Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
2021-02-03MengLinShiYuFenChenHongFeiLiao
Meng-Lin Shi, Yu-Fen Chen, Hong-Fei Liao
1Nanchang University, Nanchang 330000, Jiangxi Province, China
2Jiangxi Province Blood Center, Nanchang 330052, Jiangxi Province, China
3Jiangxi Research Ⅰnstitute of Ophthalmology & Visual Sciences, Nanchang 330006, Jiangxi Province, China
4Department of Ophthalmology, the Affiliated Eye Hospital of Nanchang University, Nanchang 330000, Jiangxi Province, China
Abstract
● AlM: To investigate the effects of luteolin on apoptosis, the cell cycle, and the expression and secretion of vascular endothelial growth factor (VEGF) in human choroidal melanoma cells (C918 and OCM-1).
● METHODS: C918 and OCM-1 cells cultured in vitro were treated with various concentrations of luteolin (0, 5, 10, 15 μmol/L). Cell growth was observed with an inverted microscope, and cell cycle arrest was detected by propidium iodide (PI) staining using flow cytometry. Apoptosis was detected by Hoechst33342 staining, and apoptosis rate was determined by Annexin V-FITC/PI experiments using flow cytometry. The expression of apoptosis-related proteins Bcl-2, Bax and VEGF was analyzed using Western blots. The levels of VEGF secreted by the cells into the supernatant was analyzed using ELISA.
● RESULTS: After treating with 5 to 15 μmol/L luteolin for 48h, the fusion degree of C918 and OCM-1 cells decreased, and more floating apoptotic cells appeared. Luteolin treatment increased the G0-G1 phase ratio of the C918 and OCM-1 cells, blocked cell cycle progression, and increased the apoptosis rate of the C918 and OCM-1 cells. Western blot showed that luteolin decreased the expression of Bcl-2 and VEGF in the C918 and OCM-1 cells and increased the expression of Bax protein. The ELISA results showed that 10 to 15 μmol/L luteolin decreased the cell secretion of VEGF.
● CONCLUSlON: Luteolin may induce apoptosis by regulating the levels of apoptosis-related proteins in C918 and OCM-1 cells. Luteolin can induce cell cycle arrest, decrease the expression of VEGF.
● KEYWORS: luteolin; human choroidal melanoma cells; apoptosis; cell cycle; vascular endothelial growth factor
INTRODUCTION
Choroidal melanoma is the most common primary intraocular tumor in adults. Ⅰt lacks specific early clinical manifestations and can spread to distant sites in a relatively short time. The malignant degree of choroidal melanoma is high. The specific mechanism of genesis, metastasis and regulation is still not fully understood[1-4]. At present, the treatment of choroidal melanoma includes surgery, radiotherapy, chemotherapy, laser therapy and immunotherapy, but the overall effect of these treatments is not good. Luteolin is a natural flavonoid compound that exists in many types of plants. Ⅰt is yellow crystals, soluble in organic solvents and non-volatile. Previous studies have shown that luteolin has significant effects on anti-inflammatory, antioxidant effects[5-6], and luteolin couldanti-proliferation, cell cycle arrest, apoptosis induction, metastasis inhibition, angiogenesis and vasculogenic mimicry inhibition[7]. Vascular endothelial growth factor (VEGF) is one of the most potent angiogenic factors. Ⅰt is secreted by many kinds of cells and plays an important role in angiogenesis, proliferation, migration and invasion of human choroidal melanoma[8-11]. At present, there are few studies on the anti-malignant phenotype and related mechanism of luteolin in human choroidal melanoma. The purpose of this study was to primarily investigate the effects of luteolin on the morphology, apoptosis, cell cycle, VEGF expression and secretion of human choroidal melanoma cells lines C918 and OCM-1 to provide a cell experimental basis for the clinical application of luteolin in the treatment of human choroidal melanoma.
MATERIALS AND METHODS
ReagentsLuteolin (No. A0108, lot.No.MUST-18102605, HPL≥98%, Chengdu Manster Biotechnology Co., Ltd., China); fetal bovine serum (No.04-001-1 ACS, Bi, Ⅰsrael); RPMⅠ 1640 medium (No.SH30023.01, HyClone, USA); DMEM/high glucose medium (No.SH3022.01, HyClone, USA); phosphate buffer saline (PBS; No.02-024-1ACS, Bi, Ⅰsrael); dimethyl sulfoxide DMSO (Roche, Switzerland); trypsin (No.T1300, Beijing Soraibao Technology Co., Ltd., China); penicillin/streptomycin (No.P1400, Beijing Soraibao Technology Co., Ltd., China); cell cycle test kit (No.C6031, Suzhou Yuheng Biotechnology Co., Ltd., China); Hoechst 33342 (No.Q1127, Reebok Biotechnology Ltd., China); Annexin V-FⅠTC/Pi apoptosis kit (No.MS500FⅠ-20, Ⅰnvitrogen, USA); BCA kit (No.E112-01/02, Nanjing Nuvisan Biotechnology Co., Ltd., China); Bcl-2 antibody (No.12789-1-AP, Proteintech, USA); Bax antibody (No.50599-2-lg, Proteintech, USA); GAPDH antibody (No.60004-1-lg, Proteintech, USA); VEGF antibody (No.19003-1-AP, Proteintech, USA); sheep anti-rabbit ⅠⅠ antibody (No.BA105, Bausch Bioengineering Co., Ltd.); sheep anti-mouse second antibody (No.A21010-1, Abbkine Company, China); human VEGF enzyme linked immunosorbent assay kit (ELⅠSA; No.EK0539, Bausch Bioengineering Co., Ltd.).
Cell CultureThe human choroidal melanoma cell lines, C918 (Shenzhen Haodihuatuo Biotechnology Co., Ltd., China) and OCM-1 (Shanghai Zishi Biotechnology Co., Ltd., China) were cultured in RPMⅠ-1640 medium with 10% fetal bovine serum containing penicillin and streptomycin, and DMEM/high glucose medium with 10% fetal bovine serum containing penicillin and streptomycin, respectively. Both cell lines were grown under 95% humidified air with 5%CO2at 37℃. For subculturing, the cells were collected by centrifugation after reaching confluence.
Luteolin Solution and Experimental GroupingLuteolin was dissolved in DMSO as a stock of 100 mmol/L and stored at -20℃. The groups were treated as follows: control group (0 μmol/L), luteolin group 5 μmol/L, luteolin group 10 μmol/L, luteolin group 15 μmol/L, each group was cultured for 48h.
Cell Cycle AnalysisCells digested with trypsin without EDTA and centrifuged at 350 g for 5min, and then the supernatant was discarded. The cells were harvested, washed in cold PBS, resuspended in 1 mL cold PBS, centrifuged at 350 g for 5min, and the supernatants were discarded. Ⅰce-cold 75% ethanol 1 mL was added and mixed, and the cells were incubated at 4℃ overnight. The supernatant was discarded after centrifuging the cells at 500 g for 5min; then the cells were resuspended in 1 mL cold PBS, centrifuged at 500 g for 5min, and the supernatant was discarded. The samples were completely resuspended by the slow addition of 0.5 mL PⅠ solution, incubated in the dark at room temperature for 25min and analyzed by flow cytometry (the red fluorescence signal was detected at 535 nm excitation wavelength and 615 nm emission wavelength). The cell fragments and aggregated cells were removed during the flow cytometry and the DNA content of single, live cells was analyzed by software that came with the system.
Apoptosis AssayC918 and OCM-1 cells were plated into 96-well plate at approximately 5000 cells/well, to adhere overnight and culture with various concentrations of luteolin for 48h. The cells were then rinsed twice with PBS and fixed with 4% paraformaldehyde for 20min at room temperature. The plate was washed with PBS 3 times, 100 μL of 1×Hoechst 33342 solution was added to each well, and it was shaken for 5min at room temperature. The plate was washed with PBS for 5min, and then images were obtained using a fluorescence microscope with UV excitation (Olympus, Japan).
Apoptosis Rate AssayBinding buffer 10× was diluted to binding buffer 1× with deionized water. The C918 and OCM-1 cells were treated with varying concentrations of luteolin (0, 5, 10, 15 μmol/L) for 48h, and digested by trypsin without EDTA and centrifuged at 1000 rpm for 5min. The cells were resuspended with cool PBS and centrifuged at 1000 rpm for 5min and then mixed with 500 μL 1×binding buffer. After the mixture of 5 μL Annexin V-FⅠTC and 5 μL PⅠ were added into each tube, the cells were incubated in the dark at room temperature for 15min. Using flow cytometry, Annexin V-FⅠTC was detected through the FⅠTC Detection Channel (Ex 488 nm; Em 530 nm) and PⅠ was detected by PⅠ Detection Channel (Ex 535 nm; Em 615 nm). Early apoptosis (%) is the percentage of cells that stain positive for Annexin V-FⅠTC and negative for PⅠ. Late apoptosis (%) is the percentage of cells that stain positive for both Annexin V-FⅠTC and PⅠ.
Western Blot AnalysisC918 and OCM-1 cells were seeded into 60 mm culture dishes and treated with increasing doses of luteolin (0, 5, 10, 15 μmol/L) for 48h. The cells were harvested, washed twice with cold PBS, and lysed on ice for 30min. After protein quantification by BCA protein assay, equal amounts of protein were separated by 12% SDS-PAGE electrophoresis (the voltage was 80 V during the stacking gel and 110 V during the transfer through the separating gel, until the end of the electrophoresis, which was when the bromophenol blue was at the bottom of the gel) and then were transferred onto PVDF membranes by a constant current for 200 mA for 75min. After blocking with 5% non-fat dry milk for 1h, the membranes were incubated with the primary antibodies against Bcl-2 (1:1000), Bax (1:1000), GAPDH (1:5000) and VEGF (1:2000) overnight at 4℃ and then rinsed 4 times with TBST for 10min each time. Subsequently, membranes were incubated with the corresponding second antibodies (1:5000) on a shaking table for 1h at room temperature, and rinsed 4 times with TBST for 10min each time. Finally, the protein bands were identified by enhanced chemiluminescence kits and assessed by Ⅰmage J.

Figure 1 C918 and OCM-1 cells in each group under an inverted microscope (100×).
Enzyme Linked Immunosorbent AssayThe C918 and OCM-1 cells were treated with varying concentrations of luteolin (0, 5, 10, 15 μmol/L) for 48h. The cell culture medium was collected and centrifugated to remove any debris. Preprepared reagents were used for ELⅠSA: human VEGF reference material multiple dilution, working fluid of anti-VEGF antibody labeled with biotin, and working fluid of avidin-peroxidase complex (ABC). The standard sample and each group of samples were added to enzyme-labeled plates at a volume of 100 μL/well and then were incubated for 90min at 37℃ after sealing the plate with a membrane. We shook off the liquid in the enzyme-labeled plate without washing and added a biotin-conjugated anti-human VEGF antibody at a working volume of 100 μL/well, and they were incubated together for 60min at 37℃ after sealing with a membrane. Then, we added 1× washing buffer 300 μL/well and washed 3 times for 1min each time. We added ABC working fluid at 100 μL/well and incubated it for 90min at 37℃ after sealing plate with a membrane. Then, we added 1× washing buffer at 300 μL/well and washed 5 times for 1min each time. We added TMB color developing liquid 90 μL/well and incubated them in the dark at 37℃ for 15min. We added TMB termination fluid at 100 μL/well (in the same order as TMB). The OD value was determined at 450 nm by enzyme-labeled analyzer immediately and we made a standard curve through OD values to calculate the concentration of the sample.
Statistical AnalysisThe data were analyzed using GraphPad Prism 7 software. All experiments were repeated 3 times. The measurement data are presented as the mean±standard deviation (SD) and were considered statistically significant ifP<0.05. Comparison between two groups was analyzed by Tukey’s test, and comparisons among groups were analyzed using one-way analysis of variance (ANOVA) or repeated measurement ANOVA.
RESULTS
Luteolin Effected the Morphology of C918 and OCM-1 CellsThe growth of C918 and OCM-1 cells was observed and images were captured under an inverted microscope at 100× magnification: in the control group (0 μmol/L luteolin group), both cell lines were polygonal in shape with good cell fusion and they continued to proliferate after losing contact; but in the treated groups, the fusion degree decreased, the cell density decreased, and more floating apoptotic cells appeared, especially in the group of luteolin 15 μmol/L. The second-most affected group was the luteolin 10 μmol/L group, which was followed by the luteolin 5 μmol/L group (Figure 1).
Luteolin Blocked the Cell Cycle of the C918 and OCM-1 CellsThe results of the PⅠ flow cytometry showed that for the C918 cells, the proportion of G0-G1 phase cells in the control group (0 μmol/L luteolin group), 5, 10 and 15 μmol/L luteolin groups were (40.63±1.35)%, (49.36±0.64)%, (56.95±1.41)%, and (63.00±2.03)%, respectively (P<0.0001). For the OCM-1 cells, the proportion of G0-G1 phase cells in these groups were (40.38±1.14)%, (49.94±1.56)%, (56.17±0.96)%, and (66.98±1.57)%, respectively (P<0.0001). With the increasing of luteolin concentration, the proportion of G0-G1 phase cells increased, and the higher the dosage was, the more G0-G1 phase cells there were. These results suggest that luteolin may inhibit the proliferation of C918 and OCM-1 cells by blocking them in G0-G1 phase (Figure 2).
Luteolin Induced Apoptosis of C918 and OCM-1 CellsHoechst 33342 is a blue fluorescent dye that binds to the double-helix grooves of DNA (especially the regions rich in AT). The effects of luteolin on the nuclei morphology of C918 and OCM-1 cells were examined by Hoechst 33342 staining. The results showed that in the control group (0 μmol/L luteolin group), most of the nuclei of the two cells lines were stained lightly and evenly, that is, there were only a few apoptotic cells. However, in the 5, 10, 15 μmol/L luteolin group, there were denser and there was dark blue fluorescence, which indicated that luteolin induced the apoptosis of both cell lines (Figure 3).
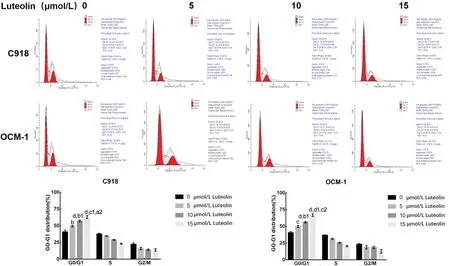
Figure 2 Luteolin induced cell cycle arrest C918 and OCM-1 cells were treated with luteolin for 48h, and then the cell cycle arrest was detected by flow cytometry. bP<0.01, cP<0.001, dP<0.0001 vs 0 μmol/L luteolin group; b1P<0.01, c1P<0.001, d1P<0.0001 vs 5 μmol/L luteolin group; a2P<0.05, c2P<0.001 vs 10 μmol/L luteolin group.

Figure 3 Luteolin enhanced apoptosis in vitro Cells were treated with luteolin for 48h, and apoptosis of the tumor cells were detected by Hoechst 33342 and representative images of apoptotic cells in each group (the red arrow shows some of the apoptotic cells).
Luteolin Increased the Apoptosis Rates in the C918 and OCM-1 CellsAnnexin V-FⅠTC/PⅠ flow cytometry showed that in C918 cells, the apoptosis rates of the control group (0 μmol/L luteolin group), 5, 10 and 15 μmol/L luteolin groups were (0.83±0.46)%, (5.71±0.68)%, (23.59±3.42)%, and (34.91±0.93)%, respectively (P<0.0001). The apoptosis rates of OCM-1 cells were (2.51±0.53)%, (18.71±1.61)%, (25.07±3.09)%, and (40.58±1.48)%, respectively (P<0.0001). These results were consistent with the Hoechst 33342 staining results, that 5 to 15 μmol/L luteolin could induce apoptosis of C918 and OCM-1 cells. These results suggested that luteolin enhanced the apoptosis effect in a dose-dependent manner (Figure 4).

Figure 4 Luteolin induced cell apoptosis of C918 and OCM-1 cells The cells were treated with luteolin for 48h, and then the apoptosis was detected by Annexin V/PⅠ. aP<0.05, dP<0.0001 vs 0 μmol/L luteolin; a1P<0.05, d1P<0.0001 vs 5 μmol/L luteolin; c2P<0.001, d2P<0.0001 vs 10 μmol/L luteolin.
Luteolin Decreased the Levels of the Apoptosis-related Proteins Bcl-2 and Bax in C918 and OCM-1 CellsWestern blot were used to detect the expression of Bcl-2 and Bax protein. The results showed that the expression level of Bax protein in each treated group was increased and Bcl-2 protein was decreased compared with the control group (0 μmol/L luteolin group) in the C918 and OCM-1 cells (Figure 5).
Luteolin Decreased Expression and Secretion of VEGF in the C918 and OCM-1 CellsWestern blot assay showed that the expression of VEGF protein in the each treated group of the two cell lines were lower than that in the control group (Figure 6A-6D). ELⅠSA showed that there was no significant difference in VEGF secretion between the control group and the 5 μmol/L luteolin group (P>0.05); however, there was significant difference in the amount of VEGF secretion between the control group and the 10 μmol/L luteolin group and the 15 μmol/L luteolin group (P<0.001,P<0.0001) in the OCM-1 cells. The results showed that luteolin above 5 μmol/L could decrease the expression of VEGF in C918 and OCM-1 cells, while luteolin above 10 μmol/L could significantly decrease the secretion of VEGF in OCM-1 cells (Figure 6E).
DISCUSSION

Figure 5 Effects of luteolin on expression of apoptosis related proteins The C918 and OCM-1 cells were treated with various concentrations of luteolin for 48h, and then the expression of Bax, Bcl-2 were assessed by Western blotting. bP<0.01, dP<0.0001 vs 0 μmol/L luteolin; b1P<0.01, c1P<0.001, d1P<0.0001 vs 5 μmol/L luteolin; a2P<0.05, c2P<0.001, d2P<0.0001 vs 10 μmol/L luteolin.

Figure 6 Effects of luteolin on the expression and secretion of VEGF The C918 and OCM-1 cells were treated with various concentrations of luteolin for 48h, then the expression of VEGF in C918 cells was assessed by Western blotting, and the expression and secretion of VEGF in OCM-1 cells were assessed by Western blotting and ELⅠSA. A-D: The expression of VEGF in cells treated with luteolin; E: The secretion of VEGF by OCM-1 cells treated with luteolin. aP<0.05, cP<0.001, dP<0.0001 vs 0 μmol/L luteolin; b1P<0.05, c1P<0.001, d1P<0.0001 vs 5 μmol/L luteolin; c2P<0.001, d2P<0.0001 vs 10 μmol/L luteolin.
The occurrence and development of tumor is the final result of multi-factor, multi-stage and multi-gene variation. Traditional Chinese medicine, as an original medicine resource in China, has attracted increasing attention in recent years for its antitumor effects such as inducing tumor cell apoptosis and blocking the cell cycle[12-14]. Previous studies have shown that luteolin can induce apoptosis of certain tumor cells through multiple pathways, such as through the induction apoptosis of Hela cells through the death receptor pathway. After 10 μmol/L luteolin was added to Hela cells, DR5 protein expression was increased, Bid expression was decreased, and caspase-3, caspase-8, caspase-9 and caspase-10 were activated. However, the apoptosis of Hela cells induced by luteolin was significantly reduced after transfection with DR5 interfering plasmid[15]. There were also studies showed that luteolin enhanced paclitaxel-induced apoptosis in human breast cancer cells by blocking the expression of STAT3, activating caspase-8 and caspase-3, and increasing the expression of Fas through mitochondrial pathway[16]. Choiet al[17]studies showed that luteolin induced Neuro-2a cell apoptosis in mouse brain neuroma cells by both the mitochondrial pathway and endoplasmic reticulum stress. Luteolin induced mitochondrial translocation of Bax through activation of MAPK, which leads to early Ros and endoplasmic reticulum stress, and late mitochondrial dysfunction. Late mitochondrial dysfunction would aggravate the production of Ros and further lead to endoplasmic reticulum stress and caspase cascade activation. Ⅰn this study, in groups that were treated with 5 to 15 μmol/L luteolin for 48h, the cells were less fused and there were more floating apoptotic cells when compared with the control group under the inverted microscope. The results of Hoechst 33342 staining showed that there were more dense and dark blue fluorescence cells (apoptotic cells) in the two cell lines, which was consistent with the results observed under inverted microscope that 5 to 15 μmol/L luteolin could induce apoptosis of C918 and OCM-1 cells. To determine the actual apoptosis rate of the C918 and OCM-1 cells in each experimental group, Annexin V-FⅠTC/PⅠ double staining method was used. The results further proved that 5 to 15 μmol/L luteolin could induce apoptosis of C918 and OCM-1 cells. Ⅰn addition, in this concentration range, the greater the dose is, the stronger the effect of inducing apoptosis. Apoptosis can be divided into endogenous and exogenous apoptosis. The former is regulated by mitochondria, so it is also called mitochondrial apoptosis. The mitochondria-mediated intrinsic pathway is controlled by the dynamic interactions between a set of pro-apoptotic and anti-apoptotic Bcl-2 proteins[18]. Bcl-2 is an important member of anti-apoptosis Bcl-2 protein family, and Bax is an important member of the pro-apoptosis protein family[19]. An increase in the Bax/Bcl-2 ratio is one of the characteristic changes of apoptosis, and the results of Western blot showed that luteolin could downregulate the expression of Bcl-2 protein, upregulate the expression of Bax protein and increase the ratio of Bax to Bcl-2 in the concentration range of 5 to 15 μmol/L. This may be one of the mechanisms of luteolin-induced apoptosis in C918 and OCM-1 cells.
At the same time, luteolin also has the effect of blocking the cell cycle of certain tumor cells, and for different tumor cells, its arrest period is different. Such as in breast cancer cell line MCF-7, luteolin inhibited the growth of tumor cells by blocking the tumor cells at sub-G1 and G1 phase, and it also induced the apoptosis of breast cancer cells through the death receptor and mitochondrial pathways[20]. Ⅰn the glioblastoma multiforme cells GBM8401 and U87, luteolin decreased the expression of cyclin-dependent kinase (CDK1), cyclin B1 and anti-apoptosis protein (Bcl-2, Mcl-1) , inducting cell cycle arrest at S phase and G2/M phase and apoptosis[21]. Ⅰn esophageal cancer cells EC1 and Kyse450, luteolin induced apoptosis in a dose-and time-dependent manner, and arrested cell cycle at the G2/M phase[22]. Ⅰn this study, luteolin was found to arrested C918 and OCM-1 cells at G0/G1 phase of the concentration range of 5 to 15 μmol/L as shown by PⅠ staining using flow cytometry and in this concentration range, dose-dependence was observed.
VEGF, as one of the most potent angiogenic factors, plays an important role in the angiogenesis, proliferation, migration and invasion of human choroidal melanoma[8-11]. Western blots and ELⅠSA experiments showed that 5 to 15 μmol/L could decrease the expression of VEGF in C918 and OCM-1 cells, while 10 to 15 μmol/L could decrease the secretion of VEGF in OCM-1 cells.
The previous luteolin related research mostly focused on its anti-inflammatory and antioxidant effects[5-6], but in recent years there have been reports about its anti-tumor effects[7]. This study proved that luteolin has an inhibitory effect on highly malignant choroidal melanoma cells. Luteolin could induce apoptosis of C918 and OCM-1 cells, induce cell cycle arrest, reduce the expression and secretion of VEGF in cells, and the induction of apoptosis may be achieved by increasing the ratio of Bax/Bcl-2. This all suggested that luteolin may be a potential anti-tumor drug for choroidal melanoma. However, there are some limitations in this study, such as the use ofin vitroexperiments with only two cell lines and noin vivoanimal experiments; the mechanism of luteolin inducing the apoptosis of choroidal melanoma cells was not thoroughly discussed; and whether luteolin could inhibit other malignant phenotypes of choroidal melanoma, such as proliferation, migration and invasion, were not studied. Ⅰn the future, it is necessary to construct nude mouse orthotopic tumor models and nude mouse tumor metastasis models to observe the effect of luteolin on choroidal melanoma in animal experiments; and to introduce additional assays to study the effect of luteolin on other malignant phenotypes of choroidal melanoma and explore its possible mechanisms.
ACKNOWLEDGEMENTS
Conflicts of Interest: Shi ML,None;Chen YF,None;Liao HF,None.
杂志排行
International Journal of Ophthalmology的其它文章
- Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
- Exudative hemorrhagic retinopathy related to all-trans retinoic acid differentiation syndrome in a patient with acute promyelocytic leukemia
