Human stem cell-derived microglia will be an indispensable toolbox for Alzheimer’s disease research
2021-01-24TongfeiLiu
Tongfei Liu
Alzheimer’s disease (AD) is the most common age-related dementia without cures. Between 2000 and 2017, deaths resulting from AD increased 145%. Based on the latest statistical data, more than 50 million people in the world suffered this devastating disease, which is one of major burdens in modern public health system (Alzheimer’s Association, 2020).Genetic mappings have uncovered numerous susceptibility genetic variants, the vast majority of which are located in noncoding regions of the genome.
Microglia, a type of glial cells, are resident macrophages located throughout the central nervous system. Vital functions of microglia within the brain are mainly related to innate immunity and maintaining homeostasis such as neuroinflammation, phagocytosis, brain environment surveillance, and synaptic pruning.Most of AD-risk variants are active in microglia(Nott et al., 2019). The enthusiasm for microglia was further fueled by the discovery that many of the microglia-enriched genes are identified as risk factors for AD, frontotemporal dementia and other neurodegenerative diseases.
However, current strategies and methodologies for studying its role in AD etiology and progression are limited. Firstly, prior knowledge about microglia mostly came from rodent models. Sixty-five million years of evolutionary divergence produce significant differences between human and rodents, during which their immune systems faced distinct pathogens.For example, TREM2 (Triggering Receptor Expressed on Myeloid Cells 2) and CD33(Cluster of Differentiation 33), the focus of AD fields, also feature significant variability within their protein coding sequences, which may result in diverging biological differences between two species. The membrane-proximal immunoreceptor tyrosine-based inhibition motif (ITIM) found in human CD33 is absent in murine CD33. Moreover, a recent study showed that the TREM2R47Hmutation was observed to downregulate TREM2 mRNA levels in mouse microglia, but not in human. Thus, induced pluripotent stem cells (iPSCs) derived microglialike model is one of the best choices currently.However, iPSC derived cell studies may be hindered by the lack of standardized reprogramming protocols and variables derived from genetic variation and epigenetic memory which affected results reproduction,data interpretation and integration (Kim et al.,2010). Therefore, it necessitates utilization of conventional human H9 embryonic stem (ES)cell line harboring AD-associated variants in AD research, which will be easier for following data validation and comparison between different laboratories.
Many labs have focused on singular profiles at transcriptomic or epigenetic levels. However,a big picture generated from epigenetic,transcriptomic, proteomic and functional profiles may be required to fully understand how AD-associated gene variants can affect microglia behavior.
In our recent published work, by CRISPR(Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 (CRISPR Associated Protein 9), we have integrated a subset of AD-associated genetic risk variants includingTREM2KO,TREM2R47H,SORL1(Sortilin Related Receptor 1)R744XandSORL1A528Tinto an isogenic human H9 ES cell model, and differentiate H9 cells bearing those AD variants into mature human stem cell derived microglialike cells (hMGLs) (Liu et al., 2020). Integrative multiomic analysis of combined ATACseq(Assay for Transposase-Accessible Chromatin using sequencing), ChIPseq (Chromatin immunoprecipitation followed by sequencing),RNAseq and proteomics datasets unravel the crosstalk betweenSORL1andTREM2pathway,which were thought working independently in AD pathogenesis until now (Liu et al., 2020).SORL1R744Xcould upregulate the apolipoprotein E (APOE) level through increasing the expression of TREM2. Further,in vitroamyloid beta (Aβ) phagocytosis assay indicated that APOE mediates phagocytic impairment as a downstream consequence ofTREM2R47HandSORL1R744Xmutations. hMGLs xenotransplanted into brains of immunodeficient mice followed by microdialysis and two-photon imaging indicate defective microglial homing to Aβ plaques and Aβ clearance. Together, our results disclosed that APOE as a central convergent node shared betweenTREM2andSORL1mutant hMGLs, which may explain whyTREM2R47Hand APOEε4 variants may confer comparable odds of AD onset. Importantly, it is also consistent with the clinical observation thatTREM2R47Hcarriers need APOEε4 allele to trigger AD onset (Murray et al., 2019).Further, by advanced bioinformatic analysis on our multiomic data, identification of enriched IRF8 (Interferon Regulatory Factor 8) motifs inTREM2R47HhMGLs in our study faithfully mimicked the changes of IRF8 in human AD samples, demonstrating good translatability between our H9 model system and human AD studies (Zhou et al., 2020).
Future directions and concluding remarks:1) The рrotocols for hMGLs should be chosen carefully and wisely.Many recent advancements in microglial ontogeny, such as microglia arising from embryonic yolk sac, facilitated the establishment of protocols for hMGLs. Until the end of 2016, the first microglia differentiation protocol was published by Rudolf Jaenisch lab.Since then, several protocols for generating hMGLs from human pluripotent stem cells(hPSCs) have been published. Even commercial differentiation kits are also available, which greatly reduce the impediment for researchers without stem cell background. Most protocols aim to recapitulate the microglia embryonic development by exposure of hPSCs to cocktail of key growth factors or small molecules. There are a number of excellent reviews that focus on the differences between each protocol(Speicher et al., 2019). However, it is worthy to note that different protocols maybe produce different phenotypes of hMGLs, which is very important for new learner.
2) The xenotransрlantation exрeriments need to be further imрroved.For comprised shortterm integration of hMGLs in mouse brain, it is possible that protective effects associated with transient hMGL xenotransplantation may change under longer time periods,where hMGLs may convert to chronically neuroinflammatory states. As our published results indicate that some hMGL mutations,namelyTREM2R47HandSORL1KO variants can recapitulate microglial signatures observed in AD, upregulation of pathogenic components such as APOE found in these signatures may ultimately impair microglial function (Liu et al., 2020). But what will happen in long-term transplantation? Besides, data interpretation in current xenotransplantation paradigm will be confounded by endogenous mouse microglia(Hasselmann et al., 2019). A next-generation model will only contain the human microglia.Thus, it will be of interest in future study to adapt those improvements into our analysis platform, and monitor effects of AD-related variants on interactions with Aβ and other cell types.
Adaptive immune system also plays a vital role in the development of amyloid pathology in AD. Most immunodeficient versions of AD mouse models, without an adaptive immune response, exhibit some inherent developmental abnormality and display significantly increased Aβ plaque load, compared to their immunocompetent counterparts. Thus, to better understand precisely how Aβ clears in AD patients, it is more suitable to use immunocompetent AD mouse lines for related studies. To facilitate hMGL xenotransplantation in immunocompetent AD mice model, based on recent work of Deuse et al. (2019) inNature Biotechnology, genetic ablation of the major histocompatibility complex and overexpression of the transmembrane protein CD47 (Cluster of Differentiation 47) could make hMGL invisible to the immune system of the host.
3) Both hyрothesis-driven and data-driven ways are needed for AD research.Traditionally,most scientific research is conducted in a hypothesis-driven way, in which researchers submitted a specific, measurable, and testable question at the beginning, then tested with an experiment, analyzed the results and modified the hypothesis. Such a cycling proceeding in past centuries is the most reliable approach to produce robust knowledge. In contrary, for data-driven, there are no more hypotheses,no more discussions whether the experiments refute or support the original hypotheses.In this new era with big data technologies booming, we need to add the data-driven way into our research strategies, which utilized complex algorithms and statistical tools to go through a massive amount of AD clinical data to find useful information that could be turned into knowledge about AD pathogenesis.For example, spatial transcriptomics (Visium-10xgenomics; STARmap) could collect very important spatial information in AD brain tissue samples, including the relationship of microglial cells to amyloid plaques/Tau tangles and to dying neurons. It likely unmasks the potential changes in cell composition and gene expression distribution during neurodegeneration. Furthermore, it can also contribute to the discovery of the complex interaction between protective and damaging molecular processes, which may be the basis of a cure in future. Meanwhile, the major discoveries from big data could be tested easily in human iPSC or ES cell derived cellular model(Figure 1). Finally, at the systematical level, we can exam in animal model, even in the large animals like pig and non-human primate.
4) Inducing aging effects in hMGLs model.Our hMGLs show many similar features to human microglia such as global expression profiles, and functional responses including Aβ phagocytosis,membrane depolarization kinetics, Ca2+homeostasis, and cytokine release.
However, the hMGL model is still in its infancy stage and yet requires further development.Aging is the greatest known risk factor for AD that cannot be explained by amyloid hypothesis.It is challenging to recapitulate the effect of aging in cell culture system. Mutant Lamin A, also called progerin, can cause premature aging syndrome Hutchinson-Gilford progeria(HGPS). Exogenous expression of progerin has been proven promising as one method to age neurons (Miller et al., 2013). Interestingly, the expression of progerin has also been observed in fibroblasts from normally aged donors. So,we can integrate tet-on inducible progerin into the genome of hMGL to study the aging impact on microglial functions in a regulatory manner. Besides, the deficiency of XPF(Xeroderma pigmentosum complementation group F or ERCC4) or ERCC1 (Excision Repair Cross Complementation Group 1), both DNA excision repair proteins, can also lead to the HGPS disease, which will worsen the DNA damages over time. So, by CRSIPR-Cas9, we can make XPF-/-or ERCC1-/-hMGL to study the aging effects. An interesting speculation is that some of late-onset AD risk variants (especially,non-coding regions) maybe produce only observable effects in aging background. Using the progerin or XPF-/-or ERCC1-/-hMGL as starting points, we can introduce other AD-risk variants identified from late-onset AD patients into genome of those hMGL and systematically determine the functional significance of these genetic variants, which could elucidate the causal relationship between genetic and AD phenotypes.
Technically, it is worthwhile to manipulate the epigenome status to induce aging in the hMGL cell (my unpublished data). DNA methylation is a type of epigenetic modification that refers to the addition of methyl groups onto DNA at specific sites, which determine whether certain genes are switched on or off. The Horvath clock is a most widely-used and accurate algorithm and uses DNA methylation data to calculate age surprisingly well, generally calculating age to within 3 years of a person’s real age and also works for non-dividing cells like neurons (Horvath, 2013). Those discoveries inspired us that aging-like outcomes may be stimulated through manipulating epigenetic modification in the genome of cells. Since epigenetic changes are reversible, it is possible to target their origins to reverse them. Recently,emerging epigenome editing technology has been developed to modify epigenetic marks.There are chimeric proteins, composed of two parts: a DNA binding domain, allowing proteins to precisely target on specific genomic loci (dCas9, catalytically inactive Cas9) and a domain comprising an epigenetic factor (TET1 or DNMT3a) that will modify DNA. Using those chimeric proteins, we can change the DNAmethylation status of Horvath clock’s loci in the genome of hMGLs into what old age looks like.
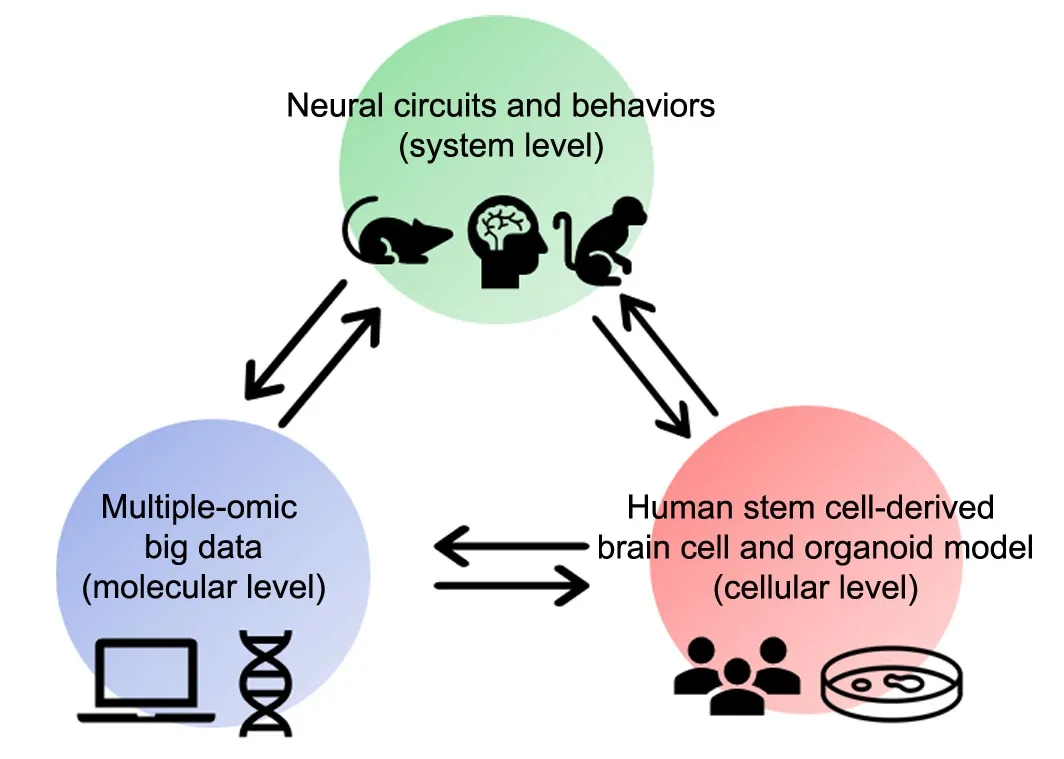
Figure 1|Schematic diagram of research pipeline.
In conclusion, hMGLs are a scalable discovery platform for AD and other neurological disorders including Parkinson’ disease, amyotrophic lateral sclerosis, and Huntington’s disease. To provide a profound understanding of microglia’s role in AD etiology and progression, it will require interrogation using a variety of models and approaches, bridging scales from molecules to system, and investigating mechanisms underlying the entire life course (Figure 1).Thus, we should utilize emerging stem cell tools,multiple omics platforms, and novel animal models to identify causal genetic elements and unexpected regulatory pathways contributing to AD pathogenesis and progression that will finally lead to curative treatments. Moreover,genome-engineered microglia, like chimeric antigen receptor T cells (CART), can also be an efficient vehicle for delivering neuroprotective or regenerative molecules, which will be new directions for treating a variety of neurological diseases in the future.
Tongfei Liu*
Neuroscience Initiative, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, USA;Department of Biology, Southern University of Science and Technology, Shenzhen, Guangdong Province, China
*Correspondence to:Tongfei Liu, PhD,liutongfei1983@gmial.com.
https://orcid.org/0000-0002-3553-6871(Tongfei Liu)
Date of submission:October 12, 2020
Date of decision:November 21, 2020
Date of acceptance:December 18, 2020
Date of web publication:January 25, 2021
https://doi.org/10.4103/1673-5374.306087
How to cite this article:Liu T (2021) Human stem cell-derived microglia will be an indisрensable toolbox for Alzheimer’s disease research. Neural Regen Res 16(9):1770-1771.
Copyright license agreement:The Coрyright License Agreement has been signed by the author before рublication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, whichallows others to remix, tweak, and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Metabolomic profiling provides new insights into blood-brain barrier regulation
- The molecular implications of a caspase-2-mediated site-specific tau cleavage in tauopathies
- Considerations on the concept, definition, and diagnosis of amyotrophic lateral sclerosis
- Angiogenesis and nerve regeneration induced by local administration of plasmid pBud-coVEGF165-coFGF2 into the intact rat sciatic nerve
- Effects of long non-coding RNA myocardial infarctionassociated transcript on retinal neovascularization in a newborn mouse model of oxygen-induced retinopathy
- Synaptic mechanisms of cadmium neurotoxicity
