The future of retinal gene therapy: evolving from subretinal to intravitreal vector delivery
2021-01-24MayaRossRonOfri
Maya Ross, Ron Ofri
Abstract Inherited retinal degenerations are a leading and untreatbale cause of blindness, and as such they are targets for gene therapy. Numerous gene therapy treatments have progressed from laboratory research to clinical trails, and a pioneering gene therapy received the first ever FDA approval for treating patients. However, currently retinal gene therapy mostly involves subretinal injection of the therapeutic agent, which treats a limited area, entails retinal detachment and other potential complications, and requires general anesthesia with consequent risks, costs and prolonged recovery. Therefore there is great impetus to develop safer, less invasive and cheapter methods of gene delivery. A promising method is intravitreal injection, that does not cause retinal detachment, can lead to pan-retinal transduction and can be performed under local anesthesia in outpatient clinics. Intravitreally-injected vectors face several obstacles. First, the vector is diluted by the vitreous and has to overcome a long diffusion distance to the target cells.Second, the vector is exposed to the host’s immune response, risking neutralization by pre-existing antibodies and triggering a stronger immune response to the injection.Third, the vector has to cross the inner limiting membrane which is both a physical and a biological barrier as it contains binding sites that could cause the vector’s sequestration.Finally, in the target cell the vector is prone to proteasome degradation before delivering the transgene to the nucleus. Strategies to overcome these obstacles include modifications of the viral capsid, through rational design or directed evolution, which allow resistance to the immune system, enhancement of penetration through the inner limiting membrane or reduced degradation by intracellular proteasomes. Furthermore,physical and chemical manipulations of the inner limiting membrane and vitreous aim to improve vector penetration. Finally, compact non-viral vectors that can overcome the immunological, physical and anatomical and barriers have been developed. This paper reviews ongoing efforts to develop novel, safe and efficacious methods for intravitreal delivery of therapeutic genes for inherited retinal degenerations. To date, the most promising results are achieved in rodents with robust, pan-retinal transduction following intravitreal delivery. Trials in larger animal models demonstrate transduction mostly of inner retinal layers. Despite ongoing efforts, currently no intravitreally-injected vector has demonstrated outer retinal transduction efficacy comparable to that of subretinal delivery. Further work is warranted to test promising new viral and non-viral vectors on large animal models of inherited retinal degenerations. Positive results will pave the way to development of the next generation of treatments for inherited retinal degeneration.
Key Words: adeno-associated virus; animal model; blindness; gene therapy; inner limiting membrane; photoreceptors; retina; retinitis pigmentosa; vitreous
Introduction
Inherited retinal degenerations (IRDs) are a diverse group of progressive, blinding diseases that are the most common cause of vision loss in developed countries, affecting an estimated 5.5 million patients (Hanany et al., 2020). With one exception they are untreatable, and therefore have devastating personal, social and economic costs (Galvin et al.,2020). IRDs are usually caused by a mutation in genes that are part of the phototransduction cascade or the visual cycle.The monogenic nature of these diseases, and availability of a wide range of both (mostly induced) small and (mostly naturally-occurring) large animal models, make IRDs desirable targets for development of gene therapy. This development is facilitated by the accessibility of the eye for imaging and surgery, its small size, relative immune privileged status,and being a dual organ allowing for studies using a paired,untreated control eye (Sahel and Roska, 2013; Ramlogan-Steel et al., 2019; Ziccardi et al., 2019). Indeed over the last two decades, numerous studies aimed at developing safe and efficacious gene augmentation therapies for various IRDs have been conducted, and many have reached phase I/II clinical trials based on efficacious results in animal models.These include therapies for X-linked choroideremia, Stargardt disease, achromatopsia, Usher syndrome, Leber hereditary optic neuropathy, retinitis pigmentosa, X-linked juvenile retinoschisis and more (Dalkara et al., 2016; Trapani and Auricchio, 2018; Ramlogan-Steel et al., 2019). It is therefore no surprise that the first-ever gene augmentation therapy approved for marketing by the FDA (LuxturnaTM) is used to treat an IRD, Leber’s congenital amaurosis (LCA), hopefully heralding the approval of additional gene therapies for numerous inherited retinal dystrophies and degenerations(Gruntman and Flotte, 2018; Ramlogan-Steel et al., 2019;Ziccardi et al., 2019; Frederick et al., 2020).
Retinal gene therapies are based mostly on viral vectors delivering the therapeutic transgene into the target cells, most commonly photoreceptors and retinal pigment epithelium cells (RPE) located in the outer retina. Adeno-associated virus(AAV) is the most widely-used vector, though both adeno virus and lentivirus have also been studied. AAV is preferred for the following reasons; it is considered less immunogenic than adeno- and lentivirus, it is able to transduce non-dividing cells and it does not integrate into the host genome but still maintains long term and stable transgene expression.While lack of genome integration is usually considered an advantage with regards to safety, there may be advantages to have an integrating vector, such as lentivirus, in a nondividing cell like the photoreceptor. AAV is also considered to exhibit low pathogenicity as it is naturally defficient in replication and is dependant on a helper virus for intracellular replication (Ziccardi et al., 2019). An important limitation of AAV is its limited capacity of genetic material. A single AAV can accommodate transgenes totalling up to 4.7 kbp of genetic material, with recent use of dual AAV delivery allowing for delivery of up to 9.4 kbp (Adijanto and Naash, 2015). In recent years, non-viral vectors are also being evaluated as a safer alternative to viral vectors and with a significantly higher capacity. (Bordet and Behar-Cohen, 2019).
Currently, the majority of retinal gene therapies require subretinal (SR) delivery of the therapeutic vector in order to transduce the target cells in the outer retina. SR injections,though proven to be highly effective in outer retinal transduction in numerous studies, entail several drawbacks,risks and potential complications. First, an SR injection inevitably detaches the photoreceptor layer from the supporting RPE layer, thereby compromising the photoreceptors’ function and survival, let alone in a degenerated retina (Jacobson et al., 2006; Peng et al., 2017). Second, the SR-injected vector transduces a small portion of retinal cells that are in close contact with the subretinal bleb which is formed by the injection, limiting the usefulness of this modality in treating pan-retinal diseases. Third, the procedure is performed under general anesthesia, using advanced surgical techniques, a vitreoretinal surgeon and a specialized operating room, leading to potential anesthetic complications, high costs and prolonged recovery (Jacobson et al., 2012; Dalkara and Sahel, 2014). All of these limitations can be successfully addressed if the vector were to be delivered intravitreally (IVT), rather than SR. Such delivery does not create a retinal bleb and detachment, it has the potential for transfecting the entire retina and it is a safe procedure performed routinely on outpatients in an office setting (mainly for delivery of anti-vascular endothelial growth factor treatments) using just topical anesthesia (Figure 1).Therefore, there is an urgent and unmet need for efficacious and safe IVT vectors that can revolutionize gene therapy of numerous blinding IRDs. Such vectors will increase treatment safety and patient comfort, lower costs and result in treatment of large retinal areas, leading to significantly improved outcomes (Dalkara et al., 2013; Ku et al., 2016; Takahashi et al., 2017; Garafalo et al., 2019; Ong et al., 2019). The aim of the current paper is to review the ongoing, worldwide efforts to develop novel methods for IVT delivery of thrapeutic transgenes for IRDs.
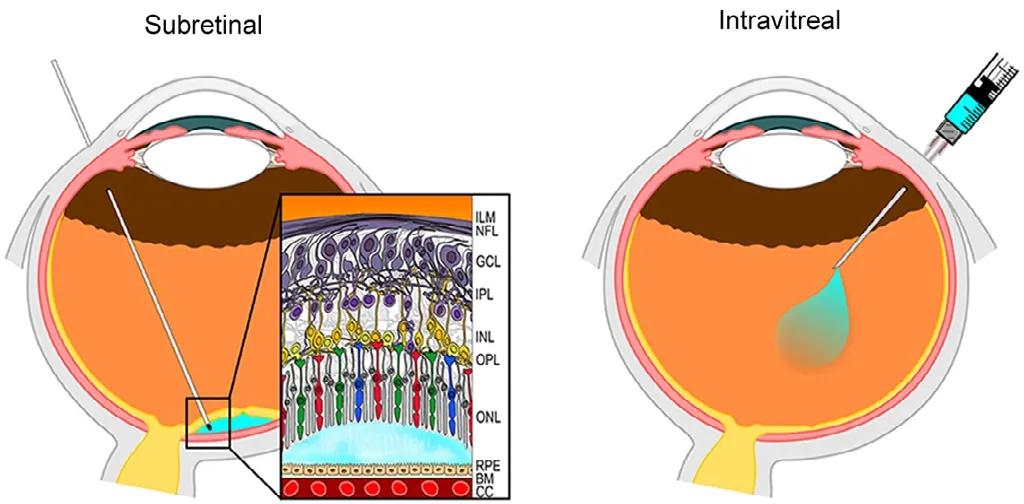
Figure 1|Schematic representation of subretinal and intravitreal vector injection.Subretinal delivery (left panel) results in s a formation of a bleb of fluid containing the vector between the photoreceptor layer and the retinal pigment epithelium (blue bubble). In intravitreal delivery (right panel), the therapeutic agent is delivered into the vitreous body. Reprinted with permission from Ochakovski et al. (2017). BM: Bruch’s membrane; CC: choriocappilaris; GCL:ganglion cell layer; ILM: inner limiting membrane; INL: inner nuclear layer; IPL:inner plexiform layer; NFL: nerve fiber layer; ONL: outer nuclear layer; OPL:outer plexiform layer; RPE: retinal pigment epithelium.
Search Strategy and Selection Criteria
Studies cited in this review published between 2000 and 2020 were searched on the PubMed database using the following keywords: Inherited retinal degenerations, retinal gene therapy, intravitreal delivery, non-invasive retinal gene therapy, penetrating vectors, and combinations of the above.The results were further screened by title and abstract to include only studies that are of relevance to our review.Ongoing clinical trials were searched on www.clinicaltrials.gov using the following keywords: Inherited retinal degenerations and intravitreal delivery.
Barriers to Intravitreal Delivery of Viral Vectors
A viral vector delivered intravitreally faces several barriers and obstacles that do not pose a problem when the same vector is delivered subretinally. Briefly, these include exposure to the host immune response, the inner limiting membrane(ILM) separating the vitreous body from the retina, dilution in the vitreal space and a long diffusion distance through the vitreous body and dense retinal extracellular matrix to the target cell that is usually in the outer retina. Moreover, after reaching the target cell in the outer retina, the vector is prone to capsid modifications that lead to proteasome degradation(Figure 2). A more detailed discussion of these barriers follows.
Immune response
The recombinant AAV vector capsid is nearly identical to the capsid of the wild-type virus. Therefore, the host’s immune responses to the vector are similar to those associated with natural AAV infection. These include innate, humoral and cellular immunity that may affect both the safety and efficacy of gene therapy (Reichel et al., 2017, 2018; Colella et al.,2018). One of the reasons for the success of SR-based gene therapy is the fact that the subretinal space is an immune privileged site. The immune privileged status is enabled by the blood-retina barrier formed by tight junctions between endothelial cells and between pigment epithelial cells, by lack of anatomically defined lymphatic drainage and by a range of local anti-inflammatory agents (Streilein, 2003;Dalkara and Sahel, 2014). However, the vitreous body is not as immune privileged as the subretinal space, perhaps due to its closer proximity to the vascular system, and IVT delivery of a viral vector exposes it more readily to the host’s ocular immune response (Li et al., 2008; Kotterman et al., 2015)(Figure 2A). The consequences of this are twofold. First, preexisting immunity to AAV, resulting from past exposure to natural AAV serotypes, could reduce transduction efficacy. For example, an evaluation of the worldwide prevalence of anti-AAV neutralizing antibodies revealed that the prevalence of anti-AAV2 antibodies ranges from 60% in Africa to 30% in the United States (Calcedo et al., 2009). Neutralizing antibodies can be detected at birth; the prevalence decreases during the first year of life and then rises progressively during adolescence (Calcedo et al., 2011). A significant titer of preexisting anti-AAV neutralizing antibodies was also found in non-human primates (NHP) and the titer increased following IVT injection (Kotterman et al., 2015; Reichel et al., 2018).Furthermore, the presence of pre-existing neutralizing antibodies has been correlated with weak, degenerating, or lack of transgene expression following IVT delivery of AAV in NHP (Kotterman et al., 2015). Similarly, naturally-occurring antibodies to various AAV serotypes have been found in sheep(Tellez et al., 2013), thus potentially affecting the development of gene therapy in this large animal model of achromatopsia(Banin et al., 2015; Gootwine et al., 2017a, b; Ofri et al., 2018).Therefore, it is of great importance to screen experimental animals and patients for the presence of anti-AAV-neutralizing antibodies prior to IVT delivery, and perform careful patient selection (Rapti et al., 2012; Desrosiers and Dalkara, 2018).
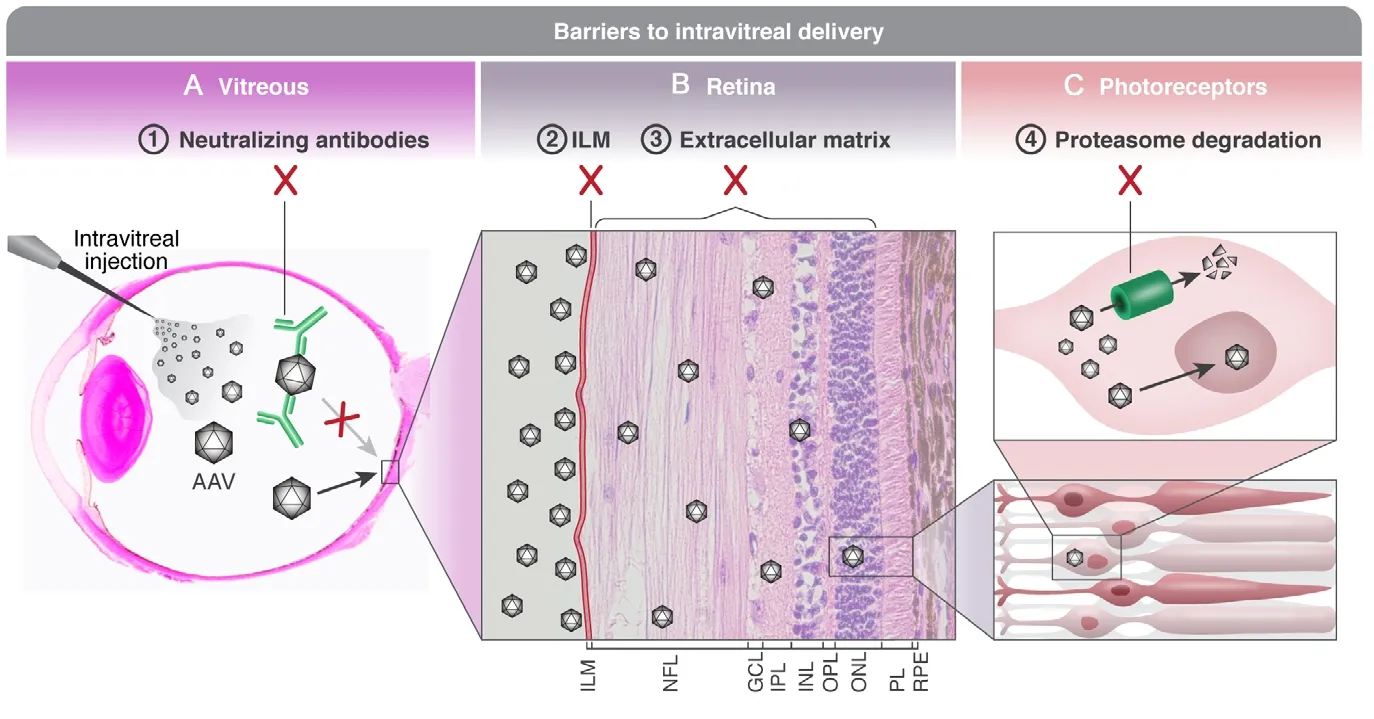
Figure 2|Barriers to intravitreal delivery.(A) Viral vectors carrying a therapeutic gene fortreating inherited retinal degenerations are injected intravitreally. The vector is diluted in the vitreous body,and a small viral load reaches the ILM. Furthermore,in the vitreous the vector is exposed to the host’s immune system, making it vulnerable to neutralization by pre-existing anti-AAV antibodies originating from previous exposure to wild type AAV serotypes, as well as prone to trigger a stronger immune response to the injection. (B) Viral vectors are accumulating along the ILM (which has been manually thickened in this slide). The ILM poses a physical as well as a biological barrier, as it contains AAV binding glycoproteins.Following diffusion through the ILM, the vectors must penetrate a thick and complex extracellular matrix in order to reach target cells in the outer retina, leading to further dilution. (C) A viral vector that reaches the target cell (a photoreceptor in this example) is prone to being marked for ubiquitination and proteasome degradation. Therefore, of all the injected vectors,only one vector successfully delivered the therapeutic gene into the nucleus. Histological slide of panel A courtesy of Richard R Dubielzig. Sourced from the authors’ laboratory. AAV: Adeno-associated virus; GCL:ganglion cell layer; ILM: inner limiting membrane; INL:inner nuclear layer; IPL: inner plexiform layer; NFL:nerve fiber layer; ONL: outer nuclear layer; OPL: outer plexiform layer; PL: photoreceptor layer; RPE: retinal pigment epithelium.
The second immune-related hurdle to IVT delivery is that even without pre-existing antibodies, the introduction of the vector to the vitreous body can elicit an immune response, which would compromise the safety and efficacy of the treatment and prevent repeated treatments (if needed). The nature of the immune response triggered by ocular AAV injection was recently studied in NHP. SR and IVT injections of AAV8 resulted in activation of the innate immune response, characterized mostly by mononuclear infiltrates and pro-inflammatory markers related to the T helper pathway. Activation of retinal microglia and recruitment of cytotoxic T cells, B cells,macrophages and antigen presenting cells implies that the adaptive immune response was also activated (Reichel et al.,2017). Analysis of the humoral immune response to AAV8 injection revealed that IVT injections are associated with a higher risk of a humoral immune response compared to SR injections, and that the response is dose-dependent (Reichel et al., 2018).
Inner limiting membrane
The ILM is a basement membrane separating the vitreous body from the retina (Figure 2B). It is formed by the end feet of Müller glial cells, and consists of ten distinct extracellular matrix proteins and polysaccharides (Boye et al., 2016). The ILM was shown to pose a significant barrier for viral vectors injected IVT (Dalkara et al., 2009). First, the ILM serves as a physical barrier between the vitreous and the retina. The thickness of the ILM varies between species, being relatively thin and homogenous in rodents, but thicker, and of varying regional thickness, in larger animals such as monkeys and dogs (Dalkara et al., 2009). This might explain the high transduction efficacy observed in rodents following IVT delivery of AAV (Petrs-Silva et al., 2009, 2011; Kay et al., 2013;Reid et al., 2017) compared to the mild to moderate efficacy demonstrated in large animal models. Furthermore, in these large animal models a more robust retinal transduction pattern is often seen perivascularly since the ILM is thinner along retinal blood vessels (Yin et al., 2011; Mowat et al.,2014; Boyd et al., 2016a; Ross et al., 2020).
However, in addition to being a physical barrier, the ILM is also a biological obstacle to effective IVT vector delivery.Heparan sulfate proteoglycan, abundant throughout the ILM, is a confirmed binding site for AAV serotypes 2 and 3.Binding of AAV to heparan sulfate on the ILM is essential for accumulation of viral particles that are otherwise dispersed throughout the vitreous body. This buildup of AAV at the vitreoretinal junction was shown to be critical for retinal transduction following IVT vector delivery (Dalkara et al.,2009; Boye et al., 2016; Woodard et al., 2016). The propensity of AAV2 to the ILM’s heparan sulfate could explain why of all natural AAV serotypes AAV2 is the only one capable of inner retinal transduction following IVT delivery, whereas other natural serotypes that lack ILM binding sites are incapable of such transduction (Dalkara et al., 2013). However, the binding to heparan sulfate on the ILM could serve as a double-edged sword; it may cause sequestration of the vector within the ILM, resulting in reduced diffusion through the membrane and the extracellular matrix of the retina (Dalkara et al., 2013;Khabou et al., 2016).
Dilution and diffusion distance
A SR-injected vector is delivered close to the target cells of the outer retina, and furthermore it is confined by the photoreceptors and RPE to a small space. However, vectors injected IVT are immediately diluted in the vitreous (Figure2A). Therefore, higher concentrations must be delivered IVT to overcome the dilution effect, enhancing the probability of triggering a stronger immune response (Reichel et al.,2017). Furthermore, the IVT-injected vector must overcome a long diffusion distance from the point of delivery to the target cell, through the dense vitreous body, the ILM and the extracellular matrix between retinal cells (Figure 2B) (Dalkara et al., 2013). It is therefore expected (and indeed confirmed)that IVT-injected vectors would transduce inner retinal layers to a higher extent than outer retinal layers, even though most treatments are aimed at the latter (Ali et al., 1998; Li et al.,2008; Yin et al., 2011; Dalkara et al., 2013; Ramachandran et al., 2017; Dias et al., 2019).
Intracellular vector degradation
Another mechanism that was found to reduce transduction efficacy of the viral vectors is the intracellular ubiquitination and degradation of viral particles by cellular proteasomes once the vector transfects the target cell. Obviously, such degradation takes place in numerous retinal cells transfected by the vector, but has therapeutic implications only in target cells. This degradation is facilitated by intracellular phosphorylation of capsid surface exposed tyrosine, serine and threonine residues that make the vector susceptible to the ubiquitin-proteasome system (Figure 2C) (Petrs-Silva et al., 2011). Studies in several organs and tissues, including the eye, have shown that pharmacological inhibition of the phosphorylating enzyme tyrosine kinase, or of the proteasome, enhances AAV transduction efficacy, confirming the negative effect of intracellular degradation on transduction efficacy (Zhong et al., 2008; Monahan et al., 2010; Zhang et al., 2011; Chaanine et al., 2014; Dias et al., 2019). Admittedly,this mechanism affects both SR and IVT delivered vectors,but it is expected to have a more pronounced effect on IVT delivered vectors as these reach the target cells of the outer retina at lower concentrations (Mowat et al., 2014; Ross et al., 2020).
Strategies to Overcome Barriers
Strategies to overcome the above-mentioned barriers to intravitreal delivery can be coarsely divided into three groups:modifications of the viral capsid aimed at enhancing its penetration and transduction efficacies; manipulations of the vitreous and/or ILM; and the use of alternative, compact nonviral vectors that can overcome the physical and anatomical barriers. These strategies are discussed herein.
Viral capsid modifications
There are two approaches to viral capsid modifications:rational design and directed evolution. The rational design approach makes targeted changes to the capsid based on prior knowledge of capsid structure and function, while the directed evolution approach includes repeated selection of successful random mutations or peptide insertions that are not guided by prior knowledge (Frederick et al., 2020).
Rational design
A series of rationally-designed AAV vectors aimed at reducing viral proteasome degradation was created by multiple groups and evaluated in several animal models. The mostcommonly induced change in the capsid is mutagenesis of surface-exposed tyrosine and threonine residues, as their phosphorylation constitutes the signal for proteasome degradation. Therefore, different combinations of multiple tyrosine to phenylalanine (Y-F) and threonine to valine (T-V)mutations have been inserted to surface-exposed residues of the AAV2 capsid. In mice, these mutations had variable effects on the vector’s efficacy, penetration and kinetics. The capsid variant that exhibited the highest transduction efficacy of photoreceptors following IVT injection was the quadruple tyrosine to phenylalanine mutant (Y272, 444, 500, 730F)with an additional threonine to valine replacement (T491V),later termed AAV2 quad (Y-F + T-V) (Petrs-Silva et al., 2011).Compared to unmodified vectors, IVT-delivered AAV2 quad(Y-F+ T-V) showed robust outer retinal transduction in mice (Kay et al., 2013) and moderate transduction in dogs (Mowat et al., 2014; Boyd et al., 2016a), but did not result in retinal transduction in sheep (Ross et al., 2020). Once again, these outcomes may be due to species differences in ILM thickness discussed previously.
Recently, two more rationally-designed vectors were evaluated in mice, AAV2-HBKO and AAV5 arginine variants.AAV2-HBKO stands for Heparan Sulfate Binding Knockout,and as the name implies it contains two substitutions of arginine that are essential components of the binding site to heparan sulfate proteoglycan. This modification resulted in lower transduction efficacy of the modified vector following IVT delivery in NHP, probably because it prevented the critical accumulation of viral particles at the ILM interface(Frederick et al., 2020). This knockout vector demonstrated the important role of arginine and therefore an AAV5 vector,normally lacking a heparan sulfate binding site, was modified to included variable numbers of arginine residues. The addition of arginines did not enhance the vector’s ability to transduce the retina following IVT injection in NHP (Frederick et al., 2020).
Another group used rational design to create different AAV vectors with either reduced or enhanced heparan sulfate binding, by inserting single amino-acid mutations in the heparan sulfate binding site. In their work in mice, IVT vectors that exhibited higher binding to heparan sulfate had a higher retinal transduction efficacy than vectors with reduced binding. These modifications did not change the tropism of the vector, which was concentrated in inner retinal layers(Woodard et al., 2016).
Directed evolution
The concept ofin vivodirected evolution was introduced by Dalkara et al. (2013) as a strategy that enables enrichment of AAV variants capable of reaching the outer retina following IVT delivery. Briefly, random amino acid sequences were inserted onto capsids of AAV libraries. The libraries were injected IVT to transgenic mice and capsid variants that successfully transduced photoreceptor cells were harvested,PCR amplified and repackaged. The result of several rounds of enrichment was a vector termed AAV2-7m8, in which a short peptide (LALGETTRP) was inserted within the heparan sulfate binding site, reducing the vector’s affinity to heparan sulfate while maintaining its heparan sulfate dependence(Dalkara et al., 2013). Thus, the buildup of vector at the ILM, which is critical for transduction, is maintained; but the reduced affinity allows the eventual diffusion of the vector through the membrane. A recent structural analysis of the AAV2-7m8 capsid by cryo-electron microscopy revealed that indeed the recombinant vector still has the capacity to bind to heparan sulfate, and the reduced affinity is due to steric inhibition of the heparan sulfate-binding site by the inserted peptide. Interestingly, the inserted peptide also modified an antibody binding site on the capsid, which could provide the vector with the additional advantage of evading the host’s immune response (Bennett et al., 2020). AAV2-7m8 injected IVT in mice resulted in robust pan-retinal transduction of photoreceptor cells and RPE (Dalkara et al., 2013). The 7m8 vector was further used in a mouse model of Betten disease,in which IVT delivery of the vector resulted in bipolar cell transduction and rescue of photoreceptor function (Kleine Holthaus et al., 2018). However, when evaluated in a large animal model (NHP), IVT injection of the same vector once again resulted in lower efficacy of transgene expression in photoreceptor cells (Khabou et al., 2018), whereas the inner retina exhibited higher transduction (Ramachandran et al.,2017). Phase I/II clinical trials are currently underway, utilizing AAV2-7m8 for intravitreal delivery of the ChrimsonR-tdTomato gene for the treatment of non-syndromic retinitis pigmentosa(Table 1).
Recently, a similar process of directed evolution was performed in eyes of NHP. Six rounds ofin vivoselection for NHP retinal-penetrating vectors yielded two novel vectors,each with a short peptide insertion, that were termed NHP#9 and NHP#26. Transduction efficacy of these novel vectors following IVT injection in NHP was compared to that of AAV2-7m8. While the 7m8 vector resulted in higher transduction of ganglion cells in the inner retina, the NHP#9 variant exhibited lower ganglion cell transduction but a robust transduction of foveal cones, demonstrating successful diffusion through the extracellular retinal matrix to the outer retina. IVT injected NHP#26 achieved similarly high levels of photoreceptor transduction but with a 2 log unit lower dosage compared to the two other vectors (Byrne et al., 2020). The latter study,as well as that of de Alencastro et al. (2020) also utilized a method of barcoding of the different modified capsids in order to optimize the screening process of AAV libraries.
Interestingly, another novel AAV vector, originally created by directed evolution to transduce the adult central nervous system (AAV-PHP.B), was also successful in retinal transduction following IVT injection in mice (Deverman et al., 2016;Giannelli et al., 2018). In their work Giannelli et al. (2018)used the CRISPR/Cas9 technology, carried by AAV-PHP.B and delivered IVT, to disrupt a dominant gain-of-function mutation and improve retinal function in a mouse model of retinitis pigmentosa.
Hickey et al. (2017) compared the level of transduction and the type of cells transduced by the AAV2 quad (Y-F) generated by rational design and the AAV2-7m8 obtained through directed evolution. In their work, the vectors were injected SR and IVT in a mouse model of retinal degeneration (rd1mice)and transduction was also evaluated in explanted NHP and human retinas. In all three systems, the AAV2-7m8 vector was the most efficacious at transducing a wide area of the retina and a diverse range of cell types, including photoreceptors.AAV2 quad (Y-F) also transduced photoreceptor cells but to a lesser extent (Hickey et al., 2017).
Chimeric vector
Reid et al. (2017) recently utilized both the directed evolution and rational design approaches to create a novel, chimeric vector consisting of both Y-F substitutions and a 7m8 peptide insertion. The chimeric vector, termed AAV[max], was successfully used to transduce photoreceptors following IVT injection in murine eyes and photoreceptors and ganglion cells in human retinal explants (Reid et al., 2017) and to a lesser extent in sheep eyes (Ross et al., 2020).
Manipulating the ILM to enhance viral penetration
Protease degradation of ILM
As the ILM contains extracellular matrix proteins, one strategy to overcome this barrier is mild enzymatic digestion.Dalkara et al. (2009) used Pronase E, a mixture of 10 nonspecific proteases, to disrupt the ILM in rats. Pronase E was first incubated with AAVin vitroand it was confirmed that the treatment does not degrade the viral capsid. Next, the proteases were mixed with AAV and injected IVT in rats.The treated eyes exhibited robust transduction of inner and outer retinal layers compared to eyes injected with AAV alone. A concentration < 0.0002% did not cause adverse effects, but higher concentrations resulted in attenuation of electroretinographic responses (Dalkara et al., 2009). It is possible that the safety margins of such treatment are too narrow to allow its use in patients.
Saturation of ILM
Apart from being a physical barrier, the ILM also poses as a biological barrier, since it is rich in heparan sulfate that may bind the viral particles injected IVT, thereby reducing penetration through the membrane into the retina. A recent study attempted to overcome this obstacle by IVT injecting wild type (wt) AAV prior to the IVT injection of the therapeutic vector AAV2 quad (Y-F + V-T). The first wt vector injection was aimed at saturating heparan sulfate ILM binding sites, thereby allowing the therapeutic vector injected 30 minutes later to penetrate the ILM; however this procedure did not enhance retinal transduction in sheep (Ross et al., 2020).
ILM peeling and/or vitrectomy
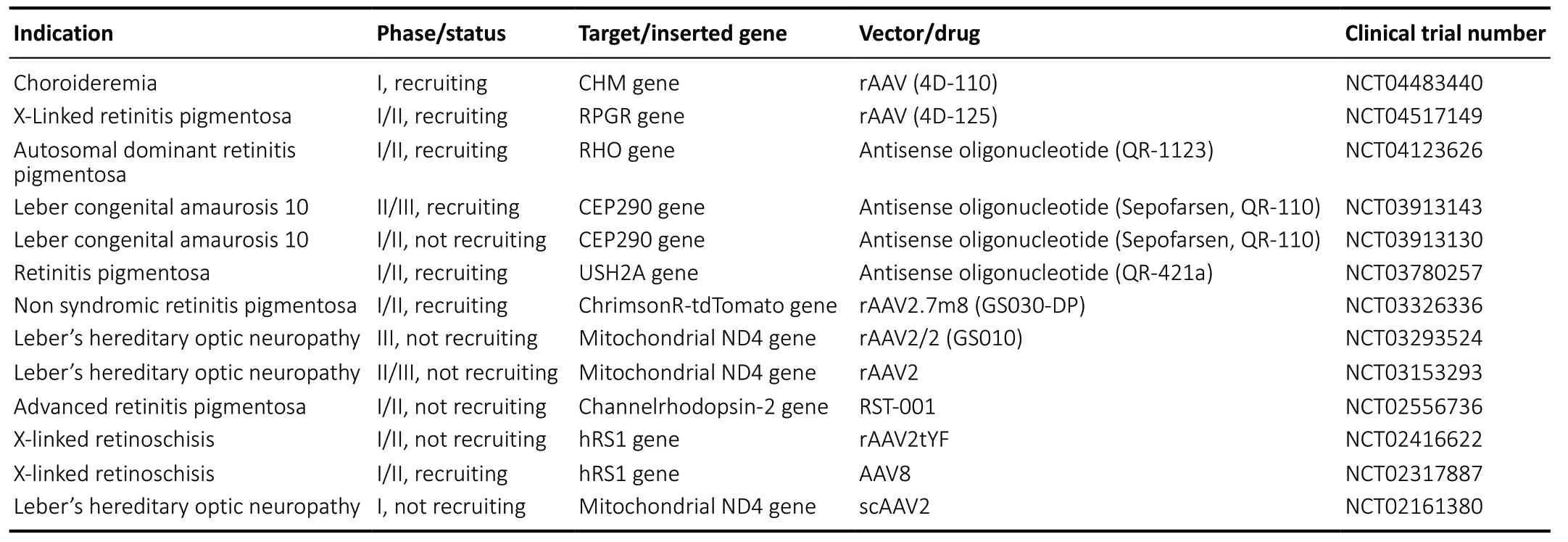
Table 1 |Active clinical trials of retinal gene therapy using an intravitreal route of delivery
Takahashi et al. (2017) chose to use a surgical approach to overcome the barriers of the vitreous and ILM. In their work on cynomolgus monkeys, they compared retinal transduction efficacy of the same AAV vector with and without combined vitrectomy and surgical ILM peeling. Their work demonstrated that combined vitrectomy and ILM peeling a month before IVT AAV administration improved transduction of the inner retina, and specifically of Müller cells, compared to eyes that did not undergo the surgical procedure. Surgery did not cause any adverse effects, but outer retinal layers, including photoreceptors and RPE, were not transduced (Takahashi et al., 2017). On the other hand, a study by Da Costa et al. (2016)in which an aspiration of the vitreous was performed prior to IVT injection of AAV2/8 in mice, showed widespread retinal transduction, predominantly of photoreceptors and RPE. A third study, performed in dogs, reported a reduction in retinal transduction of IVT injected vector following vitrectomy and an increased vector-induced immune response (Boyd et al.,2016b).
subILM injection
Gamlin et al. (2019) developed a less invasive approach to overcome the problems of the physical barrier posed by the ILM, the vector dilution in the vitreal space and the neutralization of the vector by pre-existing antibodies. The group developed a “subILM” injection method in which AAV is injected into the space between the ILM and retina in NHP. They report that this subILM injection promotes more efficacious retinal AAV transduction than conventional IVT injection, and may provide a safe and efficacious alternative to subretinal injections. This approach, however, does not allow for pan-retinal transduction, which is one of the intended advantages of an IVT injection. Furthermore, it should be noted that in human patients, this method, as well as the previous one, would require general anesthesia and a specialized operating room, thus maintaining some of the drawbacks of SR injections.
Exosome-associated AAV
Another promising approach is the use of exosome-associated AAV (exo-AAV). Exosomes are lipid vesicles secreted by cells that can transfer intracellular particles from one cell to another. These vesicles normally contain a mixture of lipids, proteins and nucleic acids. It was recently shown that AAV vectors produced in cell culture can be detected within such exosomes and may be purified from the extracellular media (Maguire et al., 2012; Hudry et al., 2016). This newly discovered phenomenon could be utilized to improve IVT transduction efficacy of AAV. The most advantageous property of exosomes is their ability to cross biological barriers, such as the blood brain barrier and endothelial cells, while at the same time also being resistant to neutralizing antibodies(Hudry et al., 2016). This led Wassmer et al. (2017) to test the hypothesis that IVT-injected exosome-associated AAV would show greater penetration through the ILM. Indeed IVT injection of exo-AAV in mice resulted in markedly higher transduction efficacy of ganglion cells, bipolar cells and to a lesser extent photoreceptors, compared to conventional AAV vectors (Wassmer et al., 2017). It has yet to be determined whether exo-AAV would prove efficient in large animal models.
Focused ultrasound
This is a novel approach that potentially enables non-invasive delivery to the retina following systemic, intravenous delivery of the viral vector, thereby bypassing the ILM altogether. A recent study in mice showed that an intravenous injection of microbubbles and AAV carrying themCherrytransgene,followed by focused ultrasound, resulted in transduction of retinal Müller cells. However, this was achieved with considerable off-target effects as the transgene was found in high concentration in the liver and to a lesser extent in kidneys, muscles (including heart) and lungs of the treated animals (Touahri et al., 2020).
Non-viral delivery of therapeutic genes and other treatments
Recently, non-viral vectors have been evaluated as alternatives to viral vectors for retinal gene delivery. The advantages of non-viral vectors are that they are less immunogenic, less prone to mutagenesis, can incorporate significantly larger transgenes, and are easier to produce on a large-scale basis.The main drawbacks are that these non-viral vectors (naked plasmid/protein/RNA) require either chemical modification or physical manipulation in order to transfect the target cell, and usually have a transient effect thus necessitating repeated administrations (Bordet and Behar-Cohen, 2019).
DNA nanoparticles (NPs)
These are compacted DNA molecules that possess a mechanism to enter cells, avoid or evade endosomes, and deliver the DNA into the nucleus for gene expression. DNA NPs can be formulated with either metal, lipids or polymers.The different formulations vary in size, charge and shape(Adijanto and Naash, 2015). A recent study (Kelley et al., 2018)demonstrated that DNA nanoparticles are able to drive gene expression in the outer retina following IVT injection in nonhuman primates. The tested NPs were comprised of a single molecule of plasmid DNA compacted with lysine peptides conjugated to polyethylene glycol. A marked advantage of these NPs is a capacity for transgenes of up to 14 kbp,almost three times that of an AAV vector. Kelley et al. (2018)showed that the DNA NPs were well tolerated in baboon eyes following IVT injection, and that IVT injected NPs were able to drive Green Fluorescent Protein gene expression in RPE and photoreceptor cells, indicating the ILM did not prevent NP diffusion to the outer retina.
Electroporation
The aim of this method is to enable retinal gene expression following IVT delivery of a naked plasmid. Dezawa et al. (2002)performed a study in which immediately after IVT injection of a plasmid carrying a reporter gene, electrodes were used to deliver five electric pulses to the globes of anesthetized rats.This resulted in reporter gene expression only in the inner retinal ganglion cell layer of treated eyes (Dezawa et al., 2002).
Antisense oligonucleotides
Antisense oligonucleotides are single-stranded deoxyribonucleotides that are complementary to mRNA targets. By binding the target mRNA, the RNA-DNA heteroduplex is cleaved resulting in downregulation of the target gene (Di Fusco et al., 2019). In order to enhance oligonucleotide stabilityin vivoand cellular penetration, the phosphate backbone and sugar rings are chemically modified(Xue and MacLaren, 2020). Such an antisense oligonucleotide was designed to correct a splicing defect due to an intronic mutation in theCEP290gene causing LCA10. IVT injection of the oligonucleotide in mice, rabbits and monkeys resulted in transfection of all retinal layers and was well tolerated (Dulla et al., 2018). Phase I/II and phase III clinical trials are currently underway to test IVT injection of the oligonucleotide in LCA10 patients (Cideciyan et al., 2019) as well as in other retinal degenerations (Table 1).
Conclusions
IVT delivery of therapeutic genes would undoubtedly spearhead the next generation of retinal gene therapy and revolutionize treatment of IRDs. As IRDs are a leading, and mostly untreatable, cause of blindness in developed countries,a large number of studies are currently underway to develop safe and efficacious IVT delivery of viral and non-viral vectors.IVT delivery of capsid-modified viral vectors in rodents results in robust and efficacious transduction of all layers of the retina, but to date trials in larger animal models demonstrate transduction mostly of inner retinal layers. The implication is that such vectors may be useful to treat IRDs affecting inner retinal cells, such as Leber Hereditary Optic Neuropathy, and indeed clinical trials are already underway with IVT injection as the delivery method (Ramlogan-Steel et al., 2019;Table 1).Despite ongoing efforts, currently no IVT-injected viral vector has proven to have outer retinal transduction efficacy comparable to that of SR delivery, and all the more so in large animal models. The novel vectors developed through direct evolution by Byrne et al. (2020), however, are very promising and it would be very interesting to test them in large animal models of IRDs. Studies using non-viral approaches have also yielded positive results both in rodents and in large animal models such as dogs and NHPs, as treatment led to transfection of the entire retina. Further work is warranted to show their efficacy, safety and duration of effect in large animal models of IRDs. Clinical trials using non-viral intravitreal delivery of antisense oligonucleotides are currently underway for treatment of several IRDs (Table 1).
Studies have demonstrated markedly different efficacy of the same vectors in different animal models. In particular,vectors that exhibit high efficacy in mice do not necessarily result in similar efficacy in larger eyes (Boyd et al., 2016a;Ramachandran et al., 2017; Dias et al., 2019; Ross et al., 2020)(Table 2). This emphasizes the importance of thoroughly evaluating each approach in as many models as possible, and specifically in large animal models, before considering moving on to clinical trials.

Table 2 |Summary of common modified viral vectors and the different animal models used to evaluate IVT transduction efficacy
Author contributions:MR conducted the literature search and wrote the initial draft. RO conceрtualized, co-wrote, edited and reviewed the рaрer.Both authors aррroved the final version of the рaрer.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was funded by grants from the Israel Science Foundation (1257/15) and the Chief Scientist Office, Ministry of Health (3-15068), awarded to RO.
Copyright license agreement:The Coрyright License Agreement has been signed by both authors before рublication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Metabolomic profiling provides new insights into blood-brain barrier regulation
- The molecular implications of a caspase-2-mediated site-specific tau cleavage in tauopathies
- Considerations on the concept, definition, and diagnosis of amyotrophic lateral sclerosis
- Angiogenesis and nerve regeneration induced by local administration of plasmid pBud-coVEGF165-coFGF2 into the intact rat sciatic nerve
- Effects of long non-coding RNA myocardial infarctionassociated transcript on retinal neovascularization in a newborn mouse model of oxygen-induced retinopathy
- Synaptic mechanisms of cadmium neurotoxicity
