP 60CSx (USA) and recorded in WGS84 datum.Specimens were preserved in 75% ethanol and muscle tissues were preserved in 95% ethanol for genetic analysis.Specimens and tissues were subsequently deposited in the zoological collection of the Zoological Museum of Lomonosov Moscow State University(ZMMU), Moscow, Russia.Measurements were taken to the nearest 0.1 mm with a Mitutoyo digital caliper.The descriptions of the morphological characteristics followed Poyarkov et al.(2018) (see Supplementary Methods for details).Staging of larvae followed Gosner (1960) and larval morphometrics followed Altig (2007), with details in the Supplementary Methods.
To reconstruct the matrilineal genealogy, we used 16S rRNA sequences ofMicrylettasp.from Lam Dong Province,as well as 16S rRNA sequences of all currently recognizedMicrylettaspecies reported in earlier phylogenetic studies of the genus (Alhadi et al., 2019; Das et al., 2019; Liu et al.,2021; Miller et al., 2021; Munir et al., 2020; Poyarkov et al.,2018; Suwannapoom et al., 2020; Yang & Poyarkov, 2021).Information on GenBank accession Nos., museum vouchers,and origin locality of sequences used in this study is summarized in Supplementary Table S1.DNA extraction and amplification protocols followed Poyarkov et al.(2018) and Suwannapoom et al.(2020) and are detailed in the Supplementary Methods.
We inferred matrilineal genealogy using Bayesian inference(BI) and maximum-likelihood (ML) approaches (see Supplementary Methods).In total, 16S rRNA gene sequences from 35 Microhylinae specimens were included in the final analysis, representing all 10 currently recognized nominal species ofMicrylettaand three outgroup taxa (Mysticellus franki,Uperodonsystoma, andKaloulapulchra) (see Supplementary Table S1).Pairwise sequence divergences(uncorrectedP-distances) were calculated using MEGA v6.0.6(Tamura et al., 2013) (see Supplementary Table S2).
The ML and BI analyses resulted in essentially identical topologies (Figure 1B).Reconstructed topologies were also congruent with earlier phylogenies of the genusMicryletta(Das et al., 2019; Miller et al., 2021; Poyarkov et al., 2018;Suwannapoom et al., 2020; Yang & Poyarkov, 2021).All species ofMicrylettaformed a well-supported clade (1.0/98;hereafter nodal support values are given for BI posterior probability (BPP)/ML ultra-fast bootstrap values (UFB),respectively).The basal clade, comprised ofM.sumatranafrom Sumatra andM.dissimulansfrom Southern Thailand,was poorly supported (0.59/89).Micrylettanigromaculatafrom northern Vietnam was reconstructed as a sister clade to all other remainingMicrylettaspecies (0.94/82).Among the remaining species,Micrylettasp.from Lam Dong Province formed a distinct clade (1.0/99), while other species were nested within a clade, though with moderate nodal support(0.94/83).Precise genealogical relationships among them were poorly resolved, thoughM.inornatafrom Sumatra and southern Myanmar was grouped withM.aishanifrom India and northern Myanmar with low nodal support (0.80/84),M.erythropodafrom southern Vietnam was grouped withM.cf.lineatafrom Tenasserim (1.0/99), andM.hekouensisfrom Yunnan Province in China was grouped withM.immaculatafrom Hainan Island and withM.steinegerifrom Taiwan Island(0.99/97).
Genetic divergence betweenMicrylettasp.from Lam Dong Province, Southern Vietnam, and all other congeners ranged fromP=2.6% (withM.aishanifrom India) toP=5.8% (withM.sumatranafrom Sumatra) (Supplementary Table S2).These values are generally higher than the formalP=3.0% threshold for the 16S rRNA gene, which is widely considered as an indicator of anuran species-level divergence (Vieites et al.,2009).At the same time, genetic distances between some recognized species of the genusMicrylettawere much lower,e.g., the divergence in 16S rRNA gene betweenM.hekouensisandM.steinegeriwas estimated as 1.2% of substitutions (which differs from the 3.2% value originally reported by Liu et al., 2021).In the case ofMicrylettasp.from Lam Dong andM.aishani(P=2.6%), the value was slightly lower than the formal threshold; however, these species are not sister lineages and differ significantly in external morphology, coloration, ecological preferences, and distribution areas (see Comparisons).The congruent morphological and molecular differences ofMicrylettasp.from other congeners are indicative of a species status, and as such we consider the Lam Dong population to be recognized as a distinct species, which we formally describe below.
Taxonomic account
Micryletta melanops sp.nov.(Figure 1; Supplementary Figures S1–S3)
Chresonymy:Micrylettacf.inornata— Poyarkov [Paiarkov] &Vassilieva (2011): 186–187, 211, Fig.5.26.
Holotype:ZMMU A7583, adult female (field No.NAP-00449),collected from a pit-fall trap within a montane evergreen pine forest in the environs of Giang Ly Ranger Station in Bidoup-Nui Ba NP, Da Nhim River Valley, Da Chais Commune, Lac Duong District, Lam Dong Province, Langbian Plateau,southern Vietnam (Figure 1A), collected by Nikolay A.Poyarkov on 9 May 2009 (N12.1843°, E108.6789°; at an elevation of 1 460 m a.s.l.).
Referred specimens:ZMMU NAP-01381-1–5, series of five tadpoles (Gosner stages 34–37, including three specimens at stage 34 (ZMMU NAP-01381-1-3, respectively); one specimen at stage 36 (ZMMU NAP-01381-4); and one specimen at stage 37 (ZMMU NAP-01381-5)) with the same collection information as the holotype.
Etymology:Specific epithet “melanops” is an adjective in the nominative case derived from the Ancient Greek “melanos” for“black” and “ops” for “eye” and is given in reference to the characteristic uniform black iris coloration found in the new species.We recommend “Black-eyed paddy frog” for the common English name and “Nhái bu mten” for the common Vietnamese name of the new species.
Diagnosis:The new species is assigned to the genusMicrylettaDubois, 1987 based on the following morphological attributes: body size small; vomerine teeth absent; tympanum small, rounded, externally visible; subarticular tubercles on fingers and toes very prominent; three well-developed metacarpal tubercles; distinct supernumerary palmar and metatarsal tubercles posterior to base of digits; first finger not reduced; webbing on fingers absent and on toes rudimentary(Alhadi et al., 2019; Das et al., 2019; Dubois, 1987; Munir et al., 2020; Poyarkov et al., 2018; Yang & Poyarkov, 2021).
Micrylettamelanopssp.nov.is distinguished from all congeners based on a combination of the following morphological characters: body size small (SVL 22.4 mm,single female); iris uniform black; snout truncate in dorsal view, nearly truncate in profile; tibiotarsal articulation of adpressed limb reaching eye level; dorsal surface smooth;supratympanic fold present, prominent; outer metatarsal tubercle absent; tips of toes weakly dilated into small discs;finger webbing absent, toe webbing rudimentary between toes II–III and III–IV; coloration of dorsal surfaces on head and body orange-red with small irregularly shaped brown patches;dorsal surface of limbs pale dark brown with small reddish speckles; flanks dark brown anteriorly, fading to grayish brown posteriorly with brown spots in groin area; lateral sides of head blackish brown, lacking white patches; coloration of ventral surfaces immaculate dark gray; tadpoles with distinct yellow blotches across tail and fin.
Description of holotype:Adult gravid female, small-sized,slightly dehydrated due to preservation in ethanol; abdomen dissected on ventral surface from pectoral girdle to middle part of belly (Supplementary Figure S1B).Body habitus moderately slender, body elongated and oval shaped (Supplementary Figure S1A,B); head wider than long (HL/HW 0.94); snout short (SL/SVL 0.11), truncate in dorsal view (Supplementary Figure S1A), nearly truncate in profile, not projecting beyond lower jaw (Supplementary Figure S1C); eyes comparatively large (EL/SVL 0.11), slightly protuberant in dorsal and lateral views, slightly shorter than snout (EL/SL 0.97), shorter than interorbital distance (EL/IOD 0.69).Top of head flat; canthus rostralis distinct, rounded; loreal region almost vertical,noticeably concave; nostril oval shaped, horizontal, lateral,located closer to tip of snout than to eye (N-EL/SVL 0.05)(Supplementary Figure S1C); interorbital distance about two times wider than internarial distance (IND/IOD 0.53), about three times wider than upper eyelid (UEW/IOD 0.33);tympanum small (TYD/SVL 0.05), round, relatively indistinct with tympanic rim not elevated above tympanal area;supratympanic fold thick, glandular, rounded, gently curving from posterior corner of eye towards axillary area(Supplementary Figure S1C).Choanae elongated and oval shaped, widely spaced; upper jaw edentate; vomerine teeth absent; tongue without papillae, spatulate, lacking posterior notch and free behind for three-quarters of its length.
Forelimbs short and slender (FLL/SVL 0.71); lower arm comparatively long and slender (LAL/SVL 0.51), hand less than half length of forelimb (HAL/FLL 0.39).Fingers slender(Supplementary Figure S2A), completely free of webbing,slightly flattened dorsoventrally, and lacking lateral skin fringes; first finger well developed, slightly shorter than second finger (1FL/2FL 0.76); relative finger lengths: I<II<VI<III; tips of all fingers rounded, not expanded to disks; subarticular tubercles on fingers rounded and very prominent, subarticular tubercle formula: 1, 1, 2, 2; nuptial pad absent; three palmar(or metacarpal, MCT) tubercles: inner MCT distinct, oval shaped and flat (IPTL/SVL 0.02); outer MCT large, rounded(OPTL/SVL 0.04); medial MCT large, prominent, drop shaped,ca.same diameter as inner MCT, located closer to outer MCT than to inner MCT; five rounded and one elongated prominent supernumerary palmar tubercle: four at base of fingers I–IV about same size as proximal subarticular tubercle, small rounded supernumerary palmar tubercle between medial MCT and tubercle at base of finger III, much smaller than MCTs;and elongated tubercle on outer proximal edge of palm, as long as outer MCT (Supplementary Figure S2A).
Hindlimbs slender and comparatively long (HLL/SVL 1.81),more than two times length of forelimb (FLL/HLL 0.39); tibia long and slender (TL/SVL 0.51), one-third of hindlimb length(TL/HLL 0.28); heels overlap when hindlimbs located at right angles to body, tibiotarsal articulation of adpressed limb reaching level of eye; foot slightly longer than tibia length(FL/TL 1.73).Relative toe lengths: I<V<II<III<IV; tarsus smooth, inner tarsal fold absent; tips of all toes rounded,weakly dilated into small disks, slightly wider than those of fingers (3FDD/4TDD 0.73); rudimentary web between toes II–III and III–IV (Supplementary Figure S2B); subarticular tubercles on toes round and prominent, subarticular tubercle formula: 1, 1, 2, 3, 2; metatarsal tubercle single: inner metatarsal tubercle oval shaped, prominent, much shorter than half of first toe (IMTL/1TOEL 0.20); outer metatarsal tubercle absent; two small supernumerary tubercles at base of toes II and III, smaller than proximal subarticular tubercles.
Skin texture and skin glands: Dorsal surface of head and body smooth, laterally with few small flat glandular tubercles,dorsal surfaces of forelimbs smooth, hindlimbs dorsally smooth with small tubercules; flanks of body with rare small tubercules; lateral surfaces of head smooth; upper eyelid lacking tubercles; supratympanic fold thick, glandular; ventral surfaces smooth.Cloacal opening unmodified, directed posteriorly.
Measurements of holotype (in mm): SVL 22.4; HL 6.5; HW 6.9; SL 2.6; EL 2.5; N-EL 1.2; TD 1.2; IND 1.9; IOD 3.6; UEW 1.2; FLL 5.9; LAL 11.5; HAL 6.2; HLL 40.6; TL 11.5; FL 19.8;IPTL 0.3; OPTL 0.9; IMTL 0.5; 1FL 2.2; 2FL 2.9; 3FL 4.1; 4FL 2.5; 1TOEL 2.4; 2TOEL 3.7; 3TOEL 5.6; 4TOEL 7.2; 5TOEL 3.2; 3FDD 0.5; 4TDD 0.7.
Holotype coloration:In life, dorsum orange-red with small irregularly shaped pale brown patches forming reticulated pattern (Figure 1C); no large black spots on dorsum; body flanks brown and lacking large dark spots or whitish mottling,dark brown anteriorly, fading to gray brown and becoming translucent towards posterior; lateral sides of head dark brown with few reddish speckles, lacking white patches on upper lips,tympanal and axillary regions immaculate dark brown;supratympanic fold ventrally edged in dark brown; dorsal surfaces of forelimbs, thighs, shanks, fingers, and toes dark brown with orange-red speckles; ventral surfaces immaculate dark gray.After 11 years in preservative, the pattern generally remained unchanged, though dark brown colors faded to gray brown, and bright reddish and orange tints faded to light gray(Supplementary Figure S1).Holotype body swollen with bicolored (dark brown and beige) eggs (Supplementary Figure S1B).
Tadpole description:Description of larval morphology is based on five tadpoles (Gosner stages 34–37) (ZMMU NAP-01381-1–5).Identification of tadpoles was confirmed by 16S rRNA partial sequencing (see Supplementary Table S1).Details of tadpole morphology are presented in Figure 1D and Supplementary Figure S3.Mean values (in mm) and standard deviations of measurements of tadpoles (n=5) at stages 34–37 are as follows: TL 13.49±1.20; BL 5.04±0.54; TaL 8.45±1.25; TB 1.52±0.12; BW 3.87±0.27; BH 3.00±0.36; MTH 3.71±0.30; SVL 5.72±0.45; SS 4.20±0.49; DF 1.25±0.10; VF 1.36±0.20; IP 3.68±0.31; SP 1.25±0.12; ED 0.98±0.07; MW 1.7±0.17.
External morphology:Body oval shaped in dorsal view,slightly elongated (BW/BL 0.77±0.04), maximal width at midbody; snout smoothly rounded.In lateral view (Figure 1D),body ovoid, with flattened and somewhat raised snout.Tail rather short, about one and a half times longer than body(TaL/BL 1.68±0.10), with narrow terminal filament on tip; ca.11 myotomes discernable in lateral view.Tail musculature well developed (TB/BW 0.39±0.01).Dorsal fin high, extending onto trunk, starting point at posterior two-thirds of body length;ventral tail fin almost same height as dorsal fin (VF/DF 1.09±0.19), both fins reach maximal height at mid-tail level,sharply tapered posteriorly into tail filament (Figure 1D).Spiracle medial with smooth free margin.Vent tube medial,vertical, slightly slanting; vent opening located at base of ventral fin.Eyes lateral, moderate (ED/BL 0.12±0.02); pupils with lateral orientation, not visible from below.Nostrils closed at stages 34–37, nostril location marked with small protuberances on snout.Mouth wide (MW/BW 0.44±0.03) with anterior orientation.From dorsal view, upper labium short,semicircular, and smooth; lower labium small, narrow, U shaped with smooth fringe (Supplementary Figure S3C).Keratinized mouthparts and lateral lobes absent.
Tadpole coloration:In life, depending on light conditions,dorsal coloration varies from dark gray to brownish gray(Figure 1D; Supplementary Figure S3A); body ventral surfaces weakly pigmented, semitranslucent, spiracle unpigmented.Tail fins grayish or brownish, well pigmented on anterior twothirds of its length, except for transparent terminal filament;edges of both dorsal and ventral tail fins more densely pigmented than proximal parts.Both tail fins proximally marked with distinct golden yellow blotches, tail with small yellow patches across; posterior portion of belly with yellow spots (Figure 1D).Iris dark brown to black.In preservative,yellowish tints fade to dark gray.
Comparisons:The new speciesMicrylettamelanopssp.nov.is unique among all currently recognizedMicrylettaspecies by having uniform black coloration of the iris in life(Supplementary Figure S4K), with all other congeners having bicolored irises with black or dark-brown background color and tiny bronze or golden speckles on upper part, and in lower part in some species (Supplementary Figure S4A–J; see Supplementary Table S3 for details).Though iris coloration has occasionally been reported as variable in some Anura groups (see Glaw & Vences, 1997), this character inMicrylettaappears to be quite stable, with no previous studies ever reporting a uniform black iris for this genus (Supplementary Figure S4).The new species can also be distinguished from its congeners by the following morphological features.Micrylettamelanopssp.nov.differs fromM.aishaniby smaller size in females (22.4 mm vs.25.6–27.3 mm);comparatively larger tympanum (TYD/EL ratio 0.47 vs.0.14–0.29); thick and distinct supratympanic fold (vs.weakly developed); smooth dorsal skin texture (vs.shagreened with minute spinules); orange-red dorsal coloration with small brown patches forming reticulate pattern (vs.brown to reddish brown with larger dark-brown or black spots and blotches);dark-brown coloration of upper jaw lacking white patches (vs.whitish patches or spots on upper jaw); tibiotarsal articulation of adpressed limb reaching level of eye (vs.level of armpit);and uniform coloration of ventral surface (vs.mottled pattern on belly).Furthermore, the ranges of the new species(Langbian Plateau in southern Vietnam) andM.aishani(northeastern India, Bangladesh, and northern Myanmar) are separated by at least 2 000 km, and their ecological preferences also differ (Das et al., 2019).The new species further differs fromM.dissimulansby orange-red dorsal coloration with small brown patches forming reticulate pattern(vs.reddish brown with large brownish spots forming camouflage pattern); smooth dorsal skin texture (vs.slightly granulated to shagreened); tibiotarsal articulation of adpressed limb reaching level of eye (vs.level of tympanum);and uniformly colored ventral surface (vs.mottled pattern on belly).Micrylettamelanopssp.nov.further differs fromM.erythropodaby outer metatarsal tubercle absent (vs.present);orange-red dorsal coloration with brown reticulate pattern (vs.variable, usually gray or beige to saturated ochre or brick red with large black spots and blotches); white patches and spots on flanks and upper lip absent (vs.present); tibiotarsal articulation of adpressed limb reaching level of eye (vs.level of tympanum); and uniform coloration of ventral surface (vs.mottled pattern on belly).The new species can be diagnosed fromM.hekouensisby slightly larger body size in females(22.4 mm vs.20.8 mm), snout truncate in dorsal view (vs.abruptly rounded), rudimentary foot webbing (vs.absent),tibiotarsal articulation of adpressed limb reaching level of eye(vs.level in front of eye), supratympanic fold distinct (vs.indistinct), orange-red dorsal coloration with small brown patches forming reticulate pattern (vs.golden-brown dorsum with brownish-black stripes and blotches), uniform dark-brown upper lip lacking white patches (vs.with white spots), absence of longitudinal dark-gray stripe on body flanks (vs.present),and uniform dark-gray belly (vs.pinkish brown with white marbling).The new species further differs fromM.lineataby orange-red dorsum with brown reticulated pattern (vs.grayish brown with three straight continuous or broken lines); white patches on lateral sides of head absent (vs.present); and dark stripe on flanks absent (vs.present, edged with weak light stripe ventrally).Compared withM.inornatasensustrictofrom Sumatra Island, Indonesia,Micrylettamelanopssp.nov.can be distinguished by generally larger body size in females (22.4 mm vs.19.5 mm); brown reticulate dorsal pattern (vs.large and distinct dark-brown spots or stripes); orange-red ground color of dorsum (vs.dark brown or brownish gray with silver tinge); and dark-brown sides of head lacking white spots or patches (vs.black with white spots along upper lip).Micryletta melanopssp.nov.differs fromM.nigromaculataby smaller size in females (22.4 mm vs.24.2–25.9 mm); smooth dorsal skin texture (vs.slightly granular with small round flattened tubercles); brown reticulate dorsal pattern (vs.generally prominent dark hourglass-shaped markings); and flanks brown, lacking black spots (vs.blackish patches edged in white on flanks).The new species further differs fromM.steinegeriby smaller size in females (22.4 mm vs.27.0–30.1 mm); tibiotarsal articulation of adpressed limb reaching eye level (vs.level of tympanum); uniform coloration of ventral surface (vs.mottled pattern on belly); orange-red ground color of dorsum (vs.dark gray to purplish brown); lateral sides of head dark brown lacking white patches or spots (vs.gray brown with series of white spots); and flanks dark brown anteriorly with brown spots in groin area (vs.gray brown with dark marbling).Micrylettamelanopssp.nov.further differs fromM.sumatranaby orange-red background coloration of dorsum (vs.golden brown); dark-brown reticulated pattern on dorsum (vs.absent); lateral sides of head dark brown lacking light spots or patches (vs.cream spots on lips, tympanum region, and axilla); and uniform coloration of ventral surface(vs.mottled pattern on belly).Finally, the new species can be diagnosed fromM.immaculataby smaller size in females(22.4 mm vs.27.7–30.1 mm); snout truncated in dorsal view(vs.rounded); tibiotarsal articulation of adpressed limb reaching level of eye (vs.level of tympanum); orange-red dorsal coloration with brown reticulate pattern (vs.uniform bronze-brown or reddish-brown dorsum, lacking pattern); and head sides dark brown, lacking white spots (vs.dark brown with white spots along upper lip).
Furthermore, the distinct yellow blotches across the tail and fin of tadpoles distinguishMicrylettamelanopssp.nov.fromM.steinegeriandM.erythropoda, which lack yellow spots and blotches in tadpoles (Chou & Lin, 1997; Vassilieva et al.,2016).
Distribution and natural history:The new species is currently only recorded from the mid-elevation montane evergreen pine forest in its type locality.Micrylettamelanopssp.nov.is an elusive species: the only adult gravid female specimen was collected from a pit-fall trap within a pine forest dominated byPinuskesiyaRoyle ex Gordon at an elevation of 1 460 m a.s.l.; despite further herpetofaunal surveys conducted in May to July 2010–2011 no additional specimens of the new species were recorded.Tadpoles were collected from a small temporary swamp at the edge of the mixed evergreen montane forest and the pine forest ca.300 m from the holotype collection site.All further information on ecology of the new species, including reproduction, male advertisement calls, diet, and predators, is lacking.
Conservation status:To date,Micrylettamelanopssp.nov.is known only from a narrow area within the environs of the Giang Ly Ranger Station within the Bidoup-Nui Ba NP, Lam Dong Province, Langbian Plateau, southern Vietnam.Our intensive surveys in similar habitats over several other forested areas of the Langbian Plateau within Lam Dong,Khanh Hoa, and Dak Lak provinces failed to discover any new populations ofMicrylettamelanopssp.nov.; it is likely that the new species has a limited distribution within the eastern edge of the Langbian Plateau.Hence, the extent of its distribution and its population trends remain unknown.Therefore, we suggest thatMicrylettamelanopssp.nov.be considered as a Data Deficient (DD) species following the IUCN’s Red List categories (IUCN Standards and Petitions Committee, 2019).
NOMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format represents a published work according to the International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5–8.6 of the Code).This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN.The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/.
Publication LSID:
urn:lsid:zoobank.org:pub:496520BA-E40F-41DE-A4B3-064450A17882
MicrylettaimmaculataLSID:
urn:lsid:zoobank.org:act:ABBC2CF0-9708-421D-B4F0-9A998FBBAC0F
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for field surveys was granted by the Bureau of Forestry, Ministry of Agriculture and Rural Development of Vietnam (permits #170/TCLN–BTTN of 07/02/2013;#400/TCLN-BTTN of 26/03/2014; #831/TCLN–BTTN of 05/07/2013) and by local administration (Lam Dong Province:#5832/UBND-LN of 22/10/12).
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
N.A.P., J.H.Y., and T.V.N.designed the study, N.A.P.performed the field work, and N.A.P., T.V.N., and V.A.G.performed data analysis.All authors wrote the text and read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Andrey N.Kuznetsov, Hoi Dang Nguyen (JRVTTC), and Thai Van Nguyen (SVW) for supporting our study.We thank Eduard A.Galoyan, Anna B.Vassilieva, Eugene S.Popov, Alina V.Alexandrova, and Olga V.Morozova for help and support during field work.The authors are grateful to Chris Oldnall for proofreading the paper.We express our sincere gratitude to Peter Geissler, L.Lee Grismer, and two anonymous reviewers for useful suggestions on an earlier version of the manuscript.
杂志排行
Zoological Research的其它文章
- Novel rhino-like SHJHhr mice with thyroid dysfunction
- The high diversity of SARS-CoV-2-related coronaviruses in pangolins alerts potential ecological risks
- Site-specific and seasonal variation in habitat use of Eurasian otters (Lutra lutra) in western China:implications for conservation
- Understanding autism spectrum disorders with animal models: applications, insights, and perspectives
- High-quality chromosome-level genome assembly of redlip mullet (Planiliza haematocheila)
- Challenging Wallacean and Linnean shortfalls:Ectatosticta spiders (Araneae, Hypochilidae)from China
