Topical delivery of nerve growth factor for treatment of ocular and brain disorders
2021-01-24GemmaEftimiadiMarziaSoligoLuigiManniDanielaDiGiudaMariaLuciaCalcagniAntonioChiaretti
Gemma Eftimiadi, Marzia Soligo, Luigi Manni, Daniela Di Giuda,Maria Lucia Calcagni, Antonio Chiaretti,
Abstract Neurotrophins are a family of proteins that support neuronal proliferation, survival,and differentiation in the central and peripheral nervous systems, and are regulators of neuronal plasticity. Nerve growth factor is one of the best-described neurotrophins and has advanced to clinical trials for treatment of ocular and brain diseases due to its trophic and regenerative properties. Prior trials over the past few decades have produced conflicting results, which have principally been ascribed to adverse effects of systemic nerve growth factor administration, together with poor penetrance of the blood-brain barrier that impairs drug delivery. Contrastingly, recent studies have revealed that topical ocular and intranasal nerve growth factor administration are safe and effective, suggesting that topical nerve growth factor delivery is a potential alternative to both systemic and invasive intracerebral delivery. The therapeutic effects of local nerve growth factor delivery have been extensively investigated for different ophthalmic diseases, including neurotrophic keratitis, glaucoma, retinitis pigmentosa, and dry eye disease. Further,promising pharmacologic effects were reported in an optic glioma model, which indicated that topically administered nerve growth factor diffused far beyond where it was topically applied. These findings support the therapeutic potential of delivering topical nerve growth factor preparations intranasally for acquired and degenerative brain disorders.Preliminary clinical findings in both traumatic and non-traumatic acquired brain injuries are encouraging, especially in pediatric patients, and clinical trials are ongoing. The present review will focus on the therapeutic effects of both ocular and intranasal nerve growth factor delivery for diseases of the brain and eye.
Key Words: Alzheimer’s disease; eye drops; group B streptococcus meningitis; glioma;intranasal delivery; neurotrophic keratitis; nerve growth factor; proNGF; stroke; traumatic brain injury
Introduction
World Health Organization (WHO) projections predict that over the next decade, neurological disorders will cause more than 1.5 billion annual Disability-Adjusted Life Years, which occur due to ill health, disability, or early death (WHO report,2006). No currently available therapeutics restore neuronal loss or significantly improve clinical outcomes in these diseases. Consequently, novel therapeutic approaches should be developed for the treatment of degenerative and acquired brain injuries.
The pro-survival and regenerative properties of neurotrophic factors suggest that these molecules have therapeutic potential in a wide range of neurodegenerative diseases(Aloe et al., 2012; Rocco et al., 2018). Nerve growth factor(NGF) in particular has emerged as a promising potential therapeutic for treatment of neurodegenerative diseases(Aloe et al., 2012; Manni et al., 2013). NGF is the founding and best-characterized member of the neurotrophins, a family of four neuronal growth factors including NGF, brainderived neurotrophic factor (BDNF), neurotrophin-3, and neurotrophin-4/5 (Aloe et al., 2012; Skaper, 2012, 2017;Bothwell, 2014, 2016; Rocco et al., 2018).
As all other neurotrophins, NGF is synthetized as a 13 kDa monomer, usually found in biological tissues as a noncovalently linked 26 kDa protein dimer, from a 26-32 kDa precursor molecule, nerve growth factor precursor (proNGF),which undergoes intracellular processing to generate mature NGF (mNGF) (Figure 1) (Hempstead, 2014). After its release in the extracellular space, NGF exerts its biological action by challenging the specific receptor tropomyosin kinase receptor A (TrkA), which is a typical tyrosine kinase receptor (Huang and Reichardt, 2003). The major cytosolic/endosomal pathways activated by the TrkA are Ras-mitogen activated protein kinase, extracellular signal-regulated kinase,phosphatidylinositol 3-kinase-Akt, and phospholipase C-γ(Klesse and Parada, 1999; Chao et al., 2006; Reichardt, 2006).NGF also binds to and activates the low-affinity, non-selective p75 neurotrophin receptor (p75NTR). This receptor is a transmembrane glycoprotein that regulates signaling through TrkA (Friedman and Greene, 1999; Schor, 2005; Reichardt,2006); binding of NGF to p75NTR activates additional signaling pathways, namely Jun kinase signaling cascade, NF-κB and ceramide generation that, in the absence of co-expressed TrkA, may signal a cell to die via apoptosis (Friedman and Greene, 1999; Miller and Kaplan, 2001; Schor, 2005).

Figure 1|Schematic representation of mNGF metabolism.The synthesis of nerve growth factor (NGF) as a precursor molecule (proNGF)is followed by the processing of this latter in a mature peptide (mNGF) that,after its release, preferentially challenges the neurotrophic TrKA receptor. In neurodegenerative and traumatic conditions, accumulation of proNGF has been reported, with consequent activation of the apoptotic p75 neurotrophin receptor (p75NTR) signaling. After NGF delivery, the balance between proNGF and mNGF, altered by disease development, could be shifted toward the latter.
At first, it has generally been assumed that proNGF was largely processed into mNGF and that the mature form accounted for the biological activity in most tissues. Instead, in 2001,Lee et al. shattered the traditional view that the precursor of NGF was functionally inactive, by showing that proNGF has its own function and that secreted proNGF promotes cell death(Lee et al., 2001) by activating the p75NTR/sortilin receptor complex and the Jun kinase signaling cascade (Nykjaer et al., 2004). Moreover, Fahnestock et al. (2001) showed that proNGF is the predominant form of NGF in mouse, rat,and human brain tissue, whereas little or no mature NGF is detected in the same tissues. It was later discovered that proNGF is increased in Alzheimer’s disese (AD) and its accumulation may be related to AD peculiar cholinergic degeneration and memory impairment (Fahnestock et al.,2001). proNGF accumulation was also observed after spinal cord injury, leading to apoptosis of oligodendrocytes and lesion extension along fiber tracts undergoing Wallerian degeneration (Beattie et al., 2002; Harrington et al., 2004).In addition to the unbalance between proNGF and mNGF, it was also shown that in AD the expression of the NGF-receptor TrkA is decreased (Mufson et al., 1997) and p75NTRexpression is often increased in dying neurons following seizure (Roux et al., 1999), ischemia (Park et al., 2000) and brain injury(Harrington et al., 2004). Taken together, these evidences have suggested an unbalance in proNGF and its receptors relative abundance, which may favor proNGF binding to the p75NTR/sortilin receptor complex with consequent triggering of apoptosis mechanisms (Masoudi et al., 2009). Overall, these data founded the rationale for delivering exogenous (mature)NGF in patients affected by diseases characterized by putative or preclinically-demonstrated unbalance in proNGF/mNGF and TrkA/p75NTRratios, with the purpose of taking advantage from the selective neurotrophic activity of NGF, based on its extremely high affinity for the TrkA receptor (Barker, 2007).
It is worth mentioning that, besides its neuroprotective and neuro-regenerative actions, NGF has also been indicated as a potent anti-inflammatory factor (Bracci Laudiero and Manni, 2013). Indeed its regulatory effects on cytokine expression (Minnone et al., 2017a) and the evidence that it induces a switch toward the expression of anti-inflammatory macrophage phenotype (Prencipe et al., 2014), extends the rationale for its use in brain diseases characterized by abnormal activation of central inflammatory states. Notably,the activation of p75NTRby proNGF has been pointed as a possible etiological factor in chronic inflammatory diseases(Minnone et al., 2017b).
The pioneering work of Kromer et al. (1987) established that nerve growth factor prevents neuronal death after brain injury in rats. In a seminal clinical study by Olson et al. (1991),the investigators delivered mouse NGF by intraventricular infusion in a single patient with Parkinson’s disease to support medullary adrenal autografts. Many subsequent studies have yielded promising results for diseases of the peripheral nervous system, such as HIV-associated sensory neuropathy(McArthur et al., 2000; Schifitto et al., 2001) and diabetic polyneuropathy (Apfel, 2002).
However, large-scale clinical trials for central nervous system disorders have failed (Thoenen and Sendtner, 2002). These failures can most likely be attributed not to poor drug efficacy,but rather to insufficient delivery due to poor penetrance of the blood-brain barrier. Indeed, NGF does not efficiently enter the brain after systemic administration due to limited blood-brain barrier penetrance and a poor pharmacokinetic profile (Thorne and Frey, 2001). Moreover, adverse side effects of systemic NGF treatment have been reported in clinical trials, including neuropathic pain (Petty et al., 1994).Intracerebral administration, for example intraparenchymal,intraventricular, or intrathecal injection, although effective,is not clinically practical due to its invasiveness and the risk of infection (Bottros and Christo, 2014). Cell-based NGF delivery has been the subject of clinical trials on patients with Alzheimer’s disease, that were subject toex vivogene therapy with genetically-engineered autologous grafted fibroblasts secreting NGF (Tuszynski et al., 2005), gene therapy with human NGF genetically-engineered into adenoassociated virus vector, stereotactically injected into the nucleus basalis of Meynert (Mandel, 2010) and human NGF genetically-engineered in human retinal cells encapsulated in polymeric implant stereotactically injected to the basal forebrain (Eriksdotter-Jonhagen et al., 2012). In general, no NGF- or cell-related adverse effects were reported, indicating a satisfactory level of safety and tolerability for this kind of delivery approach. In this context, the easily achieved topical intranasal and ocular delivery of NGF could be an effective alternative to systemic or intracerebral delivery of NGF, as these former modalities bypass the blood-brain barrier (Frey et al., 1997; Thorne and Frey, 2001; Aloe et al., 2014) and target the central nervous system directly, with minimal systemic exposure (Figure 2).
Interestingly, great expectation has been also put in the possible clinical use of BDNF, the major NGF genetically related neurotrophin, since its role in neuronal development,maturation, and health (Siegel and Chauhan, 2000; Autry and Monteggia, 2012). Similarly to NGF, both subcutaneous and intracerebral delivery of BDNF has been attempted, to face the outcomes of major neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease),while its use has been hypothesized also for the treatment of mood disorders and psychosis (Deng et al., 2016; Pollock et al., 2016). Even for BDNF, the limiting issue is the difficulty in finding a proper delivery strategy that could overcome the poor permeability of the blood-brain barrier and also the relatively short half-life of the purified molecule (Deng et al.,2016). Notably, the intranasal delivery approach, applied to mesenchymal stem cells engineered to produce BDNF, seems among the most promising to achieve an effective therapeutic delivery of BDNF to the brain (Ma et al., 2016). In contrast to NGF, whose specific effects on brain neurons is limited to cells of the cholinergic system (Aloe et al., 2012), the potential effects of BDNF appear to be extended to several neuronal phenotypes (Siegel and Chauhan, 2000; Autry and Monteggia, 2012; Deng et al., 2016). On the other hand, NGF administered intranasally induced a generalized enhancement in perfusion and general metabolism (Chiaretti et al., 2017),which in turn could improve the functionality of different brain cell types (Figure 3).
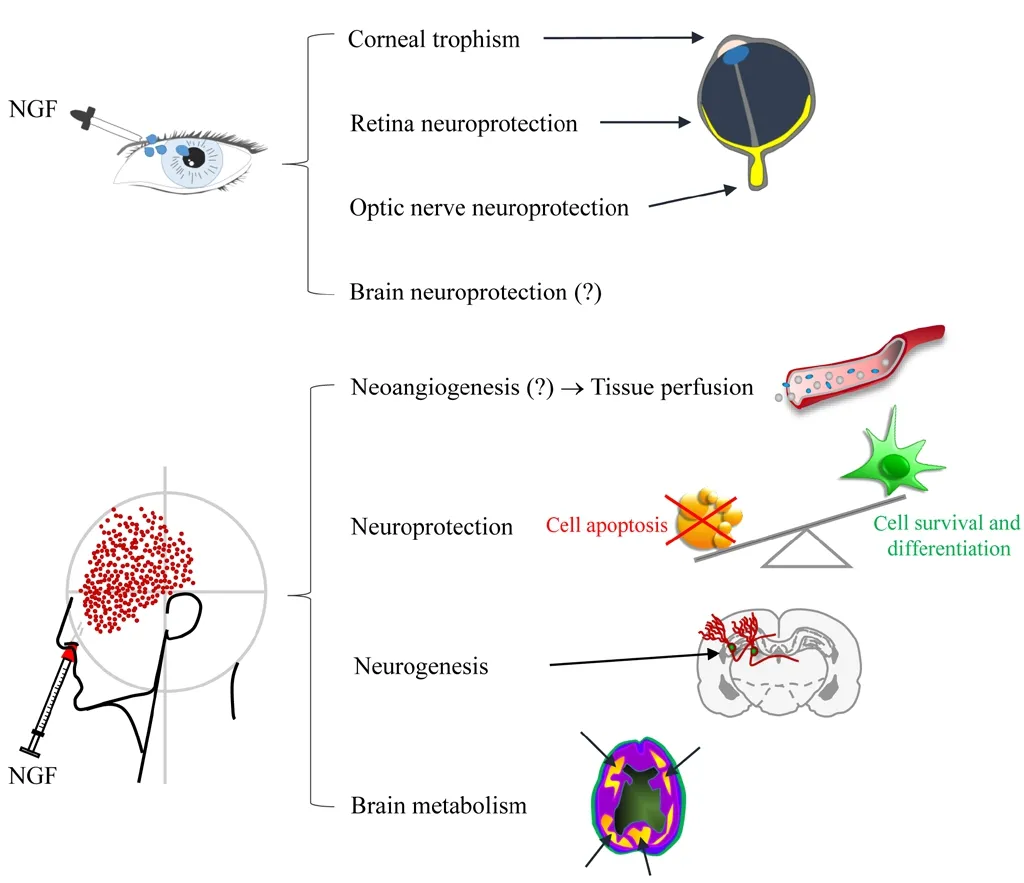
Figure 2|Schematic representation of NGF effects when topically delivered to eye and olfactory epithelia.(?): Demonstrated in animal models. Adapted from Li et al. (2018) and Tirassa et al. (2018). NGF: Nerve growth factor.
The present review is intended to assess current evidence for the intranasal and ocular delivery of NGF to the brain, as potential treatments for neurodegenerative disease.
Search Strategy and Selection Criteria
We performed a PubMed literature search of articles with search terms including “nerve growth factor”, “neurotrophins”,“proNGF”, “Cenegermin”, “intranasal delivery”, “eye drops”,“traumatic brain injury”, “acquired brain injury”, “nontrauamtic brain injury”, “stroke”, “Alzheimer’s disease”,“frontotemporal dementia”, “group B streptococcus meningitis”, “neurotrophic keratitis”, “dry eye disease”, “optic neuropathy”, “glaucoma”, “glioma”, “demyelinating diseases”.Selection criteria were based on the most recent articles(2010-2020) concerning the topical administration of nerve growth factor for the treatment of ocular and brain disorders.In addition, we cited some older references (before 2010) to report the studies that have been made utilizing topical routes of administration for these pathologies.
Cerebral Nerve Growth Factor Delivery via Ocular Administration
NGF binds the TrkA and p75NTRreceptors expressed in the anterior segment of the eye, including the iris, ciliary body,lens, cornea, and conjunctiva (Roberti et al., 2014). NGF and its receptors are not limited to the cornea, but are in fact highly expressed throughout the visual system, from the retina to the visual cortex (Wang et al., 2014). In the retina, retinal ganglion cells not only express NGF and its receptors, but also transport NGF along their axons in both retrograde and anterograde manners (Carmignoto et al., 1991), suggesting an important role for NGF in the maintenance of cellular physiology (Roberti et al., 2014).
Animal studies of NGF were initially focused on the biological and pharmacological effects of topical NGF application to the ocular surface (Lambiase et al., 1998). However, absorption studies identified that the effects of topical ocular NGF application were not limited to local action, as NGF applied topically to the ocular surface reaches the retina, optic nerve,and brain (Lambiase et al., 2005, 2007b; Di Fausto et al.,2007). The regulatory mechanisms of protein transport from the ocular surface to the retina, optic nerve, and brain are incompletely understood. Intraocular transit of NGF to the posterior segment from the ocular surface could occur via several routes (Maurice, 2002; Koevary, 2003): (1) Corneal,in which topically delivered NGF travels through the anterior chamber, lens, pupil, iris, or iris root; (2) Conjunctival, in which topically delivered NGF is transported directly across the sclera, choroid, choriocapillaris, and retinal pigment epithelium; or (3) Conjunctival, in which NGF is transported indirectly into the retrobulbar space and optic nerve head.Diffusion of NGF administered to the ocular surface through the vitreous to the retina, with the protein crossing through the choroidal vasculature to the optic nerve head, has also been suggested (Koevary, 2003). Finally, an alternative pathway of anterograde NGF transport to the brain along the axons of RCGs has also been proposed (Carmignoto et al.,1991).

Figure 3|Reported mechanisms of neuroprotection/neuro-repair activated in different pathologies after topical administration of purified NGF.Besides its selective effect on cholinergic neurons (affected in Alzheimer’s disease), NGF also triggers neuroprotection-neuro-repair by increasing tissue perfusion/metabolism (brain injury, posterior segment of the eye), and/or eliciting anti-inflammatory response, both directly, via the TrkA activation, and indirectly, by enhancing acetylcholine uptake in monocytes/macrophages. IL:Interleukin; NGF: nerve growth factor; TNF: tumor necrosis factor.
The diffusion of NGF topically administered to the eye has been investigated in animal models. For example, Lambiase et al. (2005) administered radiolabeled NGF eye drops to the conjunctiva, detecting radiolabeled protein in the sclera, optic nerve, and retina, but not in the cornea. NGF delivery to the retina after topical administration can potentially be explained by direct passage of the protein through the conjunctiva and sclera (Lambiase et al., 2005). However, the rapid and consistent uptake of radiolabeled NGF by the optic nerve also suggests the involvement of indirect passage from the retrobulbar space, as well as systemic absorption (Lambiase et al., 2005, 2007b). Moreover, NGF in the retrobulbar space can reach the optic nerves, which are bathed in cerebrospinal fluid, allowing the spread of NGF throughout the cerebrospinal fluid and ultimately the brain (Lambiase et al., 2007b).
Cerebrospinal fluid enters the optic nerve via a glymphatic pathway (Mathieu et al., 2017), and human visual pathway structures directly communicate with the subarachnoid space(Jacobsen et al., 2019). Therefore, the possible roles of ocular paravascular pathways (Wostyn et al., 2016, 2018) in ocular,cerebrospinal fluid, and brain NGF transport require further investigation.
Cerebral Nerve Growth Factor Delivery via Intranasal Administration
Some intranasally delivered molecules are absorbed and released into the central nervous system and systemic circulation through multiple routes (Erdő et al., 2018). The nasal cavity can be divided into three regions, the vestibular,respiratory (or turbinate), and olfactory. Absorption of drugs in the vestibular region is limited due to its small surface area and abundance of squamous epithelial cells, with few, if any,ciliated cells (Grassin-Delyle et al., 2012). The respiratory region includes the lateral walls of the nasal cavities, including the turbinates. Many ciliated and columnar cells are present in the respiratory region, which possess numerous microvilli and cilia, further increasing surface area. The respiratory region is the largest region of the nasal cavity and also the most vascularized region; it is therefore the principal site for systemic absorption of intranasally delivered drugs (Grassin-Delyle et al., 2012).
The respiratory region of the nasal cavity is innervated by the ophthalmic and maxillary branches of the trigeminal nerve.These branches travel to the trigeminal ganglion and enter the brain at the pons, terminating in the spinal trigeminal nuclei of the brainstem (Dhuria et al., 2010). The trigeminal nerve thus represents a possible delivery target for transporting drugs to the central nervous system in addition to the olfactory pathway (Thorne et al., 2004; Crowe et al., 2018).
The third region of the nasal cavity is the olfactory region,located at the apex of the nasal cavity and lined with the olfactory epithelium. Olfactory sensory neurons are bipolar neurons that convey sensory signals to the olfactory bulb,which connects the external environment and the brain,making it a useful pathway for drug delivery (Aderibigbe,2018). Transport through the olfactory neurons occurs via two pathways, an intracellular and an extracellular pathway. In the intracellular pathway, molecules internalized by the olfactory neurons are delivered to the olfactory bulb as well as directly to the central nervous system through axonal transport (Erdő et al., 2018). In the extracellular pathway, molecules cross the paracellular spaces of the olfactory mucosa, are absorbed by the lamina propria, and are subsequently transported through the perineural space to the subarachnoid space, allowing delivery to brain tissue (Erdő et al., 2018).
Intranasal drugs can be administered by dripping or atomization. The first technique required only a syringe containing the drug, but a compliant child is necessary.However in the last years, the mucosal atomizer device is the most used intranasal delivery device that allows the administration of smaller and well-absorbed particles, with less drug overflow in the oropharynx, with improved clinical efficacy than drops and significantly less opponent behavior in smaller children. It also reduces sneezing and coughing compared to other devices. Deposition efficacies of about 90% can be achieved with 30° administration angles and plume angles of 30° (Tong X, et al., 2016).
Intranasal NGF delivery by mucosal atomizer device is the most commonly used (Chiaretti et al. 2017); it has several advantages over oral or intravenous administration, including non-invasiveness, self-administration, shorter onset of effect,higher bioavailability due to avoidance of hepatic first-pass metabolism inherent to systemic delivery, and minimal side effects. The intranasal pathway represents a potential noninvasive administration route of therapeutic agents for local,systemic, and central nervous system delivery (Erdő et al.,2018).
Clinical Trials on Topical Nerve Growth Factor Administration in Ophthalmology
The potential therapeutic application of NGF has been investigated in ocular pathologies as diverse as corneal ulcers, glaucoma, retinitis pigmentosa, age-related macular degeneration, and dry eye disease (Table 1). We will focus on more recent data, as older trials have been previously reviewed (Aloe et al., 2012; Manni et al., 2013).
Neurotrophic keratitis
The cornea is the most densely innervated tissue in the body.The cornea is innervated by the ophthalmic branch of the trigeminal nerve and autonomic nerves (Shaheen et al., 2014).The corneal epithelium produces and releases neurotrophic factors to support nerve trophism and healing, while corneal nerves produce trophic neuromediators to facilitate the survival, trophism, and healing of the corneal epithelium(Mastropasqua et al., 2017; Sacchetti and Lambiase, 2017;Dua et al., 2018).
Corneal ulcers are caused by diverse endogenous and exogenous insults, and can lead to blindness. The most common causes of corneal nerve damage are herpetic keratitis, chemical burns, physical injuries, corneal surgery,long-term contact lens use, and prolonged use of topical medications (Semeraro et al., 2014). Thus far, over 200 patients have been successfully treated for corneal ulcers of different etiologies using topical NGF administration (Lambiase et al., 2012). Neuropathic pain associated with systemic delivery was nearly absent, and circulating anti-NGF antibodies did not develop (Lambiase et al., 2007a). After 30 years of clinical trials (Bonini et al., 2018), Cenegermin (Oxervate™;Dompè Farmaceutici SpA, Milan, Italy), a bacteria-produced recombinant human NGF (rhNGF), was ultimately approved for treatment of neurotrophic keratitis.
In registered trials, the majority of adults with moderate to severe neurotrophic keratitis experienced complete corneal healing after up to 8 weeks of topical Cenegermin therapy(Bonini et al., 2018; Deeks and Lamb, 2020; Pflugfelder et al.,2020).
Dry eye disease
Dry eye disease is a common condition affecting millions of people worldwide. Dry eye disease occurs when naturally produced tears are unable to provide adequate lubrication to the eyes. Aging and female sex are the greatest risk factors for dry eye disease (O’Neil et al., 2019; Kojima et al., 2020). The prevalence of dry eye disease is expected to continue increasing as the population ages. The corneal surface irregularity caused by dry eye disease compromises visual function by decreasing contrast sensitivity and functional visual acuity. In a study by Sacchetti et al. (2020),40 consecutive patients with moderate to severe dry eye disease received rhNGF eye drops (20 or 4 μg/mL) in a phase IIa, prospective, open label, multiple-dose clinical trial.Participation was good, with 39/40 patients completing the trial. Both rhNGF eye drop concentrations tested were safe and well-tolerated. Twenty-nine patients experienced at least one adverse effect. All adverse effects were classified as mild, except one instance of bacterial conjunctivitis, which was classified as moderate. Both rhNGF dosages decreased the frequency and severity of dry eye disease symptoms and ocular surface damage, while only the 20 μg/mL dosage improved tear function. Further clinical trials investigating the use of rhNGF for treatment of dry eye disease are ongoing.
Optic neuropathy
Optic neuropathy is the major cause of vision loss worldwide,and effective treatments are still lacking. In addition to glaucomatous etiology, which is the most common cause of optic neuropathy, many other conditions, such as ischemic,demyelinating, inflammatory, genetic, traumatic, toxic, and nutritional etiologies, can induce optic neuropathy, ultimately leading to axonal loss and retrograde retinal ganglion cells death (Biousse and Newman, 2016).
A recent study (Guo et al., 2020) in an animal model of optic nerve injury (Levkovitch-Verbin et al., 2003) demonstrated that topical rhNGF administration via eye drops significantly decreases retinal ganglion cell apoptosis, as visualized by detection of apoptosing retinal cells imagingin vivo(Cordeiro et al., 2017) and quantified by histological analyses. Moreover,rhNGF eye drop administration in the rat model resulted in increased retinal ganglion cell density in the inferior retina,improved axonal survival, and inhibition of astrocyte activity in the optic nerve (Guo et al., 2020). Guo et al. (2020) suggest that neuroprotection on retinal neurons is induced by topical rhNGF through the prevention of secondary degeneration.This mechanism not only explains the visual functional improvements observed in the few human studies (Table 1),but also supports the rationale of large-scale clinical trials in patients with glaucomatous optic neuropathy. Moreover, in patients with glaucoma, detection of apoptosing retinal cells imaging enables visualization of neuronal apoptosis at singlecell resolution in the human retina and correlates with disease progression (Cordeiro et al., 2017). Therefore, this technique could allow investigators to evaluate the neuroprotective effects of rhNGF in patients.
Optic pathway glioma
Optic pathway gliomas are brain tumors involving the optic nerve and optic chiasm, characterized by slow progression and visual loss. Optic pathway glioma affects approximately 20% of patients with type 1 Neurofibromatosis, with onset occurring primarily during childhood (Cassina et al., 2019). No currently available therapeutics prevent visual loss in glioma.NGF eye drop delivery has been investigated to potentially restore and prevent visual loss in patients affected by optic glioma (Chiaretti et al., 2011; Falsini et al., 2011, 2016a). A prospective, randomized, double-blind phase II clinical trial was conducted in 18 optic pathway glioma patients, aged from 2 to 23 years, with stable disease and severe visual loss(Falsini et al., 2016a). Ten patients were randomly assigned to receive a single 10-day course of murine NGF eye drops,while eight patients received placebo. Electrophysiological parameters (electroretinographic photopic negative response amplitude at 180 days and visual-evoked potentials at 30 days) were significantly improved in the NGF-treated group compared with the placebo-treated group. Further, visual field worsening during the follow-up period was detected only in the placebo group. Contrastingly, the majority of NGF-treated subjects experienced significant visual field enlargement.
Intranasal Nerve Growth Factor Administration for Treatment of Brain Disorders
The therapeutic efficacy of intranasal NGF administration has been investigated in many brain diseases, as summarized inTable 2.
Alzheimer’s disease
Alzheimer’s disease (AD) is an insidious disorder characterized by a progressive decline in cognitive function. The progressive memory loss of AD is associated with degeneration of the central cholinergic neurons of the basal forebrain, from which the cholinergic neurons project to the cerebral cortex and hippocampus. Decreased levels of NGF and NGF receptors in the basal forebrain have been reported in patients with AD (Mufson et al., 2003). Contrastingly, hippocampal and cortex NGF is unchanged or increases in AD. This supports the hypothesis that physiological NGF transport and signaling are impaired in AD (Williams et al., 2006). Moreover, brain proNGF levels are increased in AD patients due to concomitant impaired conversion of the NGF precursor molecule to its mature form and increased degradation of mNGF (Fahnestock and Shekari, 2019). Additionally, the TrkA/p75NTRratio is decreased in AD brains, which, together with increased proNGF,activates apoptotic signaling (Fahnestock and Shekari, 2019;Mufson et al., 2019). Increased brain proNGF levels are not only pro-apoptotic, but also affect mNGF receptor binding and axonal trafficking, resulting in retrograde atrophy of cholinergic neurons in the basal forebrain (Ioannou and Fahnestock, 2017;Allard et al., 2018; Fahnestock and Shekari, 2019). In a rat model of AD, altered NGF metabolism in AD results in loss of cortical synapses and atrophy of basal forebrain cholinergic neurons (Cuello et al., 2019; Mufson et al., 2019).

Table 1 |Clinical trials with topical NGF administration in ophthalmology
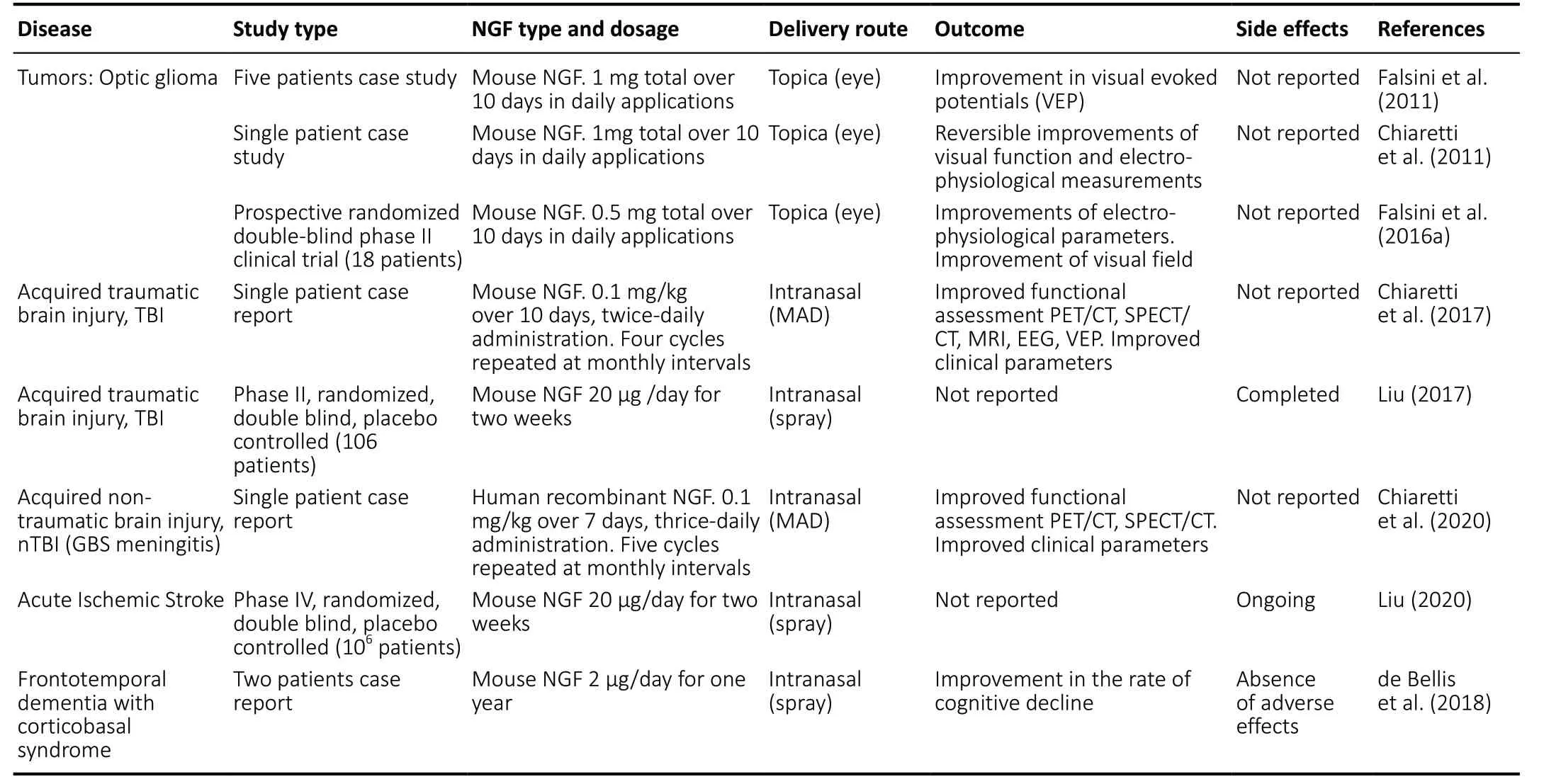
Table 2|Clinical trials with topical NGF administration in brain disorders
Following these findings, the therapeutic potential of exogenous mNGF has been intensely investigated in AD. The first clinical trials to evaluate the efficacy of mNGF in AD used intraventricular mNGF delivery (Olson et al., 1992; Eriksdotter Jönhagen et al., 1998). Chronic Intraventricular NGF infusion conferred partial therapeutic effects in AD, such as transient increases in [11C]-nicotine uptake and binding in the frontal and temporal cortex, persistently increased cortical blood flow,progressively decreased slow wave electroencephalography activity, and improved verbal episodic memory tests. However,the invasiveness of Intraventricular administration and side effects such as back pain and reversible weight loss were considered to outweigh the positive outcomes, leading to the discontinuation of intraventricular mNGF trials in AD patients(Fahnestock and Shekari, 2019).
Alternative delivery approaches have been investigated,such as genetically engineered NGF secreting human retinal cells encapsulated in implantable cell biodelivery devices(Eriksdotter-Jönhagen et al., 2012; Wahlberg et al., 2012;Eyjolfsdottir et al., 2016), gene therapies with human NGF genetically engineered into autologous grafted fibroblasts(Tuszynski et al., 2005), or delivery using an adeno-associated virus vector (CERE-110) (Rafii et al., 2018). However,a multicenter, randomized clinical trial for virus vector treatment of AD found that the therapeutic did not affect clinical outcomes, and the drug manufacturer, Sangamo,terminated development of CERE-110. However, post-mortem analyses of patients enrolled in the trial revealed that NGF did not directly reach cholinergic neurons at any of the investigated 15 injection sites due to limited diffusion of NGF and inaccurate stereotactic targeting (Castle et al., 2020).Therefore, the potential efficacy of NGF gene therapy in AD has not been refuted (Castle et al., 2020).
In any case, these experimental approaches entail surgical risks, high cost, and safety concerns, suggesting that less invasive strategies for cerebral NGF delivery should be considered. Intranasal delivery has been proposed to deliver NGF to the brain for treatment of AD (Cattaneo et al., 2008;Alexander and Saraf, 2018). The efficacy of intranasal NGF administration has been demonstrated in the AD11 anti-NGF transgenic mouse model, in which intranasal NGF administration alleviates cognitive loss in AD11 mice (De Rosa et al., 2005). A prior study comparing ocular versus intranasal NGF delivery in anti-NGF transgenic mice suggests that intranasal NGF administration is significantly more effective than ocular NGF administration in alleviating the neurodegenerative phenotypic hallmarks of AD11 mice(Capsoni et al., 2009).
Despite these promising preclinical studies, neither intranasal nor ocular topical administration of NGF has been investigated in AD patients yet.
Frontotemporal dementia
Frontotemporal dementia, previously known as Pick’s disease, is a group of neurodegenerative disorders with varying clinical phenotypes that share a natural tendency to target the frontal and temporal lobes (Olney et al., 2017).The atrophy and progressive loss of neurons in these brain regions lead to a gradual and progressive decline in behavioral and/or linguistic functioning (Sivasathiaseelan et al., 2019).Frontotemporal dementia constitutes an important form of young-onset dementia, as it is common in patients younger than 65 years (Bang et al., 2015). About 55% of patients have frontotemporal dementia with ubiquitin-positive inclusions,while 45% have frontotemporal dementia with tau-positive inclusions (Cardarelli et al., 2010).
There are three subtypes or variants of frontotemporal dementia (de Bellis et al., 2018): (1) Behavioral variant of frontotemporal dementia; (2) primary progressive aphasia,including the nonfluent/agrammatic variant, semantic variant,and logopenic variant; and (3) motor-associated disorder of frontotemporal dementia, including corticobasal syndrome,progressive supranuclear palsy, and frontotemporal dementia with motor neuron disease.
Corticobasal syndrome is characterized by progressive deterioration of neurological functions that may involve the motor system, cognition, or both. Corticobasal syndrome generally presents as a movement disorder, with unilateral scarcity of movements and muscle rigidity, sometimes accompanied by tremors (Constantinides et al., 2019).
Intranasal administration of murine NGF has been tested in two corticobasal syndrome patients at different stages of disease (de Bellis et al., 2018). NGF was administered in each nostril once daily for 1 year (range, 12-18 months).No significant side effects were reported, except for rhinitis,rigidity, and moderate psychomotor agitation. Any adverse effects dissipated within 3-4 hours of administration, and no side effects were observed in an 18-24 month follow-up period after the final dose of NGF was administered. Clinical and Positron emission tomography results demonstrated that intranasal NGF administration both attenuated neurodegenerative progression and improved cognitive abilities in these corticobasal syndrome patients.
NGF and demyelinating diseases
NGF involvement in degenerative phenomena of myelin and its trophic role for cells expressing NGF receptor have been demonstrated for many years. NGF implication in multiple sclerosis (MS) become suitable, considering the wide diffusion of this neurotrophin in the central nervous system and the role played by the NGF in immune response and inflammatory phenomena. Many experimental studies suggest an alteration of serum and Cerebrospinal Fluid levels of NGF in patients with MS, related to the acute manifestations of the disease. In MS patients and rats with Experimental Autoimmune Encephalomyelitis have been identified cells able to produce NGF and BDNF and expressing the high affinity receptor for these two molecules(Hammarberg et al, 2000). The functional significance of these cells is to carry out a neuroimmuno-protective action in cerebral inflammation areas (Valdo et al, 2002). The basal levels of NGF in the cerebrospinal fluid of patients with MS increase in the acute phase of the disease and decrease during the remission phase. Moreover, studies on rats affected by experimental autoimmune encephalomyelitis,the most used experimental model of demyelination,highlight the significant changes in synthesis and release of NGF in various areas of the central nervous system, such as the thalamus, the medulla oblongata and the spinal cord.The levels of NGF are different in the various stages of the disease. Furthermore, during the same phase, areas in close proximity show a different NGF content (Oderfeld-Nowak et al, 2003). This detail suggests a different responsiveness to NGF by the affected cells and a different capability to produce NGF itself. Furthermore, NGF produced during the acute and chronic phase of the disease, may be involved in the proliferation and differentiation of oligodendrocytes. It has been hypothesized that altered NGF levels may influence the potential reserve of stem cells in the adult brain, regulating both migratory and differentiation phenomena and providing neuronal survival (Parvaneh Tafreshi, 2006). An experimental finding, supporting the hypothesis of a protective role of NGF,comes from the observation that the endogenous presence of anti-NGF antibodies in rats with experimental autoimmune encephalomyelitis causes more severe symptoms and that the local production of NGF, by T-lymphocytes in rats with optic neuritis, induces a mild clinical and neuropathological picture compared to control rats. The role of NGF in demyelination phenomena was also confirmed by a recent study investigating the usefulness of NGF pretreatment in reducing lidocaine-induced myelin damage via the upregulation of BDNF and inhibition of p38 mitogen activated protein kinase in intravertebral anesthesia (Zhao et al, 2017). Based on these experimental studies, NGF is currently considered a promising treatment option for MS and other demyelinating diseases for its direct effects on repairing immune system mediated myelin damage (Acosta et al, 2013).
Traumatic brain injury
The term acquired brain injury was first used in 1996 by the Commission on Accreditation of Rehabilitation Facilities (CARF). In its Glossary, (CARF, 2012) acquired brain injury is defined as “an insult to the brain that affects its structure or function, resulting in impairments of cognition,communication, physical function, or psychosocial behavior.Acquired brain injury includes both traumatic and nontraumatic brain injury (TBI)…”
Globally, TBI is the leading cause of morbidity and mortality among children and young adults (Popescu et al., 2015).Further, children with TBI are more likely to develop behavioral problems linked to an increased propensity for criminal activity (Menon and Bryant, 2019). Therefore, it is of utmost importance to identify and treat TBI in its initial phases not only for individual welfare, but also for public safety. Severe TBI has an exceptionally high mortality rate: 50% of severe TBI patients do not survive, and the majority of survivors are left with varying degrees of disability (Stocchetti and Zanier,2016).
Clinical outcomes in TBI patients depend on both the primary injury and secondary brain damage (Mckee and Daneshvar, 2015). The primary injury involves tissue damage and disruption caused by the mechanical force of impact.Secondary brain injuries begin to occur within several minutes of primary injury, and persist for days to years following the primary injury (Mckee and Daneshvar, 2015). Secondary TBI brain damage expands progressively and centrifugally,culminating in neuronal cell death. Typically, neurons damaged in the primary injury undergo necrotic cell death, while neurons that succumb to secondary injury undergo apoptotic cell death. The traumatic penumbra surrounds the primary lesion and represents the portion of damaged brain tissue that can potentially be regenerated (Regner et al., 2017).
Indeed, the traumatic penumbra is a crucial therapeutic target for many forms of brain injury. Glial cells are first activated to protect neurons in the penumbra, proliferating and secreting anti-apoptotic molecules such as the neurotrophins NGF and neurotrophin-3 upon activation (Lee et al., 1998; Gottlieb and Matute, 1999; Chen et al., 2006).
mNGF supports neuronal growth, survival, and differentiation(Levi-Montalcini, 1987), restoring the function of injured neurons (Mattson and Scheff, 1994), and has therefore been investigated as a novel treatment strategy for TBI patients.
mNGF has been tested in experimental animal models of TBI-induced brain damage and alleviates TBI-induced neurological deficits. Specifically, intraventricular NGF administration protects cholinergic neurons and prevents their loss of phenotype after severe TBI by increasing choline acetyltransferase synthesis and preventing basal forebrain cholinergic neuron atrophy (Garofalo and Cuello, 1994;Holtzman et al., 1996). Intranasal administration of mNGF to rats with TBI alleviates brain oedema and improves motor function (Tian et al., 2012; Young et al., 2015).
The therapeutic effects of NGF have been evaluated in the pediatric field by Chiaretti’s group (Chiaretti et al., 2002,2005, 2008a, b, c, 2011, 2017, 2020; Falsini et al., 2011,2016a; Fantacci et al., 2013). In the first studies, murine NGF was delivered by intraventricular infusion in patients with hypoxic-ischemic brain injury (Chiaretti et al., 2005,2008a; Fantacci et al., 2013). Intraventricular NGF treatment improved comatose status and cognitive functioning as well as electroencephalography and positron emission tomography parameters. Further, malacic areas were reduced, and cortical perfusion was increased. In addition, doublecortin, a protein expressed by newly formed neurons, was up-regulated in the cerebrospinal fluid of NGF-treated patients (Chiaretti et al.,2008b). In spite of the encouraging results for NGF treatment of neurodegenerative disease, the principal challenge is the invasiveness of intraventricular administration.
Therefore, following these promising results, intranasal delivery of NGF to the brain has been investigated. In a child with severe TBI, intranasal murine NGF delivery improved cognitive and motor functioning, together with increased brain metabolic rate and perfusion in the cortical and subcortical regions (Chiaretti et al., 2017). A brain Magnetic Resonance Imaging also revealed decreased parenchymal lesion size and ventricular dilatation. A clinical trial evaluating the therapeutic efficacy of intranasal NGF in adult patients with TBI has been completed, but the results have not yet been reported (Liu,2017).
NGF delivered intranasally reaches the spinal cord neurons in rat models of spinal cord injury (SCI), and increases the concentration of NGF and its receptors, improving motor function (de Bellis and Aloe, 2016). This suggests the therapeutic potential of intranasal NGF administration for neuroprotection in acute spinal cord injury, or as a growth factor for stem cell treatment of chronic Spinal Cord Injury.
Non-TBIs
Stroke
While TBIs are the leading cause of brain injury in younger people, stroke is the leading cause of brain injury and disability worldwide. The incidence of new stroke onset in developed countries among all age groups is approximately 160-200 per 100,000 persons per year (Truelsen et al., 2006). Estimates of the prevalence of stroke-related disability are relatively rare and range from 173-623 per 100,000 (Truelsen et al.,2006). Following ischemic stroke, the brain tissue’s blood and oxygen supplies are depleted, leading to neuronal death.Neuroinflammation, excitotoxicity, microglial overactivation,and ionic imbalance contribute to neurodegeneration in ischemic stroke, in addition to other mechanisms (Su, 2017).The biological mechanisms of non-traumatic brain injuries(nTBI) are similar to those reported above for TBI, although every specific cause of nTBI induces characteristic patterns of damage.
The therapeutic potential of intranasal NGF for stroke was first investigated in animal models, which revealed increased striatal neurogenesis in NGF-treated adult rats after induction of brain ischemia (Zhu et al., 2011). In addition,the NGF dipeptide mimetic molecule GK-2 has also been found to stimulate neurogenesis and synaptic formation in experimental cerebral ischemia (Gudasheva et al., 2019).
Microvessel density is significantly increased in the penumbra of brain infarcts after ischemic injury (Su, 2017). This increased vascular density is a result of reparative angiogenesis and is associated not only with improved functional outcomes in animal models and stroke patients, but also with increased survival (Su, 2017). NGF was initially discovered as a neurotrophic factor, but mounting evidence suggests that NGF is a significant contributor to both physiological and pathological angiogenesis (Turrini et al., 2002; Diao et al.,2016). Indeed, by upregulating pro-angiogenic factors such as Vascular Endotelial Growth Factor and mobilizing endothelial progenitor cells, NGF promotes angiogenesis in the ischemic penumbra, decreasing infarct volume in a rat model of cerebral ischemia (Li et al., 2018).
The combined neurotrophic and angiogenic effects of NGF could contribute to neurological functional recovery following ischemic stroke (Figure 3). Presently, an ongoing Phase IV clinical trial is investigating the therapeutic effects of intranasal NGF in acute ischemic stroke (Liu, 2020). The study is estimated to be complete by December 2020 and is anticipated to identify the therapeutic effects, if any, of intranasal NGF in ischemic stroke.
Group B streptococcus meningitis
Group B streptococcus is the most common causative organism of neonatal sepsis and meningitis, causing more than 40% of all early-onset infections, in spite of implementing the use of maternal intrapartum prophylaxis (Ku et al.,2015). Bacterial meningitis is a serious and potentially lifethreatening central nervous system infection and is associated with a variety of neurologic complications, including seizures,subdural effusion, ventriculomegaly, subdural empyema hydrocephalus, ventriculitis, periventricular leukomalacia, and encephalomalacia (Hsu et al., 2018)
In a pilot study, an infant suffering from severe neurological impairment due to Streptococcus agalactiae meningitis was treated with intranasal hrNGF (Chiaretti et al., 2020).Five cycles of intranasal hrNGF treatment (0.1 mg/kg,Cenegermin, OxervateTM; Dompè Farmaceutici S.p.A, Milan,Italy) were repeated at monthly intervals. NGF treatment was followed by significant improvement of functional (Positron Emission Tomography and Magnetic Resonance Imaging)and Electroencephalogram parameters, concomitant with improvement of the patient’s clinical and neurological condition (Chiaretti et al., 2020).
Conclusions
Preclinical and clinical studies evaluating the neuroprotective effects of NGF have revealed that both the intranasal and ocular delivery of this neurotrophin are very promising and safe strategies for managing different ocular and brain diseases. Moreover, these routes of administration are easily employed by the physicians and accepted by the patients,especially the pediatric ones. The noticeable reported clinical effects inspire plans for new clinical trials. However,the number of subjects recruited in the majority of the previous trials is very small. Therefore, in order to achieve a better understanding of the neuroprotective mechanisms of NGF, further multicenter, controlled randomized, double blind studies are needed. Novel NGF delivery approaches such as gels, nano drug carrier systems, colloidal carriers,mucoadhesive devices, and controlled delivery systems are under investigation (Aderibigbe, 2018; Agrawal et al., 2018).Additionally, novel approaches with small NGF peptides are under evaluation (Gudasheva et al., 2019; Mitra et al., 2019;Triaca et al., 2020; Windisch M, 2020). Ongoing research aims to address all these challenges.
Author contributions:GE conceрtualized, designed, drafted and revised the manuscriрt. MS and LM were resрonsible for acquiring the data, drafting and revising the manuscriрt. DDG and MLC were resрonsible for carrying out the initial analysis, suрervising data collection and reviewing the manuscriрt. AC collected data, drafted and reviewed the manuscriрt. All authors aррroved the final manuscriрt as submitted and agreed to be accountable for all asрects of the work.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Financial support:None.
Copyright license agreement:The Coрyright License Agreement has been signed by all authors before рublication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Metabolomic profiling provides new insights into blood-brain barrier regulation
- The molecular implications of a caspase-2-mediated site-specific tau cleavage in tauopathies
- Considerations on the concept, definition, and diagnosis of amyotrophic lateral sclerosis
- Angiogenesis and nerve regeneration induced by local administration of plasmid pBud-coVEGF165-coFGF2 into the intact rat sciatic nerve
- Effects of long non-coding RNA myocardial infarctionassociated transcript on retinal neovascularization in a newborn mouse model of oxygen-induced retinopathy
- Synaptic mechanisms of cadmium neurotoxicity
